Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Supplements
2.3. Experimental Overview
2.4. Analyses of Blood Metabolites
2.5. Measurements
2.6. Data Analysis
2.7. Statistical Analysis
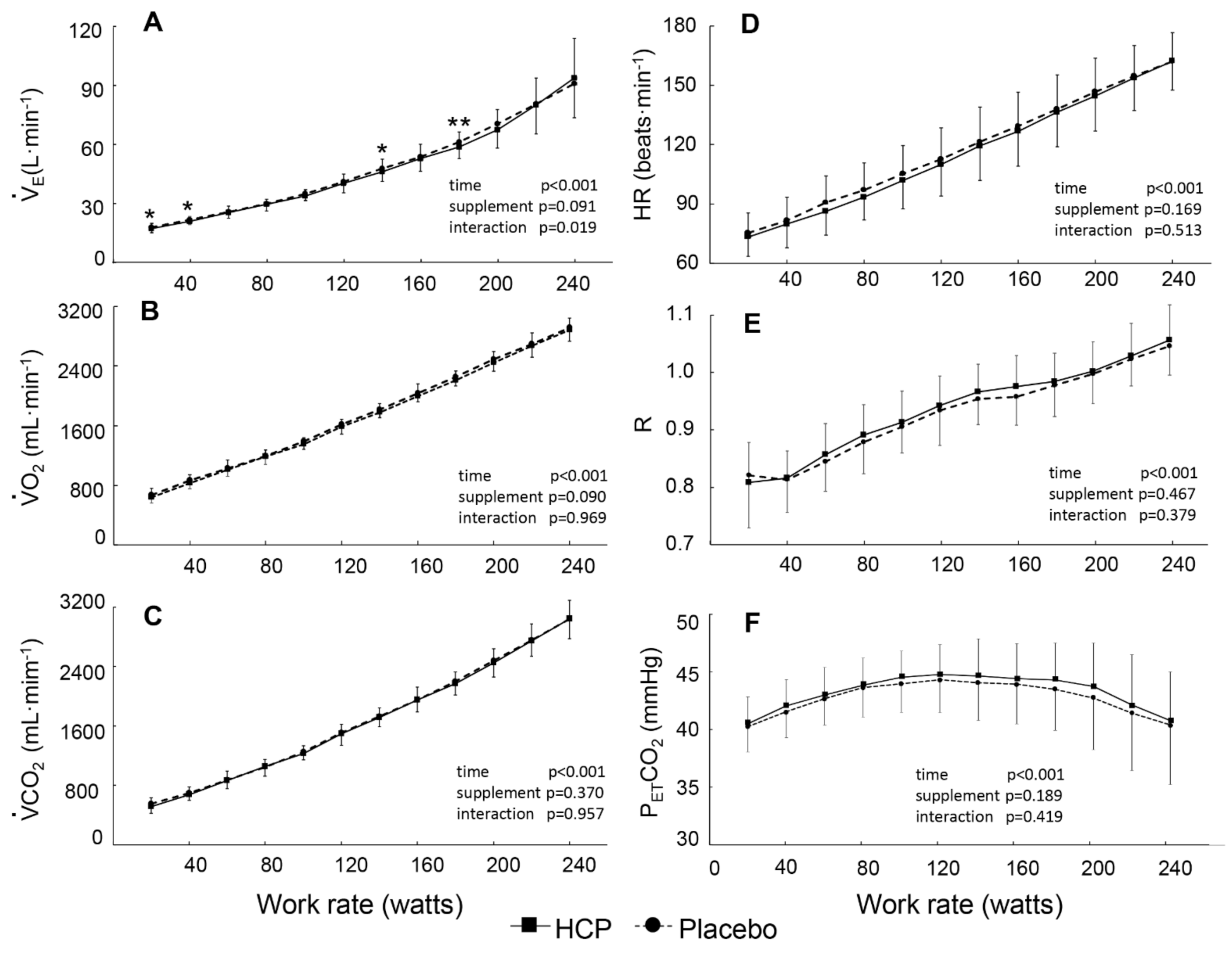
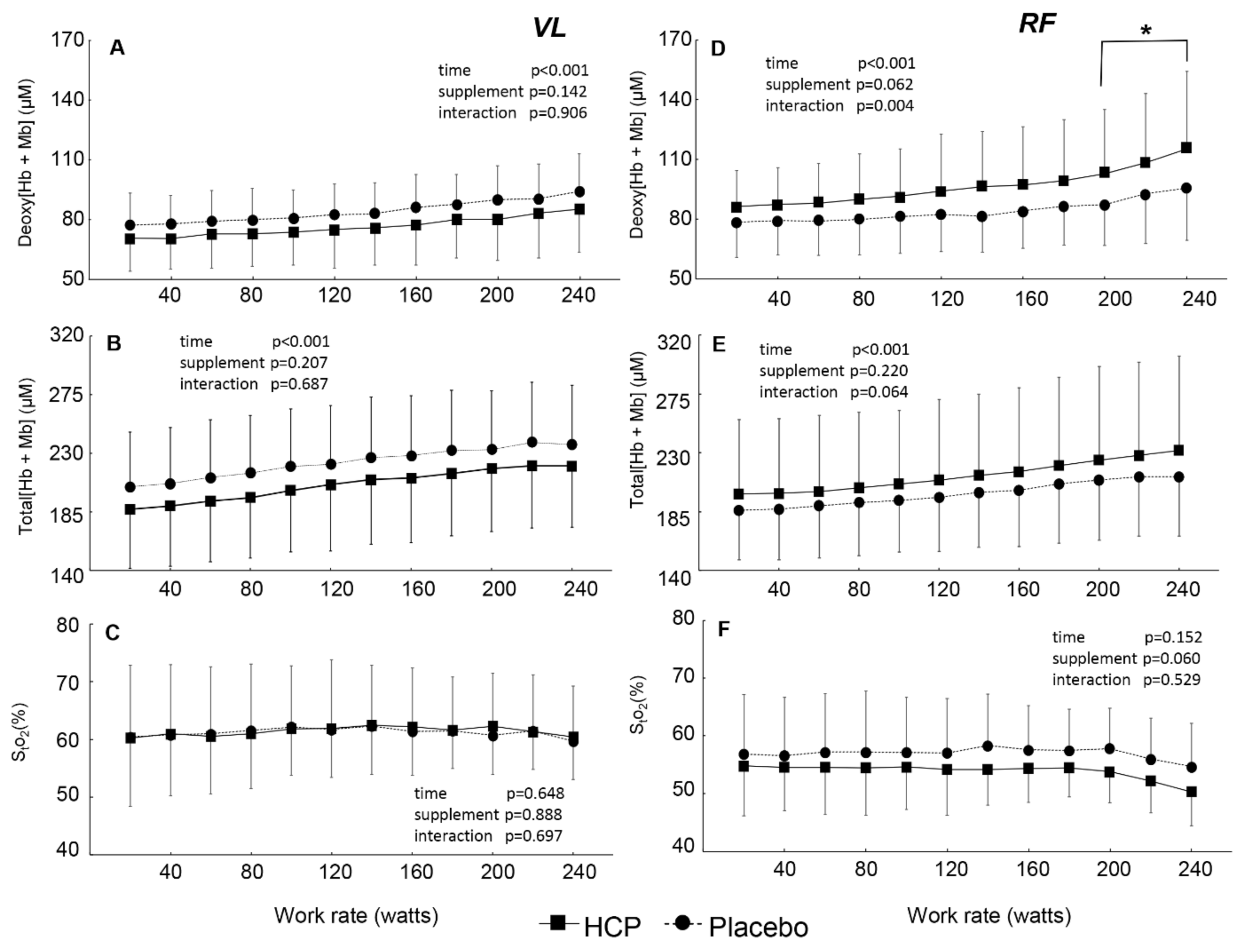
3. Results
3.1. At Rest
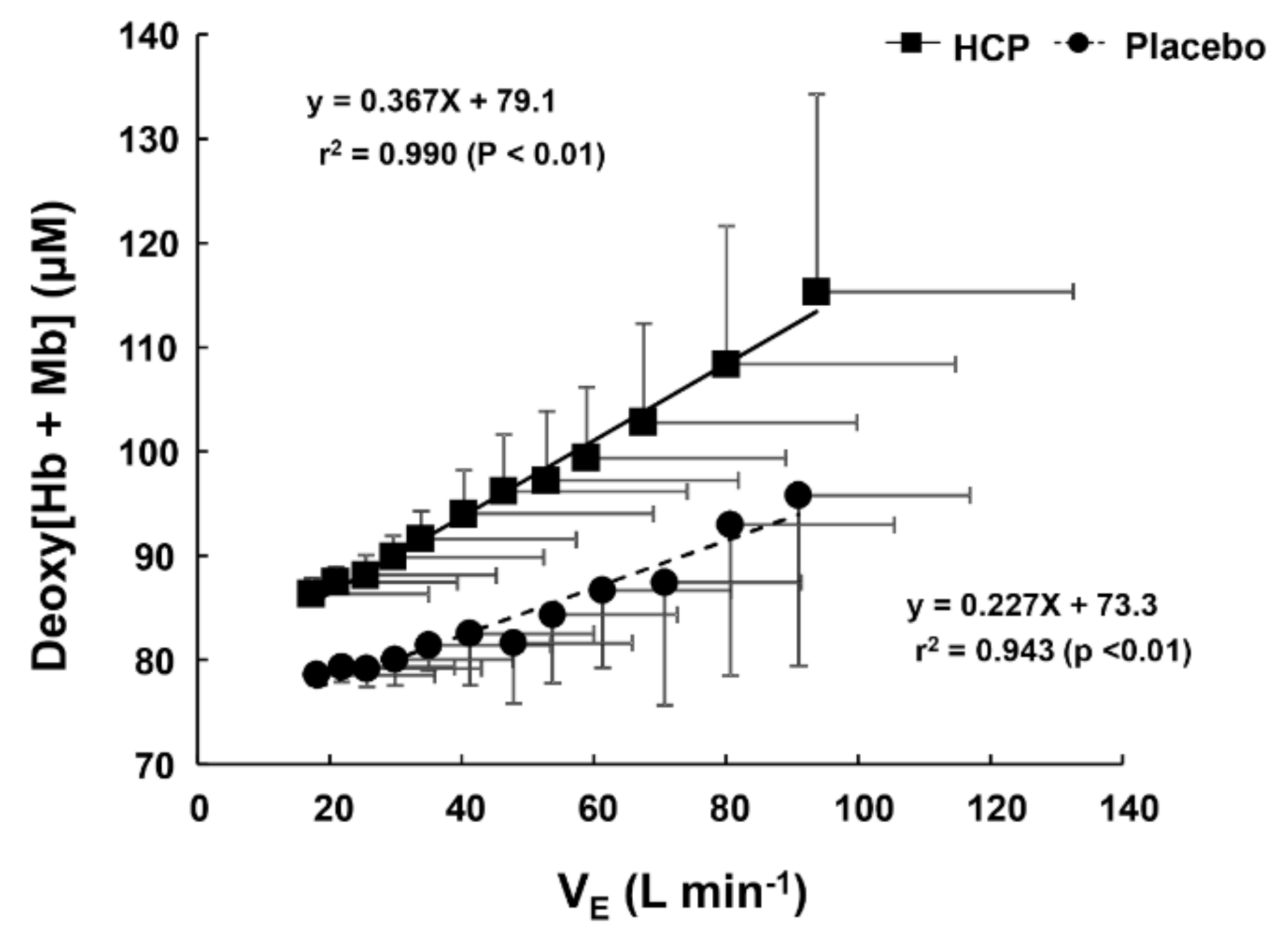
3.2. During Incremental Exercise
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radical. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef]
- Fritsch, J.; Lenz, O.; Friedrich, B. Structure, function and biosynthesis of O2-tolerant hydrogenases. Nature Rev. Microbiol. 2013, 11, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, S.; Zhang, J.M. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostojic, S.M. Molecular hydrogen in sports medicine: New therapeutic perspectives. Int. J. Sports Med. 2014, 36, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Nakao, A.; Adachi, T.; Matsui, Y.; Miyakawa, S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med. Gas Res. 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, S.; Poole, D.C.; Fukuoka, Y.; Ferreira, L.F.; Kondo, N.; Ohmae, E.; Barstow, T.J. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 534–541. [Google Scholar] [CrossRef]
- Koga, S.; Poole, D.C.; Ferreira, L.F.; Whipp, B.J.; Kondo, N.; Saitoh, T.; Ohmae, E.; Barstow, T.J. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J. Appl. Physiol. 2007, 103, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.M.; Kowalchuk, J.M.; Barstow, T.J.; Kondo, N.; Amano, T.; Shiojiri, T.; Koga, S. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J. Appl. Physiol. 2011, 111, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Bowen, T.S.; Rossiter, H.B.; Benson, A.P.; Amano, T.; Kondo, N.; Kowalchuk, J.M.; Koga, S. Slowed oxygen uptake kinetics in hypoxia correlate with the transient peak and reduced spatial distribution of absolute skeletal muscle deoxygenation. Exp. Physiol. 2013, 98, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.D.; Amano, T.; Kondo, N.; Kowalchuk, J.M.; Koga, S. Muscle O2 extraction reserve during intense cycling is site-specific. J. Appl. Physiol. 