The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review
Abstract
1. Introduction
2. Nutrition during Pregnancy and Lactation
2.1. Energy Requirement
2.2. What about Macronutrients?
2.3. Micronutrients
2.3.1. Iron and Other Minerals
2.3.2. Vitamins
2.3.3. Phytochemicals
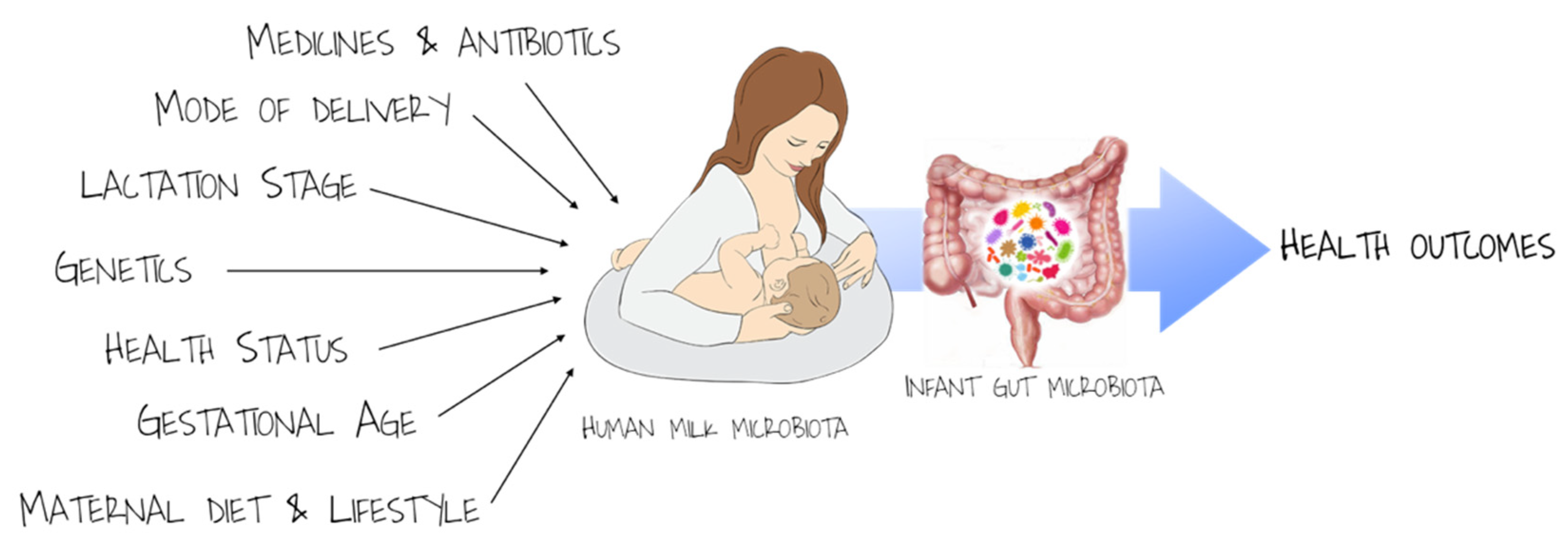
3. Human Milk: A Contribution to the Development of Infant Gut Microbiota and Immunity
4. Maternal Lifestyle and Nutritional Status during Pregnancy and Lactation and Later Health of Offspring: Some Traps
4.1. Tobacco Smoking
4.2. Obesity
4.3. Plant-Based Diet
4.4. Chemical Residues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eidelman, A.I.; Schanler, R.J. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A commentary by the espghan Committee on Nutrition. J. Pediatrics Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef]
- Mulligan, P.; White, N.R.J.; Monteleone, G.; Wang, P.; Wilson, J.W.; Ohtsuka, Y.; Sanderson, I.R. Breast milk lactoferrin regulates gene expression by binding bacterial DNA CpG motifs but not genomic DNA promoters in model intestinal cells. Pediatric Res. 2006, 59, 656–661. [Google Scholar] [CrossRef]
- Minekawa, R.; Takeda, T.; Sakata, M.; Hayashi, M.; Isobe, A.; Yamamoto, T.; Tasaka, K.; Murata, Y. Human breast milk suppresses the transcriptional regulation of IL-1β-induced NF-κB signaling in human intestinal cells. Am. J. Physiol. Cell Physiol. 2004, 287. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health A Glob. Access Sci. Source 2012, 11, 1–9. [Google Scholar] [CrossRef]
- Agostoni, C.; Baselli, L.; Mazzoni, M.B. Early nutrition patterns and diseases of adulthood: A plausible link? Eur. J. Intern. Med. 2013, 24, 5–10. [Google Scholar] [CrossRef]
- Verduci, E.; Giannì, M.L.; Di Benedetto, A. Human milk feeding in preterm infants: What has been done and what is to be done. Nutrients 2020, 12, 44. [Google Scholar] [CrossRef]
- Bode, L.; Raman, A.S.; Murch, S.H.; Rollins, N.C.; Gordon, J.I. Understanding the mother-breastmilk-infant “triad”. Science 2020, 367, 1070–1072. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Bruce, K.D. Maternal and in utero determinants of type 2 diabetes risk in the young. Curr. Diab. Rep. 2014, 14. [Google Scholar] [CrossRef]
- Catalano, P.; Demouzon, S.H. Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef]
- Scientific Opinion on Dietary Reference Values for Energy. EFSA J. 2013, 11. [CrossRef]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Energy and protein intake in pregnancy. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Saure, C.; Armeno, M.; Barcala, C.; Giudici, V.; Mazza, C.S. Excessive weight gain in exclusively breast-fed infants. J. Pediatric Endocrinol. Metab. 2017, 30, 719–724. [Google Scholar] [CrossRef]
- Forsum, E.; Lonnerdal, B. Effect of protein intake on protein and nitrogen composition of breast milk. Am. J. Clin. Nutr. 1980, 33, 1809–1813. [Google Scholar] [CrossRef]
- Grunewald, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Excessive Weight Gain during Full Breast-Feeding. Ann. Nutr. Metab. 2014, 64, 271–275. [Google Scholar] [CrossRef]
- De La Presa-Owens, S.; López-Sabater, M.C.; Rivero-Urgell, M. Fatty acid composition of human milk in Spain. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 180–185. [Google Scholar] [CrossRef]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2017, 69, 28–40. [Google Scholar] [CrossRef]
- Morrow, A.L.; Dawodu, A. Fatty Acids and Fat-Soluble Vitamins in Breast Milk: Physiological Significance and Factors Affecting Their Concentrations. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 57–67. [Google Scholar] [CrossRef]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Innis, S.M.; Friesen, R.W. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am. J. Clin. Nutr. 2008, 87, 548–557. [Google Scholar] [CrossRef]
