Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
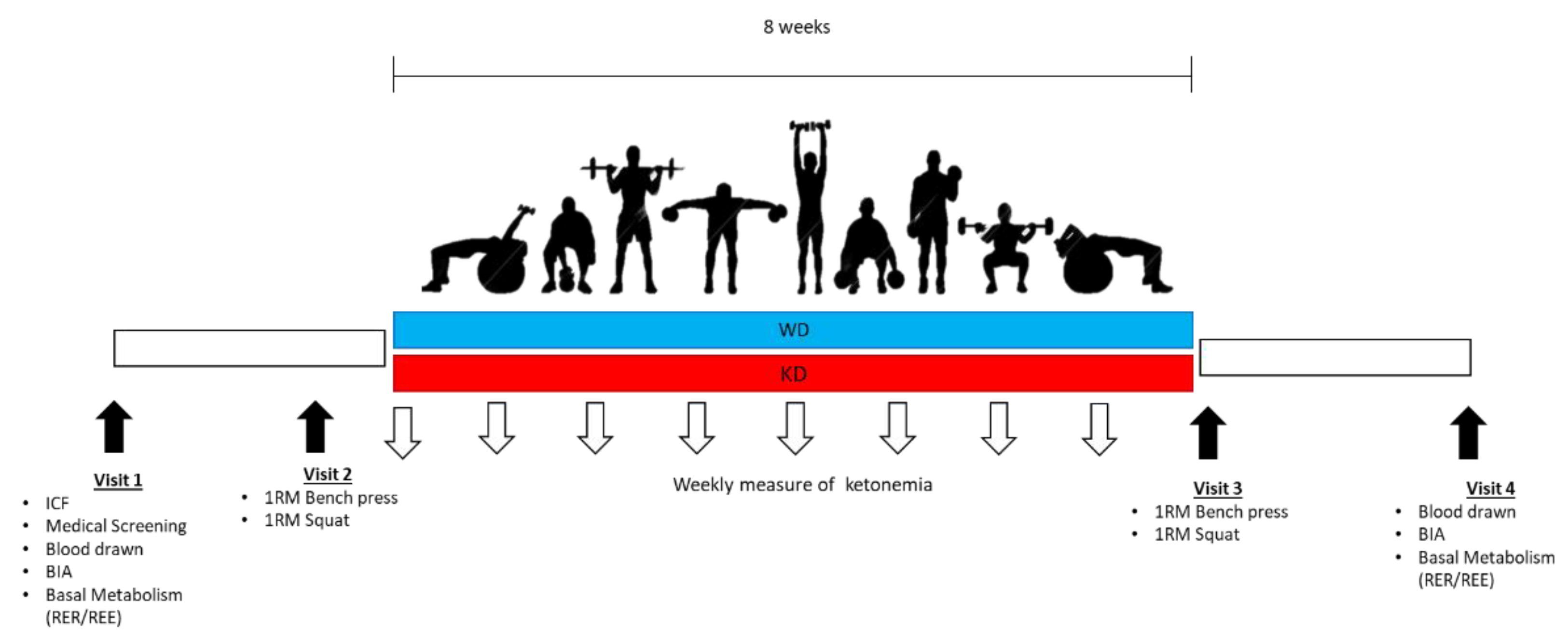
2.2. Measurements
2.3. Diet Protocols
2.4. Statistical Analysis
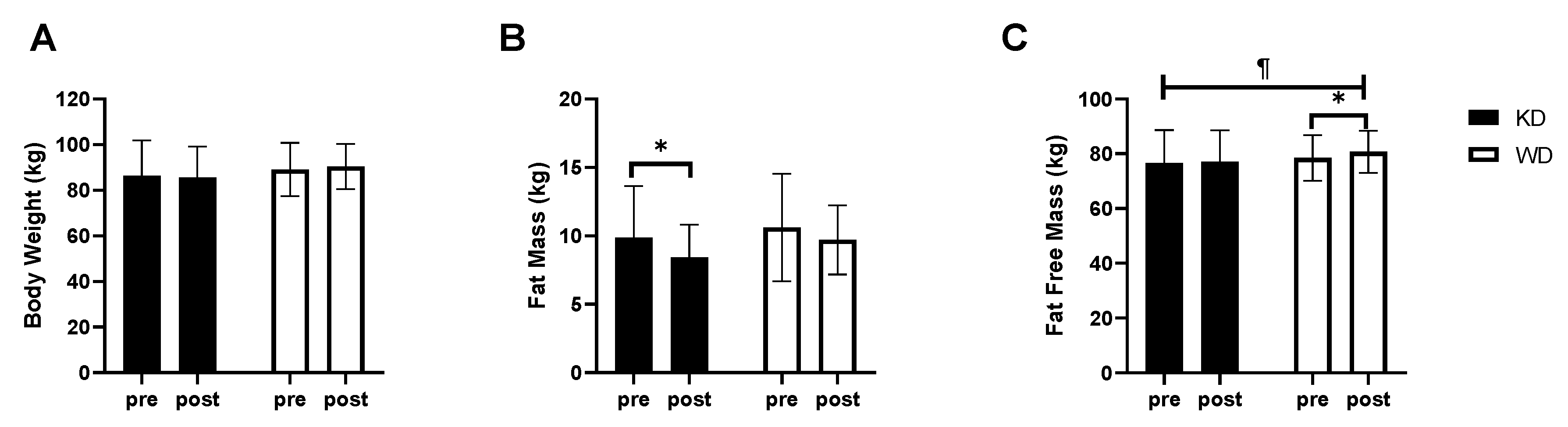