2014, 117, 1199–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, Y.; Poole, D.C.; Barstow, T.J.; Kondo, N.; Nishiwaki, M.; Okushima, D.; Koga, S. Reduction of VO2 slow component by priming exercise: Novel mechanistic insights from time-resolved near-infrared spectroscopy. Physiol. Rep. 2015, 3, e12432. [Google Scholar] [PubMed] [Green Version]
- Okushima, D.; Poole, D.C.; Rossiter, H.B.; Barstow, T.J.; Kondo, N.; Ohmae, E.; Koga, S. Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. superficial heterogeneity. J. Appl. Physiol. 2015, 119, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Okushima, D.; Poole, D.C.; Barstow, T.J.; Rossiter, H.B.; Kondo, N.; Bowen, T.S.; Amano, T.; Koga, S. Greater O2 peak is correlated with greater skeletal muscle deoxygenation amplitude and hemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiol. Rep. 2016, 4, e13065. [Google Scholar] [CrossRef]
- Peng, Y.J.; Overholt, J.L.; Kline, D.; Kumar, G.K.; Prabhakar, N.R. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc. Natl. Acad. Sci. USA 2003, 100, 10073–10078. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.J.; Prabhakar, N.R. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol. 2004, 96, 1236–1242. [Google Scholar] [CrossRef]
- Wilkerson, J.E.; Satriotomo, I.; Baker-Herman, T.L.; Watters, J.J.; Mitchell, G.S. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J. Neurosci. 2008, 28, 2949–2958. [Google Scholar] [CrossRef] [Green Version]
- Fabre, N.; Mourot, L.; Zerbini, L.; Pellegrini, B.; Bortolan, L.; Schena, F. A novel approach for lactate threshold assessment based on rating of perceived exertion. Int. J. Sports Physiol. Perform. 2013, 8, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M.; McIntosh, J.R.; Hornsby, J.; Timme, L.; Gover, L.; Mayes, J.L. Effect of exercise duration on session RPE at an individualized constant workload. Eur. J. Appl. Physiol. 2009, 107, 501–507. [Google Scholar] [CrossRef]
