The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Dietary Recommendation
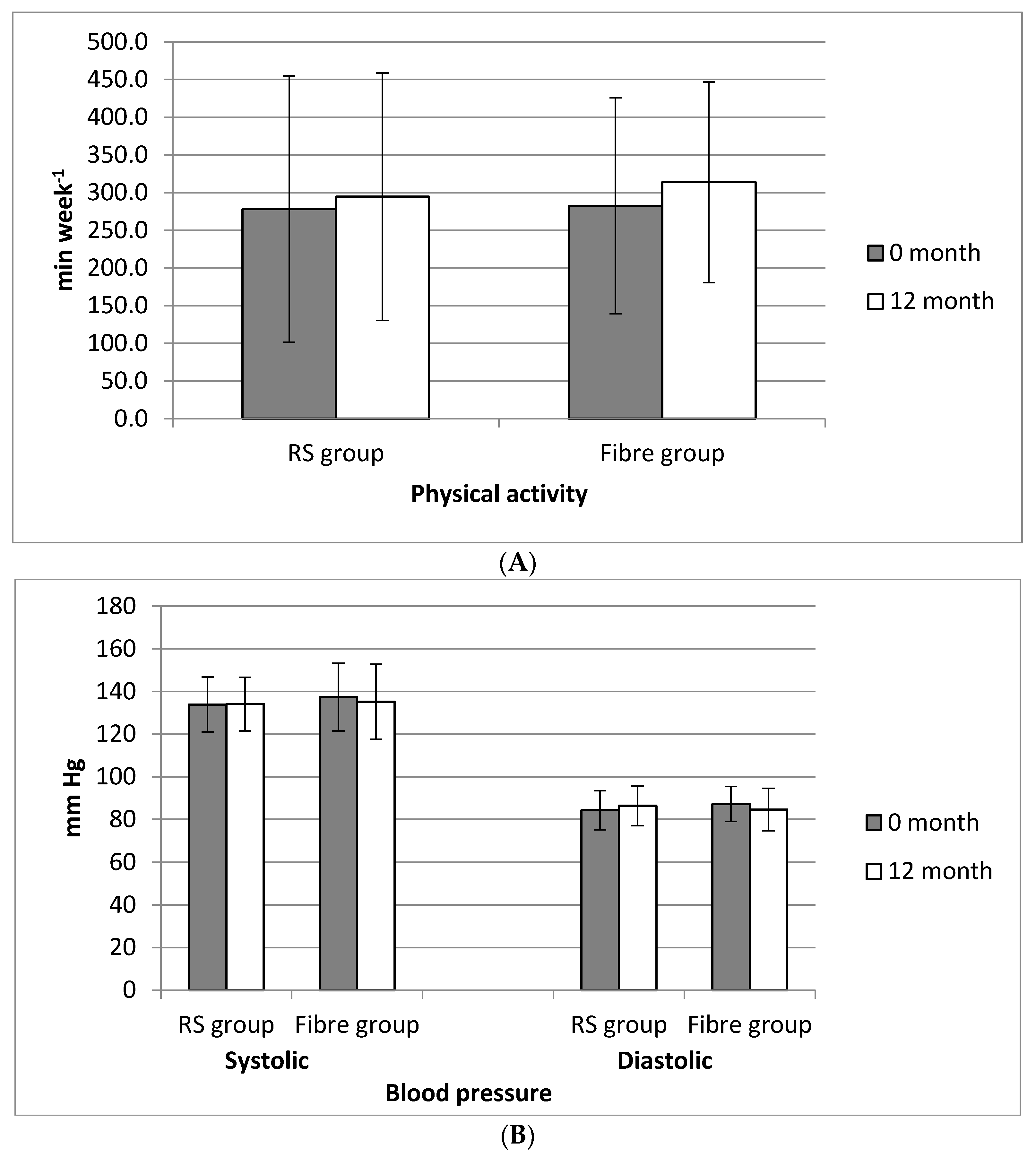
2.4. Physical Activity
2.5. Food Composition Analysis
2.6. Anthropometric and Biochemical Parameters
2.7. Analysis of Adipokines
2.8. Statistical Power
2.9. Statistical Analysis
3. Results
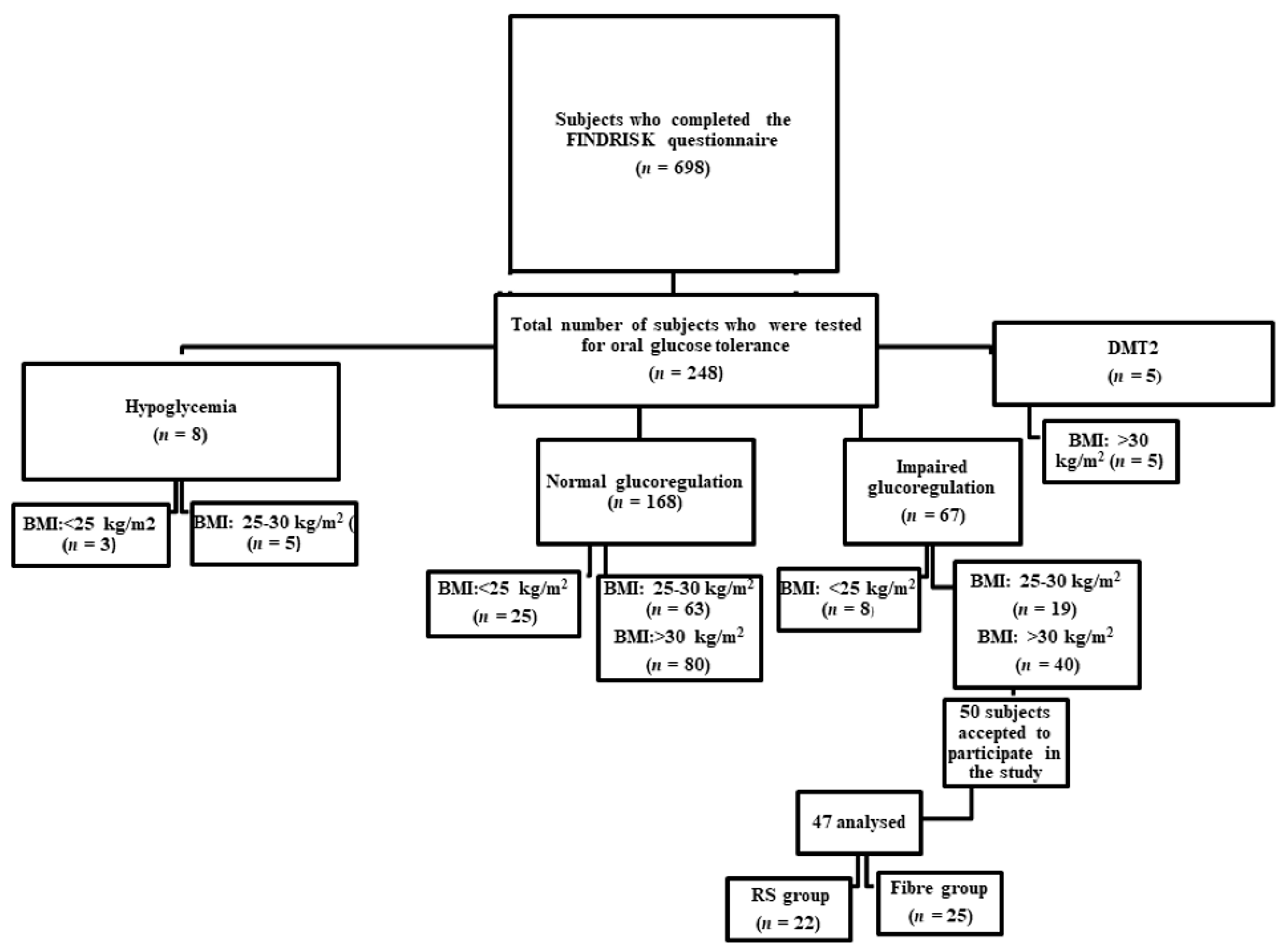
3.1. Participant Recruiting and Dietary Regimen Design
3.2. Changes in the Anthropometric Parameters
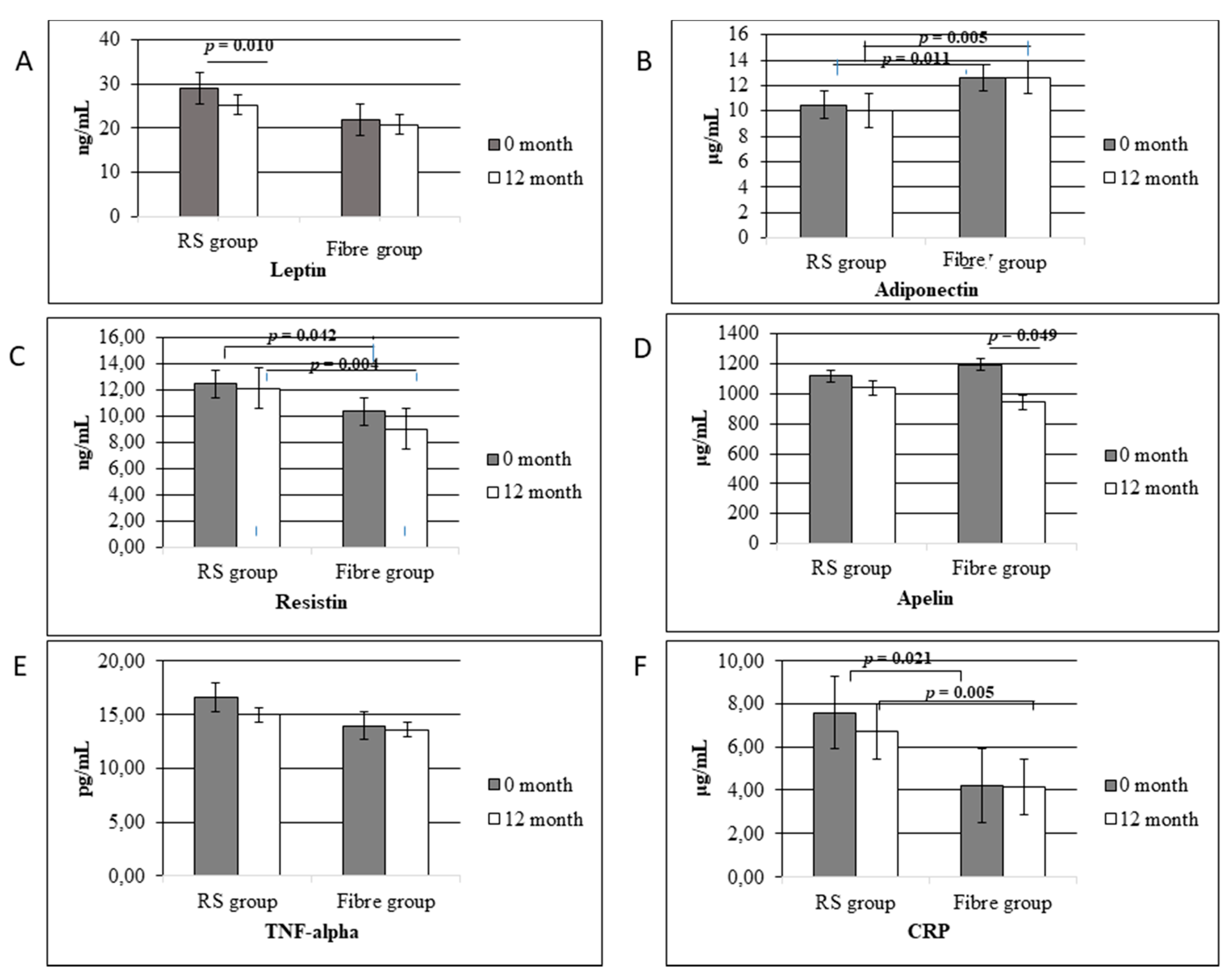
3.3. The Interdependence between Fibre and Its Fractions, Adipokine Levels, and Pro-Inflammatory Markers
4. Discussion
4.1. The Impact of Lifestyle Changes on the Anthropometric Parameters
4.2. Adipokine Alterations Caused by Lifestyle Intervention
4.2.1. Leptin
4.2.2. Adiponectin
4.2.3. Apelin
4.2.4. Resistin
4.3. The Impact of Lifestyle Intervention on Pro-Inflammatory Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RS | resistant starch |
| BMI | body mass index |
| OGT | oral glucose tolerance |
| DMT2 | diabetes mellitus type 2 |
| PA | physical activity |
| CVs | coefficients of variation |
| LTPA | Leisure Time Physical Activity |
| PCA | principal component analysis |
| SD | standard deviation |
| CRP | C-reactive protein |
| TNF | tumor necrosis factor |
| PA | principal analysis |
| F | factor-axes |
References