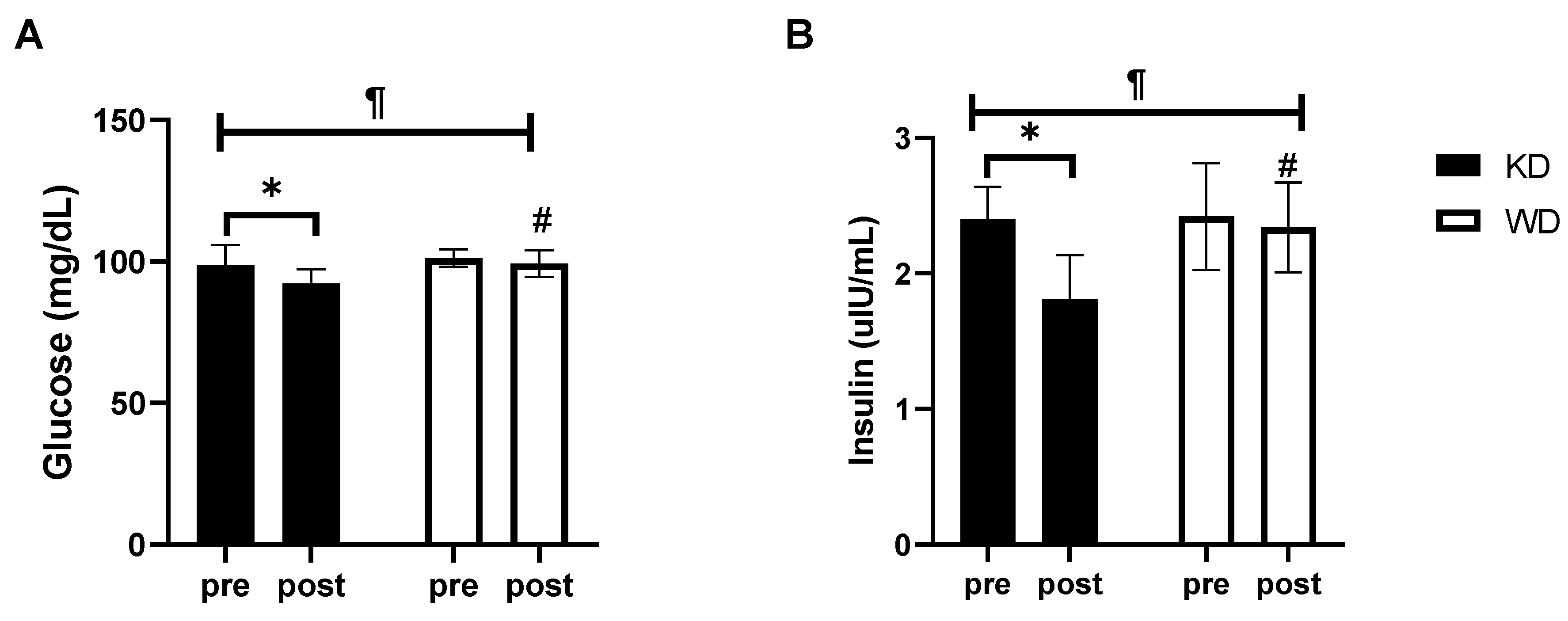
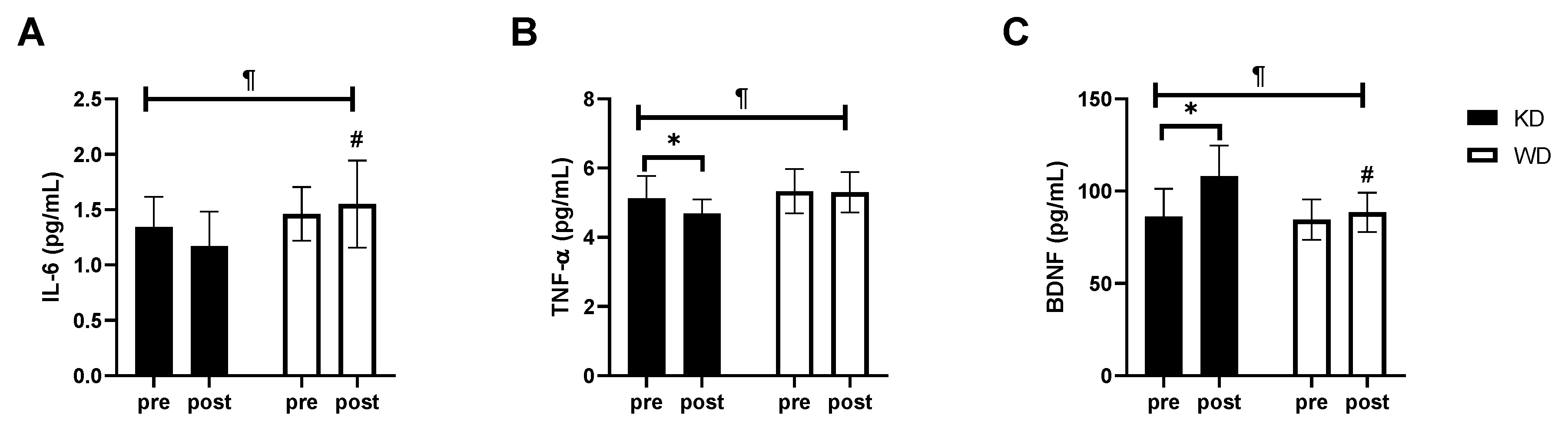
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Società Italiana di Nutrizione Umana (S.I.N.U.). Livelli di Assunzione Raccomandati di Energia e Nutrienti per la Popolazione Italiana, LARN-Revisione 2014. Available online: http://www.sinu.it (accessed on 1 December 2020).