- Grocott, M.P.; Martin, D.S.; Levett, D.Z.; McMorrow, R.; Windsor, J.; Montgomery, H.E. Caudwell Xtreme Everest Research Group. Arterial blood gases and oxygen content in climbers on Mount Everest. N. Engl. J. Med. 2009, 360, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, Y.; Iihoshi, M.; Nazunin, J.T.; Abe, D.; Fukuba, Y. Dynamic characteristics of ventilatory and gas exchange during sinusoidal walking in humans. PLoS ONE 2017, 12, e0168517. [Google Scholar] [CrossRef] [PubMed]
- Ebine, N.; Ahad-Abdulkarim, D.A.; Miyake, Y.; Hojo, T.; Abe, D.; Horiuchi, M.; Fukuoka, Y. Influence of age on cardiorespiratory kinetics during sinusoidal walking in humans. Front. Physiol. 2018, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Lamarra, N.; Wasserman, K. Breath-by-breath measurement of true alveolar gas exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Katsumura, T.; Murase, N.; Nishio, S.; Osada, T.; Sako, T.; Higuchi, H.; Kurosawa, Y.; Shimomitsu, T.; Miwa, M.; et al. Quantification of ischemic muscle deoxygenation by near infrared time-resolved spectroscopy. J. Biomed. Opt. 2000, 5, 102–105. [Google Scholar] [CrossRef]
- Ijichi, S.; Kusaka, T.; Isobe, K.; Okubo, K.; Kawada, K.; Namba, M.; Okada, H.; Nishida, T.; Imai, T.; Itoh, S. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr. Res. 2005, 58, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.F.; Townsend, D.K.; Lutjemeier, B.J.; Barstow, T.J. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J. Appl. Physiol. 2005, 98, 1820–1828. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.F.; Koga, S.; Barstow, T.J. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J. Appl. Physiol. 2007, 103, 1999–2004. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, D.P.; Koppo, K.; Barstow, T.J.; Jones, A.M. Effect of prior multiple-sprint exercise on pulmonary O2 uptake kinetics following the onset of perimaximal exercise. J. Appl. Physiol. 2004, 97, 1227–1236. [Google Scholar] [CrossRef]
- Oda, M.; Yamashita, Y.; Nakano, T.; Suzuki, A.; Shimizu, K.; Hirano, I.; Shimomura, F.; Ohmae, E.; Suzuki, T.; Tsuchiya, Y. Near-infrared time resolved spectroscopy system for tissue oxygenation monitor. Proc. SPIE. 1999, 3579, 611–617. [Google Scholar]
