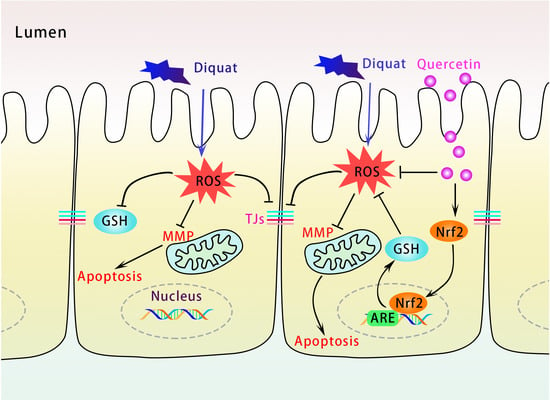
Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay
2.4. Cell Proliferation Assay
2.5. Apoptosis Analysis
2.6. Measurement of Mitochondrial Membrane Potential (MMP)
2.7. Measurement of ROS
2.8. Measurement of GSH
2.9. Immunofluorescence Staining
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
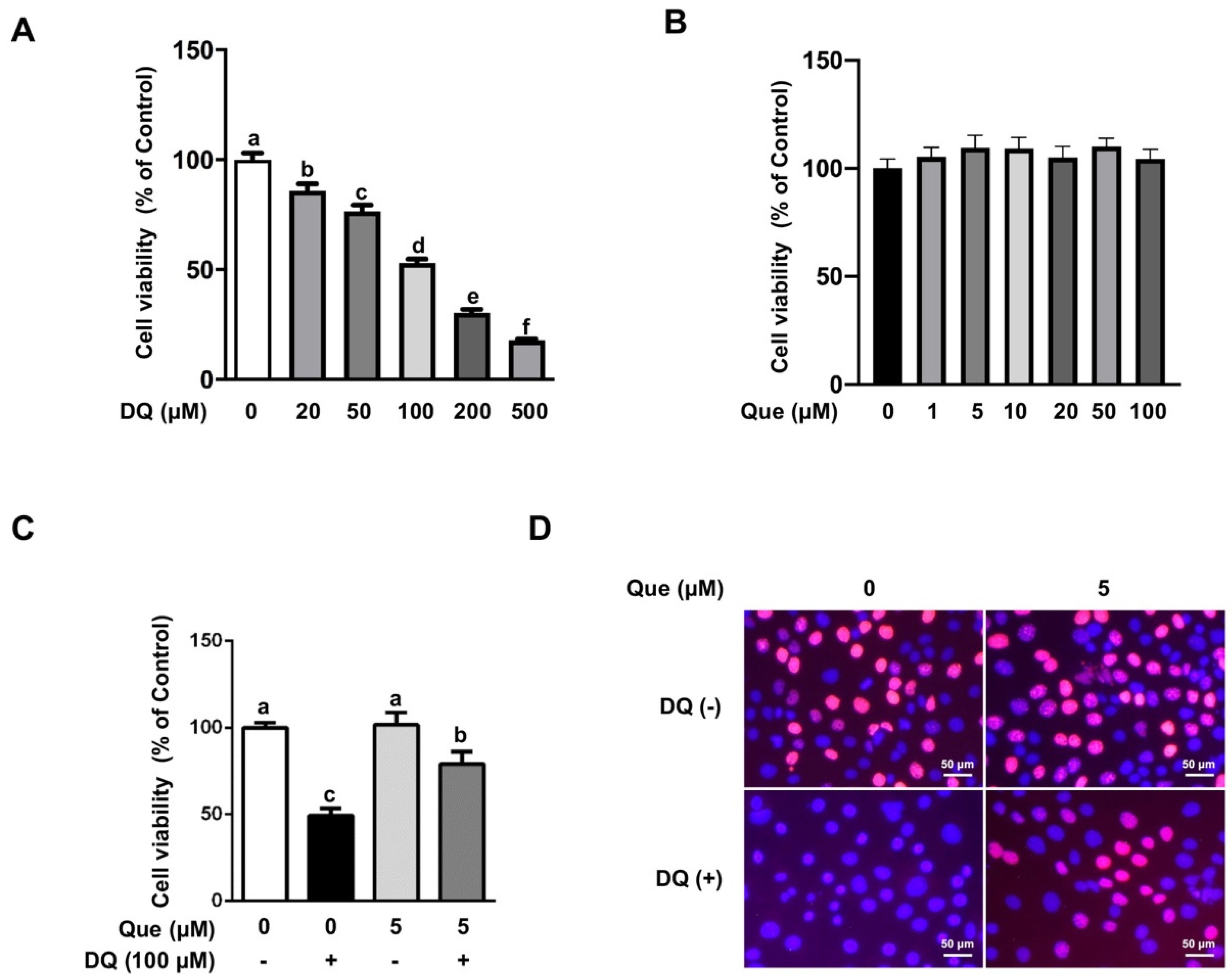
3.1. Quercetin Attenuated the Diquat-Induced Cell Viability Reduction in IPEC-1 Cells
3.2. Quercetin Attenuated Diquat-Induced Apoptosis in IPEC-1 Cells