- Lonnerdal, B. Effects of maternal dietary intake on human milk composition. J. Nutr. 1986, 116, 499–513. [Google Scholar] [CrossRef]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. N-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and meta-analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [CrossRef]
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1415. [Google Scholar] [CrossRef]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef]
- Sallis, H.; Steer, C.; Paternoster, L.; Davey Smith, G.; Evans, J. Perinatal depression and omega-3 fatty acids: A Mendelian randomisation study. J. Affect. Disord. 2014, 166, 124–131. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Tian, H.M.; Wu, Y.X.; Lin, Y.Q.; Chen, X.Y.; Yu, M.; Lu, T.; Xie, L. Dietary patterns affect maternal macronutrient intake levels and the fatty acid profile of breast milk in lactating Chinese mothers. Nutrition 2019, 58, 83–88. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Dewey, K.G.; Perez-Exposito, A.B.; Nurhasan, M.; Lauritzen, L.; Roos, N. Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Matern. Child Nutr. 2011, 7, 124–140. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef]
- Copp, K.; DeFranco, E.A.; Kleiman, J.; Rogers, L.K.; Morrow, A.L.; Valentine, C.J. Nutrition Support Team Guide to Maternal Diet for the Human-Milk-Fed Infant. Nutr. Clin. Pract. 2018, 33, 687–693. [Google Scholar] [CrossRef]
- de Waard, M.; Brands, B.; Kouwenhoven, S.M.P.; Lerma, J.C.; Crespo-Escobar, P.; Koletzko, B.; Zalewski, B.M.; van Goudoever, J.B. Optimal nutrition in lactating women and its effect on later health of offspring: A systematic review of current evidence and recommendations (EarlyNutrition project). Crit. Rev. Food Sci. Nutr. 2017, 57, 4003–4016. [Google Scholar] [CrossRef]
- Campoy, C.; Escolano-Margarit, V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Gibson, R.A.; Makrides, M. Effect of long-chain polyunsaturated fatty acid supplementation during pregnancy or lactation on infant and child body composition: A systematic review. Am. J. Clin. Nutr. 2010, 92, 857–863. [Google Scholar] [CrossRef]
- Rodríguez, G.; Iglesia, I.; Bel-Serrat, S.; Moreno, L.A. Effect of n-3 long chain polyunsaturated fatty acids during the perinatal period on later body composition. Br. J. Nutr. 2012, 107, S117–S128. [Google Scholar] [CrossRef]
- Martínez-Victoria, E.; Yago, M.D. Omega 3 polyunsaturated fatty acids and body weight. Br. J. Nutr. 2012, 107, S107–S116. [Google Scholar] [CrossRef]
- Stratakis, N.; Gielen, M.; Chatzi, L.; Zeegers, M.P. Effect of maternal n-3 long-chain polyunsaturated fatty acid supplementation during pregnancy and/or lactation on adiposity in childhood: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2014, 68, 1277–1287. [Google Scholar] [CrossRef]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Jensen, C.L.; Voigt, R.G.; Llorente, A.M.; Peters, S.U.; Prager, T.C.; Zou, Y.L.; Rozelle, J.C.; Turcich, M.R.; Fraley, J.K.; Anderson, R.E.; et al. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J. Pediatrics 2010, 157, 900–905. [Google Scholar] [CrossRef]
- Bergmann, R.L.; Bergmann, K.E.; Richter, R.; Haschke-Becher, E.; Henrich, W.; Dudenhausen, J.W. Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six-year follow-up of a prospective randomized double-blind monocenter study on low-dose DHA supplements. J. Perinat. Med. 2012, 40, 677–684. [Google Scholar] [CrossRef]