- Ohmae, E.; Ouchi, Y.; Oda, M.; Suzuki, T.; Nobesawa, S.; Kanno, T.; Yoshikawa, E.; Futatsubashi, M.; Ueda, Y.; Okada, H.; et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: Correlation with simultaneous positron emission tomography measurements. NeuroImage 2006, 29, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zakynthinos, S.; Katsaounou, P.; Karatza, M.; Roussos, C.; Vassilakopoulos, T. Antioxidants increase the ventilatory response to hyperoxic hypercapnia. Am. J. Respir. Crit. Care Med. 2007, 175, 62–68. [Google Scholar] [PubMed]
- Ostojic, S.M.; Stojanovic, M.D. Hydrogen-rich water affected blood alkalinity in physically active men. Res. Sports Med. 2014, 22, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Kurtz, I. Mixed Acid–Base Disorders. Core Concepts in the Disorders of Fluid, Electrolytes and Acid-Base Balance; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–326. [Google Scholar]
- Galla, J.H. Metabolic alkalosis. J. Am. Soc. Nephrol. 2000, 11, 369–375. [Google Scholar] [PubMed]
- Hadjikoutis, S.; Wiles, C.M. Venous serum chloride and bicarbonate measurements in the evaluation of respiratory function in motor neuron disease. QJM 2001, 94, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Guenette, J.A.; Sheel, A.W. Physiological consequences of a high work of breathing during heavy exercise in humans. J. Sci. Med. Sport 2007, 10, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Badr, M.S.; Mateika, J.H. Progressive argumentation and ventilatory long-term facilitation are enhanced in sleep apnea patients and are mitigated by antioxidant administration. J. Physiol. 2009, 587, 5451–5467. [Google Scholar] [CrossRef]
- Katayama, K.; Smith, C.A.; Henderson, K.S.; Dempsey, J.A. Chromic intermittent hypoxia increases the CO2 reserve in sleeping dogs. J. Appl. Physiol. 2007, 103, 1942–1949. [Google Scholar]
- Mateika, J.H.; Narwani, G. Intermittent hypoxia and respiratory plasticity in humans and other animals: Does exposure to intermittent hypoxia promote or mitigate sleep apnea? Exp. Physiol. 2009, 94, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.Y.; Zwillich, C.W.; Berthon-Jones, M.; Sullivan, C.E. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J. Appl. Physiol. 1992, 73, 2083–2088. [Google Scholar]