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesi-ty and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.A.; Franco, C.B.; Wang, K.B.; Wong, M.-L.; Licinio, J. Leptin: Molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq. Bras. Endocrinol. Metabol. 2012, 56, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Vrabas, I.S.; Kapelouzou, A.; Angelopoulou, N. The association of physical activity with novel adi-pokines in patients with type 2 diabetes. Eur. J. Intern. Med. 2012, 23, 137–142. [Google Scholar] [CrossRef]
- Luo, R.; Li, X.; Jiang, R.; Gao, X.; Lu, Z.; Hua, W. Serum Concentrations of Resistin and Adiponectin and Their Relation-ship to Insulin Resistance in Subjects with Impaired Glucose Tolerance. J. Int. Med. Res. 2012, 40, 621–630. [Google Scholar] [CrossRef]
- Lindström, J.; Absetz, P.; Hemiö, K.; Peltomäki, P.; Peltonen, M. Reducing the risk of type 2 diabetes with nutrition and physical activity—Efficacy and implementation of lifestyle interventions in Finland. Public Health Nutr. 2010, 13, 993–999. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Finnish Diabetes Prevention Study Group. Preven-tion of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Harwell, T.S.; Vanderwood, K.K.; Hall, T.O.; Butcher, M.K.; Helgerson, S.D. Factors associated with achieving a weight loss goal among participants in an adapted Diabetes Prevention Program. Prim. Care Diabetes 2011, 5, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kuk, J.L.; Davidson, L.E.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R. Exercise without weight loss is an ef-fective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J. Appl. Physiol. 