- Paoli, A.; Cenci, L.; Fancelli, M.; Parmagnani, A.; Fratter, A.; Cucchi, A.; Bianco, A. Ketogenic diet and phytoextracts. Comparison of the efficacy of Mediterranean, zone and tisanoreica diet on some health risk factors. Agro Food Ind. Hi-Tech 2010, 21, 24–29. [Google Scholar]
- Rubini, A.; Bosco, G.; Lodi, A.; Cenci, L.; Parmagnani, A.; Grimaldi, K.; Zhongjin, Y.; Paoli, A. Effects of Twenty Days of the Ketogenic Diet on Metabolic and Respiratory Parameters in Healthy Subjects. Lung 2015, 193, 939–945. [Google Scholar] [CrossRef] [PubMed]
- McPherson, P.A.; McEneny, J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J. Physiol. Biochem. 2012, 68, 141–151. [Google Scholar] [CrossRef]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F., Jr. Liver and kidney metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger principles of biochemistry. Worth Publ. 2000, 41, 113–158. [Google Scholar]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; Bianco, A.; Lodi, A.; Cenci, L.; Parmagnani, A. Medium term effects of a ketogenic diet and a Mediterranean diet on resting energy expenditure and respiratory ratio. BMC Proc. 2012, 6, P37. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Whitfield, J.; Heikura, I.A.; Ross, M.L.; Tee, N.; Forbes, S.F.; Hall, R.; McKay, A.K.; Wallett, A.M.; Sharma, A.P. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Heikura, I.A.; Burke, L.M.; Hawley, J.A.; Ross, M.L.; Garvican-Lewis, L.; Sharma, A.P.; McKay, A.K.; Leckey, J.J.; Welvaert, M.; McCall, L. A short-term ketogenic diet impairs markers of bone health in response to exercise. Front. Endocrinol. 2020, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.; Burke, L.M.; McKay, A.K.; Heikura, I.A.; Hall, R.; Fensham, N.; Sharma, A.P. Acute Ketogenic Diet and Ketone Ester Supplementation Impairs Race Walk Performance. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cancellara, P.; Pompei, P.; Moro, T. Ketogenic diet and skeletal muscle hypertrophy: A frenemy relationship? J. Hum. Kinet. 2019, 68, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Jabekk, P.T.; Moe, I.A.; Meen, H.D.; Tomten, S.E.; Hostmark, A.T. Resistance training in overweight women on a ketogenic diet conserved lean body mass while reducing body fat. Nutr. Metab. (Lond.) 2010, 7, 17. [Google Scholar] [CrossRef]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benitez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef]
- Tzur, A.; Roberts, B.M. The ketogenic diet for bodybuilders and physique athletes. Strength Cond. J. 2020, 42, 108–115. [Google Scholar] [CrossRef]
- Helms, E.R.; Aragon, A.A.; Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. J. Int. Soc. Sports Nutr. 2014, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.J.; Simper, T.; Barker, M.E. Nutritional strategies of high level natural bodybuilders during competition preparation. J. Int. Soc. Sports Nutr. 2018, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; MacDougall, J.D.; Tarnopolsky, M.A.; Stauber, W.T.; Elorriaga, A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J. Appl. Physiol. (1985) 1995, 78, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Janmale, T.; Draper, N.; Gieseg, S.P. Measurement of changes in urinary neopterin and total neopterin in body builders using SCX HPLC. Pteridines 2014, 25, 53–63. [Google Scholar] [CrossRef]
- Roth, S.M.; Martel, G.F.; Ivey, F.M.; Lemmer, J.T.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. High-volume, heavy-resistance strength training and muscle damage in young and older women. J. Appl. Physiol. (1985) 2000, 88, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Fernandez, M.L. Effects of resistance training on the inflammatory response. Nutr. Res. Pract. 2010, 4, 259–269. [Google Scholar] [CrossRef]
- Fabricatore, A.N.; Wadden, T.A.; Higginbotham, A.J.; Faulconbridge, L.F.; Nguyen, A.M.; Heymsfield, S.B.; Faith, M.S. Intentional weight loss and changes in symptoms of depression: A systematic review and meta-analysis. Int. J. Obes. (Lond.) 2011, 35, 1363–1376. [Google Scholar] [CrossRef]