- Brei, C.; Stecher, L.; Much, D.; Karla, M.-T.; Amann-Gassner, U.; Shen, J.; Ganter, C.; Karampinos, D.C.; Brunner, S.; Hauner, H. Reduction of the n–6:n–3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: Follow-up results from a randomized controlled trial up to 5 y of age. Am. J. Clin. Nutr. 2016, 103, 1472–1481. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef]
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human milk omega-3 fatty acid composition is associated with infant temperament. Nutrients 2019, 11, 2964. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of Maternal Diet on Human Milk Composition Among Lactating Women in Latvia. Medicina 2019, 55, 173. [Google Scholar] [CrossRef]
- Gay, M.C.; Koleva, P.T.; Slupsky, C.M.; du Toit, E.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide Variation in Human Milk Metabolome: Indicators of Breast Physiology and Maternal Lifestyle? Nutrients 2018, 10, 1151. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. The Journal of Nutrition Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F.; Hart, G.W. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- Maessen, S.E.; Derraik, J.G.B.; Binia, A.; Cutfield, W.S. Perspective: Human Milk Oligosaccharides: Fuel for Childhood Obesity Prevention. Adv. Nutr. 2020, 11, 35–40. [Google Scholar] [CrossRef]
- Lagström, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Mäkelä, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 2020, 111, 769–778. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Puddu, M.; Peroni, D.G.; Fanos, V. The Human Breast Milk Metabolome in Overweight and Obese Mothers. Front. Immunol. 2020, 11, 1533. [Google Scholar] [CrossRef]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatrics 2019, 7, 297. [Google Scholar] [CrossRef]
- Fischer Fumeaux, C.J.; Garcia-Rodenas, C.L.; De Castro, C.A.; Courtet-Compondu, M.C.; Thakkar, S.K.; Beauport, L.; Tolsa, J.F.; Affolter, M. Longitudinal Analysis of Macronutrient Composition in Preterm and Term Human Milk: A Prospective Cohort Study. Nutrients 2019, 11, 1525. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Calvo, M.V.; Megino-Tello, J.; Aguayo-Maldonado, J.; Jiménez-Flores, R.; Fontecha, J. Effect of gestational age (preterm or full term) on lipid composition of the milk fat globule and its membrane in human colostrum. J. Dairy Sci. 2020, 103, 7742–7751. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics 2014, 14, 216. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Overview of nutrients in humanmilk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Koletzko, B.; Bauer, C.P.; Bung, P.; Cremer, M.; Flothkötter, M.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Rasenack, R.; et al. German National Consensus Recommendations on Nutrition and Lifestyle in Pregnancy by the “Healthy Start-Young Family Network”. Ann. Nutr. Metab. 2013, 63, 311–322. [Google Scholar] [CrossRef]
- Allen, L.H. Anemia and iron deficiency: Effects on pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 1280S–1284S. [Google Scholar] [CrossRef]
- Khambalia, A.Z.; Collins, C.E.; Roberts, C.L.; Morris, J.M.; Powell, K.L.; Tasevski, V.; Nassar, N. Iron deficiency in early pregnancy using serum ferritin and soluble transferrin receptor concentrations are associated with pregnancy and birth outcomes. Eur. J. Clin. Nutr. 2016, 70, 358–363. [Google Scholar] [CrossRef]
- Alwan, N.A.; Hamamy, H. Maternal Iron Status in Pregnancy and Long-Term Health Outcomes in the Offspring. J. Pediatric Genet. 2015, 4, 111–123. [Google Scholar] [CrossRef]