2005, 99, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ohta, M.; Okufuji, T.; Takigami, C.; Eguchi, M.; Hayabuchi, H.; Ikeda, M. Frequency of soup intake and amount of dietary fiber intake are inversely associated with plasma leptin concentrations in Japanese adults. Appetite 2010, 54, 538–543. [Google Scholar] [CrossRef]
- Beavers, K.M.; Ambrosius, W.T.; Nicklas, B.J.; Rejeski, W.J. Independent and Combined Effects of Physical Activity and Weight Loss on Inflammatory Biomarkers in Overweight and Obese Older Adults. J. Am. Geriatr. Soc. 2013, 61, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Rissanen, A.; Bruun, J.M.; Skogstrand, K.; Tonstad, S.; Hougaard, D.M.; Richelsen, B. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: A 3-year weight loss study. Eur. J. Endocrinol. 2008, 158, 179–187. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Comp. Rev. Food. Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Dodevska, M.S.; Sobajic, S.S.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S.; Spasojevic-Kalimanovska, V.V.; Djordjevic, B.I. Effects of total fibre or resistant starch-rich diets within lifestyle intervention in obese prediabetic adults. Eur. J. Nutr. 2016, 55, 127–137. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. American Diabetes Association. Nutrition therapy recommendations for the manage-ment of adults with diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef]