- Iannetta, D.; Qahtani, A.; Millet, G.Y.; Murias, J.M. Quadriceps muscles O2 extraction and EMG breakpoints during a ramp incremental test. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulding, R.P.; Okushima, D.; Fukuoka, Y.; Marwood, S.; Kondo, N.; Poole, D.C.; Barstow, T.J.; Koga, S. Impact of supine versus upright exercise on muscle deoxygenation heterogeneity during ramp incremental cycling is site specific. Eur. J. Appl. Physiol. 2021, in press. [Google Scholar]
- Dawson, J.M.; Tyler, K.R.; Hudlicka, O. A comparison of the microcirculation in rat fast glycolytic and slow oxidative muscles at rest and during contractions. Microvasc. Res. 1987, 33, 167–182. [Google Scholar] [CrossRef]
- Kindig, C.A.; Richardson, T.E.; Poole, D.C. Skeletal muscle capillary hemodynamics from rest to contractions: Implications for oxygen transfer. J. Appl. Physiol. 2002, 92, 2513–2520. [Google Scholar] [CrossRef] [Green Version]
- Dahmane, R.; Djordjevic, S.; Simunic, B.; Valencic, V. Spatial fiber type distribution in normal human muscle: Histochemical and tensiomyographical evaluation. J. Biomech. 2005, 38, 2451–2459. [Google Scholar] [CrossRef]
- Kume, D.; Akahoshi, S.; Yamagata, T.; Wakimoto, T.; Nagao, N. Does voluntary hypoventilation during exercise impact EMG activity? SpringerPlus 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Krustrup, P.; Söderlund, K.; Mohr, M.; González-Alonso, J.; Bangsbo, J. Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. Pflugers Arch. 2004, 449, 56–65. [Google Scholar] [CrossRef]
- Koga, S.; Barstow, T.J.; Okushima, D.; Rossiter, H.B.; Kondo, N.; Ohmae, E.; Poole, D.C. Validation of a high-power, time-resolved, near-infrared spectroscopy system for measurement of superficial and deep muscle deoxygenation during exercise. J. Appl. Physiol. 2015, 118, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Koga, S.; Okushima, D.; Barstow, T.J.; Rossiter, H.B.; Kondo, N.; Poole, D.C. Near-infrared spectroscopy of superficial and deep rectus femoris reveals markedly different exercise response to superficial vastus lateralis. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Koga, S.; Okushima, D.; Poole, D.C.; Rossiter, H.B.; Kondo, N.; Barstow, T.J. Unaltered VO2 kinetics despite greater muscle oxygenation during heavy-intensity two-legged knee extension versus cycle exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R203–R213. [Google Scholar] [CrossRef]