- Krebs, N.F.; Domellöf, M.; Ziegler, E. Balancing benefits and risks of iron fortification in resource-rich countries. J. Pediatric 2015, 167, S20–S25. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Gayemmagami, S.J. Association between zinc, copper, and iron concentrations in breast milk and growth of healthy infants in Tabriz, Iran. Biol. Trace Elem. Res. 2010, 135, 174–181. [Google Scholar] [CrossRef]
- Nakamori, M.; Ninh, N.X.; Isomura, H.; Yoshiike, N.; Hien, V.T.T.; Nhug, B.T.; Van Nhien, N.; Nakano, T.; Khan, N.C.; Yamamoto, S. Nutritional status of lactating mothers and their breast milk concentration of iron, zinc and copper in rural Vietnam. J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 338–345. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.-M.; Lee, J.-E.; Cho, M.S.; Kang, B.S.; Choi, H.; Kim, Y. Association of Maternal Diet With Zinc, Copper, and Iron Concentrations in Transitional Human Milk Produced by Korean Mothers. Clin. Nutr. Res. 2016, 5, 15–25. [Google Scholar] [CrossRef]
- Khambalia, A.; Latulippe, M.E.; Campos, C.; Merlos, C.; Villalpando, S.; Picciano, M.F.; O’Connor, D.L. Milk folate secretion is not impaired during iron deficiency in humans. J. Nutr. 2006, 136, 2617–2624. [Google Scholar] [CrossRef]
- Maru, M.; Birhanu, T.; Tessema, D.A. Calcium, magnesium, iron, zinc and copper, compositions of human milk from populations with cereal and “enset” based diets. Ethiop. J. Health Sci. 2013, 23, 90–97. [Google Scholar]
- Trumpff, C.; Vandevijvere, S.; Moreno-Reyes, R.; Vanderpas, J.; Tafforeau, J.; Van Oyen, H.; De Schepper, J. Neonatal thyroid-stimulating hormone level is influenced by neonatal, maternal, and pregnancy factors. Nutr. Res. 2015, 35, 975–981. [Google Scholar] [CrossRef]
- Czech-Kowalska, J.; Latka-Grot, J.; Bulsiewicz, D.; Jaworski, M.; Pludowski, P.; Wygledowska, G.; Chazan, B.; Pawlus, B.; Zochowska, A.; Borszewska-Kornacka, M.K.; et al. Impact of vitamin D supplementation during lactation on vitamin D status and body composition of mother-infant pairs: A MAVID randomized controlled trial. PLoS ONE 2014, 9, e107708. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hulsey, T.C.; Fanning, D.; Ebeling, M.; Hollis, B.W. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: A 6-month follow-up pilot study. Breastfeed. Med. 2006, 1, 59–70. [Google Scholar] [CrossRef]
- Basile, L.A.; Taylor, S.N.; Wagner, C.L.; Horst, R.L.; Hollis, B.W. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed. Med. 2006, 1, 27–35. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Cawley, S.; Mullaney, L.; McKeating, A.; Farren, M.; McCartney, D.; Turner, M.J. A review of European guidelines on periconceptional folic acid supplementation. Eur. J. Clin. Nutr. 2016, 70, 143–154. [Google Scholar] [CrossRef]
- Kodentsova, V.M.; Vrzhesinskaya, O.A. Evaluation of the vitamin status in nursing women by vitamin content in breast milk. Bull. Exp. Biol. Med. 2006, 141, 323–327. [Google Scholar] [CrossRef]
- Salmenpera, L. Vitamin C nutrition during prolonged lactation: Optimal in infants while marginal in some mothers. Am. J. Clin. Nutr. 1984, 40, 1050–1056. [Google Scholar] [CrossRef]
- Lietz, G.; Henry, C.J.K.; Mulokozi, G.; Mugyabuso, J.K.L.; Ballart, A.; Ndossi, G.D.; Lorri, W.; Tomkins, A. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. Am. J. Clin. Nutr. 2001, 74, 501–509. [Google Scholar] [CrossRef]
- da Silva, A.G.C.L.; de Sousa Rebouças, A.; Mendonça, B.M.A.; Silva, D.C.N.E.; Dimenstein, R.; Ribeiro, K.D.D.S. Relationship between the dietary intake, serum, and breast milk concentrations of vitamin A and vitamin E in a cohort of women over the course of lactation. Matern. Child Nutr. 2019, 15. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US); 2006 Vitamin C. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544628/ (accessed on 20 July 2020).