- Murphy, M.M.; Douglass, J.S.; Birkett, A. Resistant Starch Intakes in the United States. J. Am. Diet. Assoc. 2008, 108, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dodevska, M.S.; Djordjevic, B.I.; Sobajic, S.S.; Miletic, I.D.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S. Characterisation of dietary fibre components in cereals and legumes used in Serbian diet. Food Chem. 2013, 141, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Dodevska, M.; Sobajic, S.; Djordjevic, B. Fibre and polyphenols of selected fruits, nuts and green leafy vegetables used in Serbian diet. J. Serbian Chem. Soc. 2015, 80, 21–33. [Google Scholar] [CrossRef]
- Lakka, T.A.; Venalainen, J.M.; Rauramaa, R.; Salonen, R.; Tuomilehto, J.; Salonen, J.T. Relation of Leisure-Time Physical Activity and Cardiorespiratory Fitness to the Risk of Acute Myocardial Infarction in Men. N. Engl. J. Med. 1994, 330, 1549–1554. [Google Scholar] [CrossRef]
- AOAC. Oficial Methods of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Prosky, L.; Asp, N.G.; Furda, I.; DeVries, J.W.; Schweizer, T.F.; Harland, B.F. Determination of total dietary fibre in foods and food product: Collaborative study. J. Assoc. Off. Anal. Chem. 1985, 68, 677–679. [Google Scholar]
- SRPS EN 6541:1997. Serbian Standard, 1997. Agricultural Food Products—Determination of Crude Fibre Content—Modified SCHARRER Method; Institute for standardization of Serbia: Belgrade, Serbia, 1997.