- Altar, C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–61. [Google Scholar] [CrossRef]
- Duman, R.S.; Malberg, J.; Nakagawa, S.; D’Sa, C. Neuronal plasticity and survival in mood disorders. Biol. Psychiatry 2000, 48, 732–739. [Google Scholar] [CrossRef]
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 polyunsaturated fatty acids (omega-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar. Drugs 2015, 13, 996–1009. [Google Scholar] [CrossRef]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Bostock, E.C.; Kirkby, K.C.; Taylor, B.V. The Current Status of the Ketogenic Diet in Psychiatry. Front. Psychiatry 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- LeSuer, D.A.; McCormick, J.H.; Mayhew, J.L.; Wasserstein, R.L.; Arnold, M.D. The accuracy of prediction equations for estimating 1-RM performance in the bench press, squat, and deadlift. J. Strength Cond. Res. 1997, 11, 211–213. [Google Scholar]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Apong, P.E. Nutrition and Dietary Recommendations for Bodybuilders. In Nutrition and Enhanced Sports Performance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 737–750. [Google Scholar]
- Rossow, L.M.; Fukuda, D.H.; Fahs, C.A.; Loenneke, J.P.; Stout, J.R. Natural bodybuilding competition preparation and recovery: A 12-month case study. Int. J. Sports Physiol. Perform. 2013, 8, 582–592. [Google Scholar] [CrossRef]
- Robinson, S.L.; Lambeth-Mansell, A.; Gillibrand, G.; Smith-Ryan, A.; Bannock, L. A nutrition and conditioning intervention for natural bodybuilding contest preparation: Case study. J. Int. Soc. Sports Nutr. 2015, 12, 20. [Google Scholar] [CrossRef]
- Bowler, A.L.; Polman, R. Role of a Ketogenic Diet on Body Composition, Physical Health, Psychosocial Well-Being and Sports Performance in Athletes: A Scoping Review. Sports 2020, 8, 131. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- Ruth, M.R.; Port, A.M.; Shah, M.; Bourland, A.C.; Istfan, N.W.; Nelson, K.P.; Gokce, N.; Apovian, C.M. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism 2013, 62, 1779–1787. [Google Scholar] [CrossRef]
- Johnston, C.S.; Tjonn, S.L.; Swan, P.D.; White, A.; Hutchins, H.; Sears, B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am. J. Clin. Nutr. 2006, 83, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Fleming, R.D.; Wolfe, R.R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Roberson, R.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.F.; Kahn, B.B.; et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; Carlson, M.G.; Hill, J.; Nurjhan, N. Regulation of free fatty acid metabolism by insulin in humans: Role of lipolysis and reesterification. Am. J. Physiol. Endocrinol. Metab. 2006, 263, E1063–E1069. [Google Scholar] [CrossRef]
- Cangemi, R.; Friedmann, A.J.; Holloszy, J.O.; Fontana, L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 2010, 9, 236–242. [Google Scholar] [CrossRef]
- Volek, J.S.; Gomez, A.L.; Love, D.M.; Avery, N.G.; Sharman, M.J.; Kraemer, W.J. Effects of a high-fat diet on postabsorptive and postprandial testosterone responses to a fat-rich meal. Metabolism 2001, 50, 1351–1355. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Dickinson, S.L.; Henschel, B.; Ebbeling, C.B.; Allison, D.B. Do Lower-Carbohydrate Diets Increase Total Energy Expenditure? An Updated and Reanalyzed Meta-Analysis of 29 Controlled-Feeding Studies. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition Recommendations for Bodybuilders in the Off-Season: A Narrative Review. Sports 2019, 7, 154. [Google Scholar] [CrossRef]