- Tsopmo, A. Phytochemicals in human milk and their potential antioxidative protection. Antioxidants 2018, 7, 32. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.A.; Hamulka, J.; Grabowicz-Chadrzyńska, I.; Bryś, J.; Wesolowska, A. Association between breastmilk LC PUFA, carotenoids and psychomotor development of exclusively breastfed infants. Int. J. Environ. Res. Public Health 2019, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.; Danneskiold-Samsøe, N.B.; Brejnrod, A.; Hoffmann, C.; Cabral, V.P.; de Iaucci, J.M.; Sales, C.H.; Fisberg, R.M.; Cortez, R.V.; Brix, S.; et al. The human milk microbiota is modulated by maternal diet. Microorganisms 2019, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Gomez-Gallego, C.; Garcia-Mantrana, I.; Salminen, S.; Collado, M.C. The human milk microbiome and factors influencing its composition and activity. Semin. Fetal Neonatal Med. 2016, 21, 400–405. [Google Scholar] [CrossRef]
- Dreyer, J.L.; Liebl, A.L. Early colonization of the gut microbiome and its relationship with obesity. Hum. Microbiome J. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 2017, 32, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 2017, 39, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Van Den Elsen, L.W.J.; Rekima, A.; Verhasselt, V. Early-Life Nutrition and Gut Immune Development. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 137–149. [Google Scholar] [CrossRef]
- Reijneveld, S.A.; Brugman, E.; Hirasing, R.A. Infantile colics: Maternal smoking as potential risk factor. Arch. Dis. Child. 2000, 83, 302–303. [Google Scholar] [CrossRef]
- Ekblad, M.; Korkeila, J.; Lehtonen, L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. Int. J. Paediatr. 2015, 104, 12–18. [Google Scholar] [CrossRef]
- Agostoni, C.; Marangoni, F.; Grandi, F.; Lammardo, A.M.; Giovannini, M.; Riva, E.; Galli, C. Original communication earlier smoking habits are associated with higher serum lipids and lower milk fat and polyunsaturated fatty acid content in the first 6 months of lactation. Eur. J. Clin. Nutr. 2003, 57, 1466–1472. [Google Scholar] [CrossRef]
- Hopkinson, J.M.; Schanler, R.J.; Fraley, J.K.; Garza, C. Milk production by mothers of premature infants: Influence of cigarette smoking. Pediatrics 1992, 90, 934–938. [Google Scholar]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 1–7. [Google Scholar] [CrossRef]
- Harrod, C.S.; Reynolds, R.M.; Chasan-Taber, L.; Fingerlin, T.E.; Glueck, D.H.; Brinton, J.T.; Dabelea, D. Quantity and timing of maternal prenatal smoking on neonatal body composition: The healthy start study. J. Pediatrics 2014, 165, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Blatt, K.; Moore, E.; Chen, A.; Van Hook, J.; Defranco, E.A. Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstet. Gynecol. 2015, 125, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Szlagatys-Sidorkiewicz, A.; Martysiak-Zurowska, D.; Krzykowski, G.; Zagierski, M.; Kamińska, B. Maternal smoking modulates fatty acid profile of breast milk. Acta Paediatr. Int. J. Paediatr. 2013, 102. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Galli, C.; Riva, E.; Colombo, C.; Giovannini, M.; Marangoni, F. Reduced docosahexaenoic acid synthesis may contribute to growth restriction in infants born to mothers who smoke. J. Pediatrics 2005, 147, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Pirini, F.; Guida, E.; Lawson, F.; Mancinelli, A.; Guerrero-Preston, R. Nuclear and mitochondrial DNA alterations in newborns with prenatal exposure to cigarette smoke. Int. J. Environ. Res. Public Health 2015, 12, 1135–1155. [Google Scholar] [CrossRef] [PubMed]