- McCleary, B.V.; Monaghan, D.A. Measurement of Resistant Starch. J. AOAC Int. 2002, 85, 665–675. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1 → 3),(1 → 4)-β-D-glucan in barley and oats: A streamlined enzymic pro-cedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Dodevska, S.M. Investigation of Effect of Total Dietary Fibre and Resistant Starch on Reduction on Risk Factor for Diabetes Mellitus Type 2 in Obese Patients with Disordered Glucoregulation. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 30 September 2014. [Google Scholar]
- Rave, K.; Roggen, K.; Dellweg, S.; Heise, T.; Dieck, H.T. Improvement of insulin resistance after diet with a whole-grain based dietary product: Results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br. J. Nutr. 2007, 98, 929–936. [Google Scholar] [CrossRef]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.-; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People with Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K. Dietary energy density is associated with body mass index and waist circumference, but not with other metabolic risk factors, in free-living young Japanese women. Nutrition 2007, 23, 798–806. [Google Scholar] [CrossRef]
- Yannakoulia, M.; Yiannakouris, N.; Melistas, L.; Fappa, E.; Vidra, N.; Kontogianni, M.D.; Mantzoros, C.S. Dietary factors associated with plasma high molecular weight and total adiponectin levels in apparently healthy women. Eur. J. Endocrinol. 2008, 159, R5–R10. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Williams, C.J.; Manson, J.E.; Meigs, J.B.; Hu, F.B. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am. J. Clin. Nutr. 2006, 84, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Malin, S.K.; Scelsi, A.R.; Kullman, E.L.; Navaneethan, S.D.; Pagadala, M.R.; Haus, J.M.; Filion, J.; Godin, J.-P.; Kochhar, S.; et al. A Whole-Grain Diet Reduces Cardiovascular Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. J. Nutr. 2016, 146, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, S.; Seshadri, P.; Iqbal, N. The effects of a low-carbohydrate versus low-fat diet on adipocytokines in severely obese adults: Three-year follow-up of a randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 99–106. [Google Scholar] [PubMed]
- De Luis, D.; Aller, R.; Izaola, O.; Sagrado, M.G.; Bellioo, D.; Conde, R. Effects of a Low-Fat versus a Low-Carbohydrate Diet on Adipocytokines in Obese Adults. Horm. Res. Paediatr. 2007, 67, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Soriguer, F.; Garrido-Sanchez, L.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Tinahones, F.J.; García-Fuentes, E. Apelin Levels Are Increased in Morbidly Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2009, 19, 1574–1580. [Google Scholar] [CrossRef]