- Roberts, B.M.; Helms, E.R.; Trexler, E.T.; Fitschen, P.J. Nutritional Recommendations for Physique Athletes. J. Hum. Kinet. 2020, 71, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.; Phillips, S.M. Nutrition guidelines for strength sports: Sprinting, weightlifting, throwing events, and bodybuilding. J. Sports Sci. 2011, 29, S67–S77. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Schenk, S.; Barkan, A.L.; Horowitz, J.F. Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E306–E312. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Muller, D.C.; Sorkin, J.D.; Andres, R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: The Baltimore Longitudinal Study on Aging. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 667–674. [Google Scholar]
- Valtuena, S.; Salas-Salvado, J.; Lorda, P.G. The respiratory quotient as a prognostic factor in weight-loss rebound. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 811–817. [Google Scholar] [CrossRef]
- Tagliabue, A.; Bertoli, S.; Trentani, C.; Borrelli, P.; Veggiotti, P. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A 6-month prospective observational study. Clin. Nutr. 2012, 31, 246–249. [Google Scholar] [CrossRef]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; Ravussin, E. Raising the bar on the low-carbohydrate diet Reply. Am. J. Clin. Nutr. 2016, 104, 1488–1490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hurley, B.F.; Seals, D.R.; Hagberg, J.M.; Goldberg, A.C.; Ostrove, S.M.; Holloszy, J.O.; Wiest, W.G.; Goldberg, A.P. High-density–lipoprotein cholesterol in bodybuilders v powerlifters. Negative effects of androgen use. JAMA 1984, 252, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Santora, L.J.; Marin, J.; Vangrow, J.; Minegar, C.; Robinson, M.; Mora, J.; Friede, G. Coronary calcification in body builders using anabolic steroids. Prev. Cardiol. 2006, 9, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Al-Zaid, N.S.; Mathew, T.C.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol. Cell. Biochem. 2006, 286, 1. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Hussein, T.; Asfar, S.K.; Behbahani, A.; Khoursheed, M.A.; Al-Sayer, H.M.; Bo-Abbas, Y.Y.; Al-Zaid, N.S. Long-term effects of a ketogenic diet in obese patients. Exp. Clin. Cardiol. 2004, 9, 200–205. [Google Scholar]
- Klement, R.J.; Frobel, T.; Albers, T.; Fikenzer, S.; Prinzhausen, J.; Kämmerer, U. A pilot case study on the impact of a self-prescribed ketogenic diet on biochemical parameters and running performance in healthy and physically active individuals. Nutr. Med. 2013, 1, 10. [Google Scholar]
- Ness, G.C. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Cell. Biochem. 1983, 53, 299–306. [Google Scholar]
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Investig. 1972, 51, 1537–1546. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Lodi, A.; Karsten, B.; Bosco, G.; Gomez-Lopez, M.; Brandao, P.P.; Bianco, A.; Paoli, A. The Effects of Different High-Protein Low-Carbohydrates Proprietary Foods on Blood Sugar in Healthy Subjects. J. Med. Food 2016, 19, 1085–1095. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Felig, P. The glucose-alanine cycle. Metabolism 1973, 22, 179–207. [Google Scholar] [CrossRef]
- Vozarova, B.; Stefan, N.; Lindsay, R.S.; Saremi, A.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002, 51, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Brousil, J.; Gazis, A.; Jackson, L.; Mansell, P.; Bennett, A.; Aithal, G.P. Elevated serum alanine transaminase in patients with type 1 or type 2 diabetes mellitus. QJM Int. J. Med. 2006, 99, 871–876. [Google Scholar] [CrossRef]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor (BDNF). Mol. Cell. Endocrinol. 2016, 430, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Lewis, D.R.; Wiegand, S.J.; Lindsay, R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997, 56, 131–137. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2019, 62, 64–77. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Swink, T.D.; Vining, E.P.; Freeman, J.M. The ketogenic diet: 1997. Adv. Pediatr. 1997, 44, 297–329. [Google Scholar] [PubMed]