- Knopik, V.S.; MaCcani, M.A.; Francazio, S.; McGeary, J.E. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev. Psychopathol. 2012, 24, 1377–1390. [Google Scholar] [CrossRef]
- Lee, K.W.K.; Richmond, R.; Hu, P.; French, L.; Shin, J.; Bourdon, C.; Reischl, E.; Waldenberger, M.; Zeilinger, S.; Gaunt, T.; et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ. Health Perspect. 2015, 123, 193–199. [Google Scholar] [CrossRef]
- Anderson, T.M.; Lavista Ferres, J.M.; You Ren, S.; Moon, R.Y.; Goldstein, R.D.; Ramirez, J.M.; Mitchell, E.A. Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics 2019, 143. [Google Scholar] [CrossRef]
- Ino, T. Maternal smoking during pregnancy and offspring obesity: Meta-analysis. Pediatrics Int. 2010, 52, 94–99. [Google Scholar] [CrossRef]
- Oken, E.; Levitan, E.B.; Gillman, M.W. Maternal smoking during pregnancy and child overweight: Systematic review and meta-analysis. Int. J. Obes. 2008, 32, 201–210. [Google Scholar] [CrossRef]
- Al Mamun, A.; Lawlor, D.A.; Alati, R.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am. J. Epidemiol. 2006, 164, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Koshy, G.; Delpisheh, A.; Brabin, B.J. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur. J. Public Health 2011, 21, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Gerstein, H.C.; Holloway, A.C. Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 2010, 116, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Salvi, S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur. J. Pediatrics 2009, 168, 897–905. [Google Scholar] [CrossRef]
- Merritt, T.A.; Mazela, J.; Adamczak, A.; Merritt, T. The Impact of Second-Hand Tobacco Smoke Exposure on Pregnancy Outcomes, Infant Health, and the Threat of Third-Hand Smoke Exposure to Our Environment and to Our Children. Available online: https://pubmed.ncbi.nlm.nih.gov/23421018/ (accessed on 25 October 2020).
- Mennella, J.A.; Yourshaw, L.M.; Morgan, L.K. Breastfeeding and smoking: Short-term effects on infant feeding and sleep. Pediatrics 2007, 120, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 2014, 64, 26–34. [Google Scholar] [CrossRef]
- Vickers, M.H. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Gray, C.; Li, M.; Segovia, S.A.; Vickers, M.H. Early life nutrition and energy balance disorders in offspring in later life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef]
- Enstad, S.; Cheema, S.; Thomas, R.; Fichorova, R.N.; Martin, C.R.; O’Tierney-Ginn, P.; Wagner, C.L.; Sen, S. The impact of maternal obesity and breast milk inflammation on developmental programming of infant growth. Eur. J. Clin. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Williams, C.B.; MacKenzie, K.C.; Gahagan, S. The effect of maternal obesity on the offspring. Clin. Obstet. Gynecol. 2014, 57, 508–515. [Google Scholar] [CrossRef]