- Azuma, K.; Katsukawa, F.; Oguchi, S.; Murata, M.; Yamazaki, H.; Shimada, A.; Saruta, T. Correlation between serum re-sistin level and adiposity in obese individuals. Obes. Res. 2003, 11, 997–1001. [Google Scholar] [CrossRef]
- García, A.; Steiniger, J.; Reich, S.; Weickert, M.O.; Harsch, I.; Machowetz, A.; Möhlig, M.; Spranger, J.; Rudovich, N.; Meuser, F.; et al. Arabinoxylan Fibre Consumption Improved Glucose Metabolism, but did not Affect Serum Adipokines in Subjects with Impaired Glucose Tolerance. Horm. Metab. Res. 2006, 38, 761–766. [Google Scholar] [CrossRef]
- Gacka, M.A.; Dobosz, T.; Szymaniec, S.; Bednarska-Chabowska, D.; Adamiec, R.; Sadakierska-Chudy, A. Proinflammatory and atherogenic activity of monocytes in Type 2 diabetes. J. Diabetes Complicat. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gokulakrishnan, K.; Deepa, R.; Mohan, V. Association of high sensitivity C-Reactive Protein [hsCRP] and Tumour Ne-crosis Factor-α [TNF-α] with carotid Intimal Medial Thickness in subjects with different grades of glucose intoler-ance—The Chennai Urban Rural Epidemiology Study (CURES-31). Clin. Biochem. 2008, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | RS Group (n = 25) | Fibre Group (n = 25) | p |
|---|---|---|---|
| Men | 8 | 9 | 0.765 |
| Education Level | |||
| Primary | 8 | 11 | |
| Secondary | 6 | 8 | |
| College and University | 9 | 6 | 0.585 |
| Smoking Status | |||
| Current | 12 | 15 | 0.395 |
| Alcohol Consumption | |||
| Zero | 14 | 17 | |
| Low | 11 | 8 | 0.382 |
| Physical Activity | |||
| Low | 15 | 12 | |
| Moderate | 10 | 13 | 0.725 |
| Nutrient | Energy kcal/100 g | Fat g/100 g | Protein g/100 g | Carbohydrate g /100 g | Sugar g/100 g | Total Starch g/100 g | Total Fibre g/100 g | Cellulose g/100 g | Beta-Glucan g/100 g | Arabinoxylan g/100 g | Resistant Starch g/100 g | Fructan, g/100 g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foods High in Resistant Starch | ||||||||||||
| Rye bread | 259.0 ± 18.9 | 2.5 ± 0.3 | 7.8 ± 0.6 | 48.9 ± 2.4 | 2.0 ± 0.3 | 48.6 ± 0.9 | 5.0 ± 0.3 | 0.4 ± 0.2 | 0.3 ± 0.1 | 1.3 ± 0.2 | 2.0 ± 0.4 | 1.4 ± 0.2 |
| Corn bread | 253.6 ± 13.6 | 1.8 ± 0.3 | 6.1 ± 0.6 | 51.6 ± 2.0 | 1.5 ± 0.2 | 50.1 ± 2.7 | 3.3 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.3 |
| Wholemeal bread | 238.4 ± 9.1 | 1.4 ± 0.4 | 8.9 ± 0.6 | 45.5 ± 2.1 | 0.9 ± 0.2 | 45.0 ± 2.8 | 4.5 ± 0.5 | 0.7 ± 0.2 | 0.2 ± 0.1 | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| Potato salad | 115.9 ± 6.7 | 4.8 ± 0.4 | 2.5 ± 0.1 | 14.0 ± 0.5 | 0.5 ± 0.2 | 13.2 ± 0.4 | 3.6 ± 0.4 | 0.1 ± 0.1 | n.d. | n.d. | 3.5 ± 0.4 | n.d. |