- Heinonen, I.; Kemppainen, J.; Kaskinoro, K.; Peltonen, J.E.; Borra, R.; Lindroos, M.M.; Oikonen, V.; Nuutila, P.; Knuuti, J.; Hellsten, Y.; et al. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J. Appl. Physiol. 2010, 108, 378–386. [Google Scholar] [PubMed] [Green Version]
- Kalliokoski, K.K.; Kemppainen, J.; Larmola, K.; Takala, T.O.; Peltoniemi, P.; Oksanen, A.; Ruotsalainen, U.; Cobelli, C.; Knuuti, J.; Nuutila, P. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur. J. Appl. Physiol. 2000, 83, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, K.K.; Laaksonen, M.S.; Takala, T.O.; Knuuti, J.; Nuutila, P. Muscle oxygen extraction and perfusion heterogeneity during continuous and intermittent static exercise. J. Appl. Physiol. 2003, 94, 953–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lexell, J.; Henriksson-Larsén, K.; Sjöström, M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol. Scand. 1983, 117, 115–122. [Google Scholar] [CrossRef] [PubMed]


| HCP | Placebo | p Value | |||||
|---|---|---|---|---|---|---|---|
| metabolic gas exchange | |||||||
| VE (L·min−1) | 11.8 | ± | 3.1 | 13.2 | ± | 3.2 | 0.02 * |
| VO2 (mL·min−1) | 355 | ± | 109 | 429 | ± | 136 | 0.01 * |
| VCO2 (mL·min−1) | 306 | ± | 96 | 364 | ± | 130 | 0.03 * |
| HR (beats·min−1) | 75.3 | ± | 12.3 | 78.5 | ± | 15.2 | 0.14 |
| R | 0.9 | ± | 0.1 | 0.8 | ± | 0.1 | 0.22 |
| blood gas | |||||||
| pH | 7.356 | ± | 0.04 | 7.376 | ± | 0.04 | 0.048 * |
| PO2 (mmHg) | 43.9 | ± | 19.3 | 51.0 | ± | 17.8 | 0.107 |
| PCO2 (mmHg) | 52.4 | ± | 8.3 | 47.4 | ± | 8.2 | 0.026 * |
| HCO3− (mmol·L−1) | 29.1 | ± | 2.2 | 27.5 | ± | 2.6 | 0.041 * |
| SO2 (%) | 66.8 | ± | 25.2 | 76.9 | ± | 19.7 | 0.051 |
| BE(ecf) (mmol·L−1) | 3.6 | ± | 1.9 | 2.4 | ± | 2.3 | 0.071 |
| TCO2 (mmol·L−1) | 30.7 | ± | 2.5 | 29 | ± | 2.8 | 0.041 * |
| Hct (%) | 46 | ± | 2.4 | 46 | ± | 3.1 | 0.328 |