- Oliveira, E.; Marano, D.; Do Amaral, Y.N.D.V.; Abranches, A.; Soares, F.V.M.; Moreira, M.E.L. Overweight modifies the nutritional composition of human milk? A systematic review. Cienc. Saude Coletiva 2020, 25, 3969–3980. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Sims, C.R.; Piccolo, B.D.; Andres, A. Maternal adiposity alters the human milk metabolome: Associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 2020, 112, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Amaral, Y.; Marano, D.; Oliveira, E.; Moreira, M.E. Impact of pre-pregnancy excessive body weight on the composition of polyunsaturated fatty acids in breast milk: A systematic review. Int. J. Food Sci. Nutr. 2020, 71, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Ziegler, E.E.; Grathwohl, D. Fast growth of infants of overweight mothers: Can it be slowed down? Ann. Nutr. Metab. 2014, 64, 19–24. [Google Scholar] [CrossRef]
- Inostroza, J.; Haschke, F.; Steenhout, P.; Grathwohl, D.; Nelson, S.E.; Ziegler, E.E. Low-protein formula slows weight gain in infants of overweight mothers. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 70–77. [Google Scholar] [CrossRef]
- Ellsworth, L.; Perng, W.; Harman, E.; Das, A.; Pennathur, S.; Gregg, B. Impact of maternal overweight and obesity on milk composition and infant growth. Matern. Child Nutr. 2020, 16. [Google Scholar] [CrossRef]
- Yang, Z.; Huffman, S.L. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern. Child Nutr. 2013, 9, 105–119. [Google Scholar] [CrossRef]
- Totzauer, M.; Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Verduci, E.; ReDionigi, A.; Hoyos, J.; Langhendries, J.P.; Gruszfeld, D.; Socha, P.; et al. Effect of Lower Versus Higher Protein Content in Infant Formula Through the First Year on Body Composition from 1 to 6 Years: Follow-Up of a Randomized Clinical Trial. Obesity 2018, 26, 1203–1210. [Google Scholar] [CrossRef]
- Mäkelä, J.; Linderborg, K.; Niinikoski, H.; Yang, B.; Lagström, H. Breast milk fatty acid composition differs between overweight and normal weight women: The STEPS Study. Eur. J. Nutr. 2013, 52, 727–735. [Google Scholar] [CrossRef]
- Linderborg, K.M.; Kalpio, M.; Mäkelä, J.; Niinikoski, H.; Kallio, H.P.; Lagström, H. Tandem mass spectrometric analysis of human milk Triacylglycerols from normal weight and overweight mothers on different diets. Food Chem. 2014, 146, 583–590. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lönnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Frasquet-Darrieux, M.; Gaud, M.-A.; Christin, P.; Boquien, C.-Y.; Millet, C.; Herviou, M.; Darmaun, D.; Robins, R.J.; Ingrand, P.; et al. Higher Leptin but Not Human Milk Macronutrient Concentration Distinguishes Normal-Weight from Obese Mothers at 1-Month Postpartum. PLoS ONE 2016, 11, e0168568. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatric Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Giannì, M.L.; Morniroli, D.; Leone, L.; Roggero, P.; Agostoni, C.; De Cosmi, V.; Mosca, F. Hormones in breast milk and effect on infants’ growth: A systematic review. Nutrients 2019, 11, 1845. [Google Scholar] [CrossRef]
- Kirchberg, F.F.; Grote, V.; Gruszfeld, D.; Socha, P.; Closa-Monasterolo, R.; Escribano, J.; Verduci, E.; Mariani, B.; Langhendries, J.P.; Poncelet, P.; et al. Are all breast-fed infants equal? Clustering metabolomics data to identify predictive risk clusters for childhood obesity. J. Pediatric Gastroenterol. Nutr. 2019, 68, 408–415. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Vegan Diets: Review of Nutritional and Health Benefits and Risks. 2018. Available online: https://www.blv.admin.ch/blv/en/home/das-blv/organisation/kommissionen/eek/vor-und-nachteile-vegane-ernaehrung.html (accessed on 14 January 2021).