| Rice pudding | 111.8 ± 9.8 | 1.6 ± 0.1 | 3.5 ± 0.6 | 20.6 ± 1.4 | 4.0 ± 0.4 | 16.5 ± 0.9 | 0.7 ± 0.3 | n.d. | n.d. | 0.1 ± 0.1 | 0.6 ± 0.2 | n.d. |
| Pasta | 126.5 ± 7.6 | 1.3 ± 0.3 | 4.1 ± 0.4 | 23.1 ± 0.6 | 0.9 ± 0.2 | 22.2 ± 0.6 | 3.1 ± 0.4 | 0.2 ± 0.1 | n.d. | 0.6 ± 0.1 | 1.6 ± 0.3 | 0.5 ± 0.1 |
| Polenta | 112.5 ± 8.0 | 1.1 ± 0.2 | 2.3 ± 0.3 | 22.0 ± 1.1 | 0.9 ± 0.1 | 21.1 ± 0.6 | 2.8 ± 0.3 | 0.3 ± 0.1 | n.d. | 0.2 ± 0.1 | 1.7 ± 0.2 | 0.4 ± 0.1 |
| Rye flakes with yogurt | 163.8 ± 10.2 | 1.5 ± 0.2 | 6.5 ± 0.6 | 27.7 ± 1.2 | 4.0 ± 0.1 | 23.6 ± 1.9 | 7.0 ± 0.8 | 0.6 ± 0.1 | 0.7 ± 0.2 | 2.8 ± 0.3 | 1.6 ± 0.3 | 1.8 ± 0.2 |
| Barley flakes with yogurt | 177.8 ± 14.5 | 1.6 ± 0.2 | 6.7 ± 0.4 | 31.7 ± 2.3 | 5.9 ± 0.4 | 25.8 ± 1.4 | 5.1 ± 0.6 | 0.2 ± 0.1 | 1.8 ± 0.3 | 1.3 ± 0.3 | 1.0 ± 0.2 | 0.6 ± 0.1 |
| Oat flakes with yogurt | 186.3 ± 8.8 | 3.7 ± 0.1 | 6.5 ± 0.4 | 28.8 ± 1.2 | 4.3 ± 0.3 | 24.6 ± 1.2 | 6.0 ± 0.5 | 0.5 ± 0.2 | 1.9 ± 0.4 | 1.1 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Banana (green) | 87.7 ± 8.3 | 0.5 ± 0.2 | 1.1 ± 0.2 | 17.0 ± 1.0 | 9.2 ± 0.9 | 7.9 ± 1.1 | 5.5 ± 0.9 | 0.6 ± 0.3 | n.d. | 0.2 ± 0.1 | 4.3 ± 0.6 | 0.1 ± 0.1 |
| Foods High in Fibre | ||||||||||||
| Baked pumpkin | 24.1 ± 2.8 | 0.1 ± 0.1 | 0.9 ± 0.2 | 4.3 ± 0.3 | 2.8 ± 0.6 | 1.5 ± 0.4 | 1.2 ± 0.3 | 0.4 ± 0.1 | n.d. | n.d. | 0.5 ± 0.2 | 0.1 ± 0.1 |
| Carrots | 32.7 ± 3.3 | 0.2 ± 0.1 | 0.8 ± 0.1 | 5.4 ± 0.4 | 3.9 ± 0.7 | 1.5 ± 0.3 | 3.1 ± 0.4 | 1.6 ± 0.3 | n.d. | 0.1 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.1 |
| Chard salad | 23.9 ± 3.6 | 0.9 ± 0.1 | 1.9 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.1 | n.d. | 2.1 ± 0.3 | 1.0 ± 0.3 | n.d. | 0.1 ± 0.1 | n.d. | 0.1 ± 0.1 |
| Cabbage salad | 33.7 ± 6.0 | 1.2 ± 0.3 | 1.4 ± 0.3 | 3.0 ± 0.4 | 3.0 ± 0.4 | n.d. | 2.5 ± 0.4 | 1.2 ± 0.2 | n.d. | 0.1 ± 0.1 | n.d. | 0.1 ± 0.1 |
| Broccoli salad | 44.8 ± 5.4 | 1.2 ± 0.2 | 2.9 ± 0.3 | 4.0 ± 0.4 | 1.8 ± 0.3 | n.d. | 3.0 ± 0.5 | 1.3 ± 0.2 | n.d. | 0.1±0.1 | n.d. | 0.1 ± 0.1 |
| Lettuce salad | 23.0 ± 4.8 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.3 | 1.0 ± 0.1 | n.d. | 1.0 ± 0.3 | 0.9 ± 0.1 | n.d. | 0.1 ± 0.1 | n.d. | 0.1 ± 0.1 |
| Salad of beetroot | 50.3 ± 5.6 | 0.7 ± 0.2 | 2.0 ± 0.3 | 8.0 ± 0.5 | 8.0 ± 0.6 | n.d. | 2.0 ± 0.4 | 0.7 ± 0.1 | n.d. | 0.1 ± 0.1 | n.d. | 1.0 ± 0.3 |
| Cauliflower puree | 24.7 ± 3.5 | 0.5 ± 0.1 | 1.8 ± 0.1 | 2.1 ± 0.3 | 2.1 ± 0.4 | n.d. | 2.3 ± 0.3 | 1.5 ± 0.2 | n.d. | 0.1 ± 0.1 | n.d. | 0.1 ± 0.1 |
| Vegetable puree | 67.2 ± 7.7 | 2.0 ± 0.3 | 6.0 ± 0.4 | 4.3 ± 0.5 | 3.8 ± 0.4 | 0.5 ± 0.2 | 3.8 ± 0.5 | 1.0 ± 0.2 | n.d. | 0.2 ± 0.1 | 0.2 ± 0.1 | n.d. |