- Lodi, A.; Zarantonello, L.; Bisiacchi, P.S.; Cenci, L.; Paoli, A. Ketonemia and Glycemia Affect Appetite Levels and Executive Functions in Overweight Females during Two Ketogenic Diets. Obesity (Silver Spring) 2020, 28, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Helms, E.R.; Fitschen, P.J.; Aragon, A.A.; Cronin, J.; Schoenfeld, B.J. Recommendations for natural bodybuilding contest preparation: Resistance and cardiovascular training. J. Sports Med. Phys. Fit. 2015, 55, 164–178. [Google Scholar]
- Hackett, D.A.; Johnson, N.A.; Chow, C.-M. Training practices and ergogenic aids used by male bodybuilders. J. Strength Cond. Res. 2013, 27, 1609–1617. [Google Scholar] [CrossRef]
| KD (n = 9) | WD (n = 10) | p-Value | |
|---|---|---|---|
| Age (y) | 26.22 ± 5.09 | 31.67 ± 10.39 | 0.16 |
| Weight (kg) | 86.39 ± 15.42 | 89.04 ± 11.73 | 0.68 |
| BMI (kg/m2) | 26.97 ± 1.86 | 26.66 ± 2.04 | 0.73 |
| Lean mass (%) | 88.88 ± 2.66 | 88.38 ± 3.06 | 0.71 |
| KD (n = 9) | WD (n = 10) | |
|---|---|---|
| Total Energy intake (kcal/day) | 3443.70 ± 545.94 | 3529.71 ± 374.06 |
| Protein (kcal) | 863.89 ± 154.19 | 890.40 ± 117.30 |
| Carbohydrates (kcal) | 175.00 ± 28.17 | 1952.50 ± 209.43 * |
| Fat (kcal) | 2379.81 ± 393.78 | 707.10 ± 63.66 * |
| Protein (g) | 215.97 ± 38.55 | 222.60 ± 29.33 |
| Carbohydrates (g) | 43.75 ± 7.04 | 488.13 ± 52.36 * |
| Fat (g) | 264.42 ± 43.75 | 78.57 ± 7.07 * |
| Protein (%) | 24.65 ± 1.24 | 25.03 ± 0.91 |
| Carbohydrates (%) | 5.00 ± 0.00 | 55.00 ± 0.00 * |
| Fat (%) | 68.00 ± 2.27 | 19.97 ± 0.91 * |
| KD (n = 9) | WD (n = 10) | Diet p-Value | Time p-Value | Time × Diet p-Value | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Bench press 1 RM (kg) | 129.78 ± 20.98 | 134.44 ± 17.14 * | 136.40 ± 11.27 | 141.40 ± 10.24 * | ns | 0.0009 | ns |
| Squat 1 RM (kg) | 181.33 ± 36.52 | 187.78 ± 37.41 * | 176.10 ± 27.87 | 187.00 ± 26.96 * | ns | <0.0001 | ns |
| Basal metabolism (REE) (Kcal/day) | 2014.67 ± 324.04 | 2052.56 ± 317.99 | 2069.10 ± 229.32 | 2125.20 ± 206.08 * | ns | 0.0006 | ns |
| Respiratory exchange ratio (RER) | 0.82 ± 0.01 | 0.79 ± 0.02 * | 0.83 ± 0.01 | 0.83 ± 0.02 # | 0.0022 | 0.0002 | 0.0001 |
| KD (n = 9) | WD (n = 10) | Diet p-Value | Time p-Value | Time × Diet p-Value | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Lipid profile | |||||||
| Total Cholesterol (mg/dL) | 194.78 ± 8.88 | 187.89 ± 10.15 * | 193.90 ± 18.18 | 190.60 ± 16.73 | ns | 0.0071 | ns |
| HDL (mg/dL) | 57.22 ± 3.33 | 60.00 ± 3.33 * | 52.50 ± 6.11 | 51.80 ± 5.05 # | 0.0072 | ns | 0.0039 |
| LDL (mg/dL) | 113.33 ± 8.88 | 108.00 ± 10.28 | 118.60 ± 20.64 | 116.20 ± 18.39 | ns | ns | ns |
| TG (mg/dL) | 121.00 ± 26.70 | 99.22 ± 19.72 * | 114.70 ± 13.21 | 112.70 ± 13.06 | ns | <0.0001 | 0.0003 |
| Transaminase | |||||||
| Aspartate transaminase (AST) (mg/dL) | 38.78 ± 2.82 | 38.44 ± 2.54 | 39.10 ± 3.67 | 39.30 ± 4.11 | ns | ns | ns |
| Alanine amino transferase (ALT) (mg/dL) | 43.11 ± 6.51 | 38.56 ± 3.30 * | 42.10 ± 6.59 | 44.80 ± 5.92 | ns | ns | 0.0086 |
| Anabolic Hormones | |||||||
| Testosterone total (nmol/L) | 21.76 ± 5.33 | 19.32 ± 4.09 * | 20.96 ± 5.13 | 21.27 ± 4.91 | ns | 0.0094 | 0.0016 |
| IGF-1 (ng/mL) | 213.33 ± 39.41 | 181.50 ± 25.93 * | 222.40 ± 34.27 | 219.80 ± 23.42 # | ns | 0.0050 | 0.0124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. https://doi.org/10.3390/nu13020374
Paoli A, Cenci L, Pompei P, Sahin N, Bianco A, Neri M, Caprio M, Moro T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients. 2021; 13(2):374. https://doi.org/10.3390/nu13020374
Chicago/Turabian StylePaoli, Antonio, Lorenzo Cenci, PierLuigi Pompei, Nese Sahin, Antonino Bianco, Marco Neri, Massimiliano Caprio, and Tatiana Moro. 2021. "Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders" Nutrients 13, no. 2: 374. https://doi.org/10.3390/nu13020374
APA StylePaoli, A., Cenci, L., Pompei, P., Sahin, N., Bianco, A., Neri, M., Caprio, M., & Moro, T. (2021). Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients, 13(2), 374. https://doi.org/10.3390/nu13020374