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. Vegan Diet Position of the German Nutrition Society (DGE). Ernaehrungs Umschau Int. 2016, 4. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) committee on nutrition. J. Pediatric Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice: Vegetarian infant and child nutrition. Eur. J. Pediatrics 2011, 170, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the establishment and operation of Human Milk Banks in Europe: A consensus statement from the European Milk Bank Association (EMBA). Front. Pediatrics 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Barbero, A.H.; Borrás-Novel, C.; Casanova, M.A.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Tutusaus, M.P.; Martínez, S.F.; Roig, M.D.G.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Karcz, K.; Królak-Olejnik, B. Vegan or vegetarian diet and breast milk composition—A systematic review. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Vos, P.; Shahab-Ferdows, S.; Hampel, D.; Allen, L.H.; Perrin, M.T. Vitamin B-12 content in breast milk of vegan, vegetarian, and nonvegetarian lactating women in the United States. Am. J. Clin. Nutr. 2018, 108, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battino, M. Planning Well-Balanced Vegetarian Diets in Infants, Children, and Adolescents: The VegPlate Junior. J. Acad. Nutr. Diet. 2019, 119, 1067–1074. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Sonawane, B.; Mattison, D.; McCally, M.; Garg, A. Chemical contaminants in breast milk and their impacts on children’s health: An overview. Environ. Health Perspect. 2002, 110. [Google Scholar] [CrossRef]
- Kim, H.; Chuvakova, T.; Kazbekova, G.; Hayward, D.; Tulenova, A.; Petreas, M.X.; Wade, T.J.; Benedict, K.; Cheng, Y.Y.; Grassman, J. Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakhstan: Sources of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposures in an agricultural region of southern Kazakhstan. Environ. Health Perspect. 1999, 107, 447–457. [Google Scholar] [CrossRef]
- van den Berg, M.; Kypke, K.; Kotz, A.; Tritscher, A.; Lee, S.Y.; Magulova, K.; Fiedler, H.; Malisch, R. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Arch. Toxicol. 2017, 91, 83–96. [Google Scholar] [CrossRef]
- Currie, J.; Putnam, H. Early-Life Origins of Lifecycle Well-Being: Research and Policy Implications. J. Policy Anal. Manag. 2015, 34, 208–242. [Google Scholar] [CrossRef] [PubMed]
- Lipman, T.H.; Lobo, M.L. Special Issue on Social Determinants of Health. J. Pediatr. Nurs. 2017, 37, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gadson, A.; Akpovi, E.; Mehta, P.K. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin. Perinatol. 2017, 41, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kozhimannil, K.B.; Vogelsang, C.A.; Hardeman, R.R.; Prasad, S. Disrupting the Pathways of Social Determinants of Health: Doula Support during Pregnancy and Childbirth. J. Am. Board Fam. Med. 2016, 29, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Andermann, A. Taking action on the social determinants of health in clinical practice: A framework for health professionals. CMAJ 2016, 188, E474–E483. [Google Scholar] [CrossRef] [PubMed]
- Géa-Horta, T.; Silva, R.D.C.R.; Fiaccone, R.L.; Barreto, M.L.; Velásquez-Meléndez, G. Factors associated with nutritional outcomes in the mother-child dyad: A population-based cross-sectional study. Public Health Nutr. 2016, 19, 2725–2733. [Google Scholar] [CrossRef]
- Temple Newhook, J.; Newhook, L.A.; Midodzi, W.K.; Murphy Goodridge, J.; Burrage, L.; Gill, N.; Halfyard, B.; Twells, L. Poverty and Breastfeeding: Comparing Determinants of Early Breastfeeding Cessation Incidence in Socioeconomically Marginalized and Privileged Populations in the FiNaL Study. Health Equity 2017, 1, 96–102. [Google Scholar] [CrossRef]
- WHO. Protecting, Promoting, and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: The Revised Baby-Friendly Hospital Initiative 2018; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Olędzka, G.; Szostak-Węgierek, D.; Weker, H.; Wesołowska, A. Maternal nutrition and body composition during breastfeeding: Association with human milk composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef]
| Maternal Obesity | |
|---|---|
| Side Effects on Mother | Side Effects on Infant |
| spontaneous abortion | Type 2 diabetes |
| gestational diabetes | Obesity |
| pre-eclampsia | Cardiovascular diseases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. https://doi.org/10.3390/nu13020486
Verduci E, Giannì ML, Vizzari G, Vizzuso S, Cerasani J, Mosca F, Zuccotti GV. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients. 2021; 13(2):486. https://doi.org/10.3390/nu13020486
Chicago/Turabian StyleVerduci, Elvira, Maria Lorella Giannì, Giulia Vizzari, Sara Vizzuso, Jacopo Cerasani, Fabio Mosca, and Gian Vincenzo Zuccotti. 2021. "The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review" Nutrients 13, no. 2: 486. https://doi.org/10.3390/nu13020486
APA StyleVerduci, E., Giannì, M. L., Vizzari, G., Vizzuso, S., Cerasani, J., Mosca, F., & Zuccotti, G. V. (2021). The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients, 13(2), 486. https://doi.org/10.3390/nu13020486