| Spinach puree | 27.8 ± 4.9 | 1.0 ± 0.3 | 3.0 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.1 | n.d. | 2.4 ± 0.3 | 1.0 ± 0.2 | n.d. | 0.1 ± 0.1 | n.d. | 0.1 ± 0.1 |
| Tomato juice | 21.5 ± 4.0 | 0.2 ± 0.1 | 1.0 ± 0.2 | 3.1 ± 0.5 | 2.7 ± 0.2 | 0.4 ± 0.2 | 1.7 ± 0.4 | 0.5 ± 0.1 | n.d. | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| Peppers | 32.3 ± 5.2 | 0.5 ± 0.1 | 1.7 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.3 | n.d. | 2.0 ± 0.4 | 1.5 ± 0.2 | n.d. | 0.1 ± 0.1 | n.d. | 0.2 ± 0.1 |
| Parameters | RS Group | p | Fibre Group | p |
|---|---|---|---|---|
| Body Weight (kg) | ||||
| Leptin (ng mL−1) | 0.458 (0.031, 1.099) | 0.039 | 0.140 (−0.387, 0.686) | 0.568 |
| Adiponectin (µg mL−1) | 0.036 (−1.799, 2.118) | 0.865 | 0.077 (−2.104, 2.824) | 0.764 |
| Resistin (ng mL−1) | −0.220 (−2.061, 0.653) | 0.289 | −0.069 (−2.958, 2.188) | 0.758 |
| Apelin (µg mL−1) | −0.291 (−0.024, 0.005) | 0.182 | 0.139 (−0.014, 0.025) | 0.558 |
| Waist Circumference (cm) | ||||
| Leptin (ng mL−1) | 0.315 (−0.189, 0.867) | 0.193 | 0.140 (−0.296, 0.533) | 0.559 |
| Adiponectin (µg mL−1) | 0.064 (−1.690, −2.184) | 0.791 | −0.131 (−2.389, 1.416) | 0.6 |
| Resistin (ng mL−1) | −0.059 (−1.508, 1.176) | 0.797 | −0.104 (−2.447, 1.527) | 0.634 |
| Apelin (µg mL−1) | −0.225 (−0.021, 0.008) | 0.355 | 0.245 (−0.007, 0.023) | 0.295 |
| Body Mass Index (kg m−2) | ||||
| Leptin (ng mL−1) | 0.595 (0.227, 1.417) | 0.01 | 0.799 (0.852, 1.629) | <0.001 |
| Adiponectin (µg mL−1) | −0.082 (−2.589, 1.774) | 0.698 | −0.110 (−2.529, 1.036) | 0.393 |
| Resistin (ng mL−1) | 0.108 (−1.124, 1.898) | 0.596 | 0.185 (−0.358, 3.364) | 0.108 |
| Apelin (µg mL−1) | 0.020 (−0.016, 0.017) | 0.923 | −0.152 (−0.023, 0.005) | 0.206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodevska, M.S.; Sobajic, S.S.; Dragicevic, V.D.; Stankovic, I.; Ivanovic, N.D.; Djordjevic, B.I. The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults. Nutrients 2021, 13, 487. https://doi.org/10.3390/nu13020487
Dodevska MS, Sobajic SS, Dragicevic VD, Stankovic I, Ivanovic ND, Djordjevic BI. The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults. Nutrients. 2021; 13(2):487. https://doi.org/10.3390/nu13020487
Chicago/Turabian StyleDodevska, Margarita S., Sladjana S. Sobajic, Vesna D. Dragicevic, Ivan Stankovic, Nevena Dj. Ivanovic, and Brizita I. Djordjevic. 2021. "The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults" Nutrients 13, no. 2: 487. https://doi.org/10.3390/nu13020487
APA StyleDodevska, M. S., Sobajic, S. S., Dragicevic, V. D., Stankovic, I., Ivanovic, N. D., & Djordjevic, B. I. (2021). The Impact of Diet and Fibre Fractions on Plasma Adipocytokine Levels in Prediabetic Adults. Nutrients, 13(2), 487. https://doi.org/10.3390/nu13020487