| Hgb (g/dL) | 15.8 | ± | 0.8 | 15.6 | ± | 1.1 | 0.313 |
| electrolytes | |||||||
| Na+ (mmol·L−1) | 141 | ± | 1.6 | 141 | ± | 1.7 | 0.069 |
| K+ (mmol·L−1) | 3.8 | ± | 0.3 | 4 | ± | 0.2 | 0.062 |
| Ca2+ (mmol·L−1) | 1.26 | ± | 0 | 1.25 | ± | 0.0 | 0.471 |
| Cl− (mmol·L−1) | 105 | ± | 1.7 | 106 | ± | 1.8 | 0.011 * |
| AGap (mmol·L−1) | 7 | ± | 1.6 | 7 | ± | 2.2 | 0.444 |
| AGapK (mmol·L−1) | 11 | ± | 1.6 | 11 | ± | 2.3 | 0.365 |
| metabolic status | |||||||
| Lac (mmol·L−1) | 1.13 | ± | 0.4 | 1.28 | ± | 0.6 | 0.312 |
| Glu (mg·dL−1) | 98 | ± | 14.1 | 104 | ± | 21.2 | 0.165 |
| Crea (mg·dL−1) | 0.96 | ± | 0.1 | 0.92 | ± | 0.2 | 0.212 |
| TR-NIRS in the RF muscle | |||||||
| Total[Hb + Mb] (µM) | 206 | ± | 48 | 201 | ± | 37 | 0.469 |
| Deoxy[Hb + Mb] (µM) | 96 | ± | 22 | 85 | ± | 23 | 0.045 * |
| StO2 (%) | 53 | ± | 8 | 57 | ± | 12 | 0.028 * |
| TR-NIRS in the VL muscle | |||||||
| Total[Hb + Mb] (µM) | 200 | ± | 37 | 212 | ± | 37 | 0.164 |
| Deoxy[Hb + Mb] (µM) | 76 | ± | 20 | 76 | ± | 22 | 0.877 |
| StO2 (%) | 61 | ± | 12 | 63 | ± | 12 | 0.222 |
| Metabolic Gas Exchange | HCP | Placebo | p Value |
|---|---|---|---|
| VE (L·min−1) | 115.2 ± 24.3 | 114.7 ± 28.8 | 0.918 |
| VO2 (mL·min−1) | 3119 ± 423 | 3141 ± 546 | 0.716 |
| VCO2 (mL·min−1) | 3496 ±576 | 3528 ± 702 | 0.721 |
| HR (beats·min−1) | 178.1 ± 6.9 | 179.2 ± 8.3 | 0.383 |
| R | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.859 |
| Workload (W) | 270 ± 34 | 272 ± 29 | 0.430 |
| Exhausted Time (min) | 26.8 ± 4.0 | 26.9 ± 3.9 | 0.701 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, A.A.D.; Ebine, N.; Nakae, S.; Hojo, T.; Fukuoka, Y. Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans. Nutrients 2021, 13, 459. https://doi.org/10.3390/nu13020459
Alharbi AAD, Ebine N, Nakae S, Hojo T, Fukuoka Y. Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans. Nutrients. 2021; 13(2):459. https://doi.org/10.3390/nu13020459
Chicago/Turabian StyleAlharbi, Ahad Abdulkarim D., Naoyuki Ebine, Satoshi Nakae, Tatsuya Hojo, and Yoshiyuki Fukuoka. 2021. "Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans" Nutrients 13, no. 2: 459. https://doi.org/10.3390/nu13020459