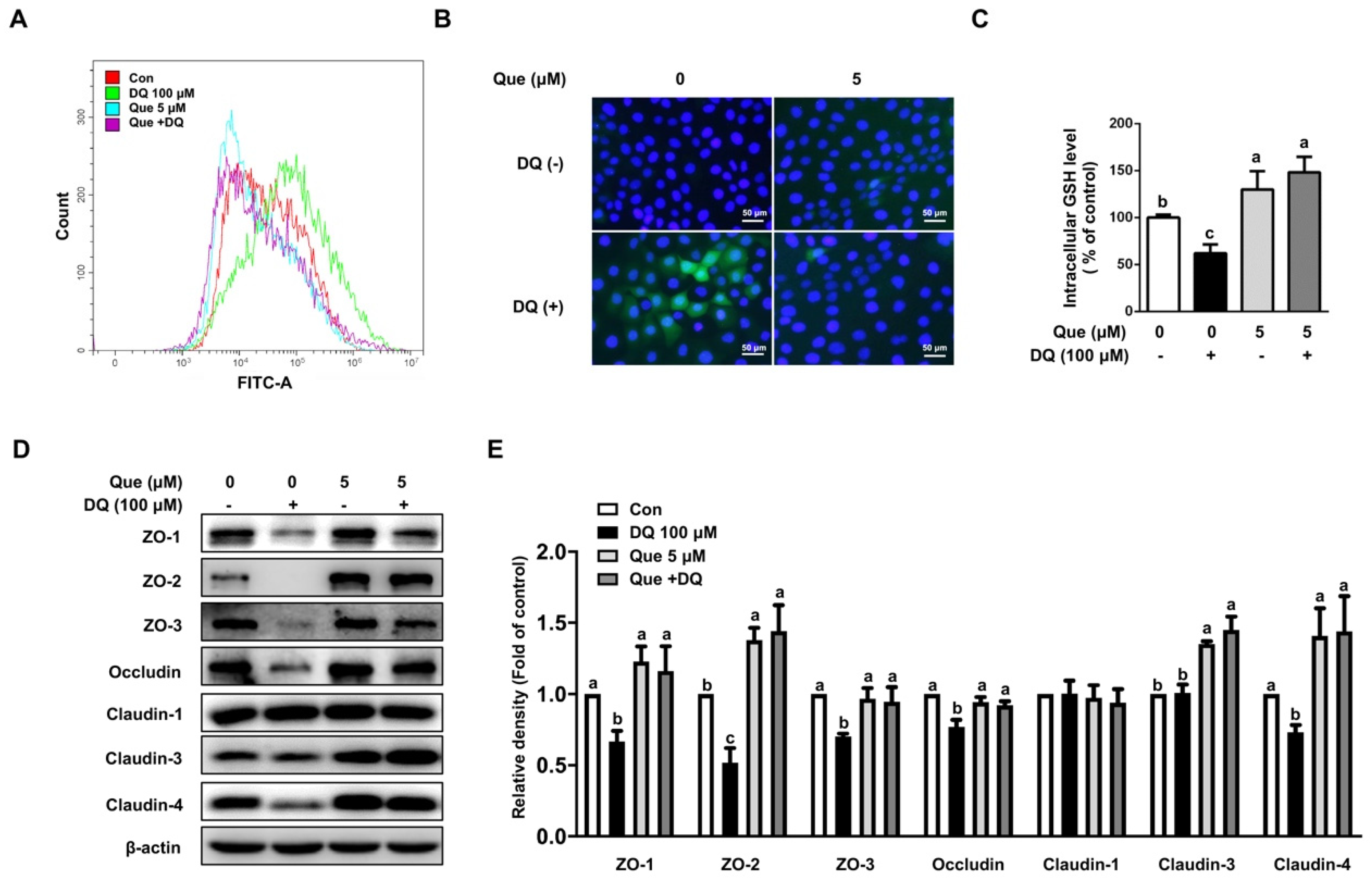
3.3. Quercetin Attenuated Diquat-Induced ROS Accumulation and GSH Deprivation in IPEC-1 Cells
3.4. Quercetin Improved the Abundance of Tight Junction Proteins in Diquat-Induced IPEC-1 Cells
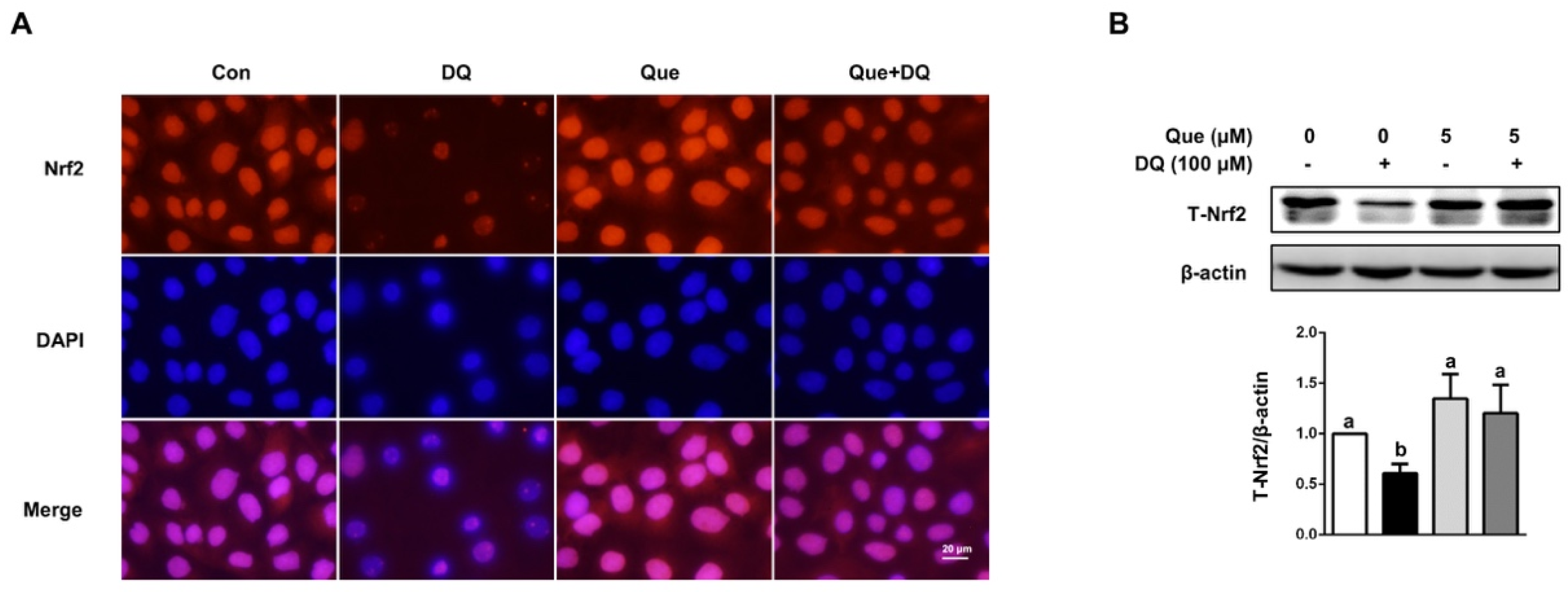
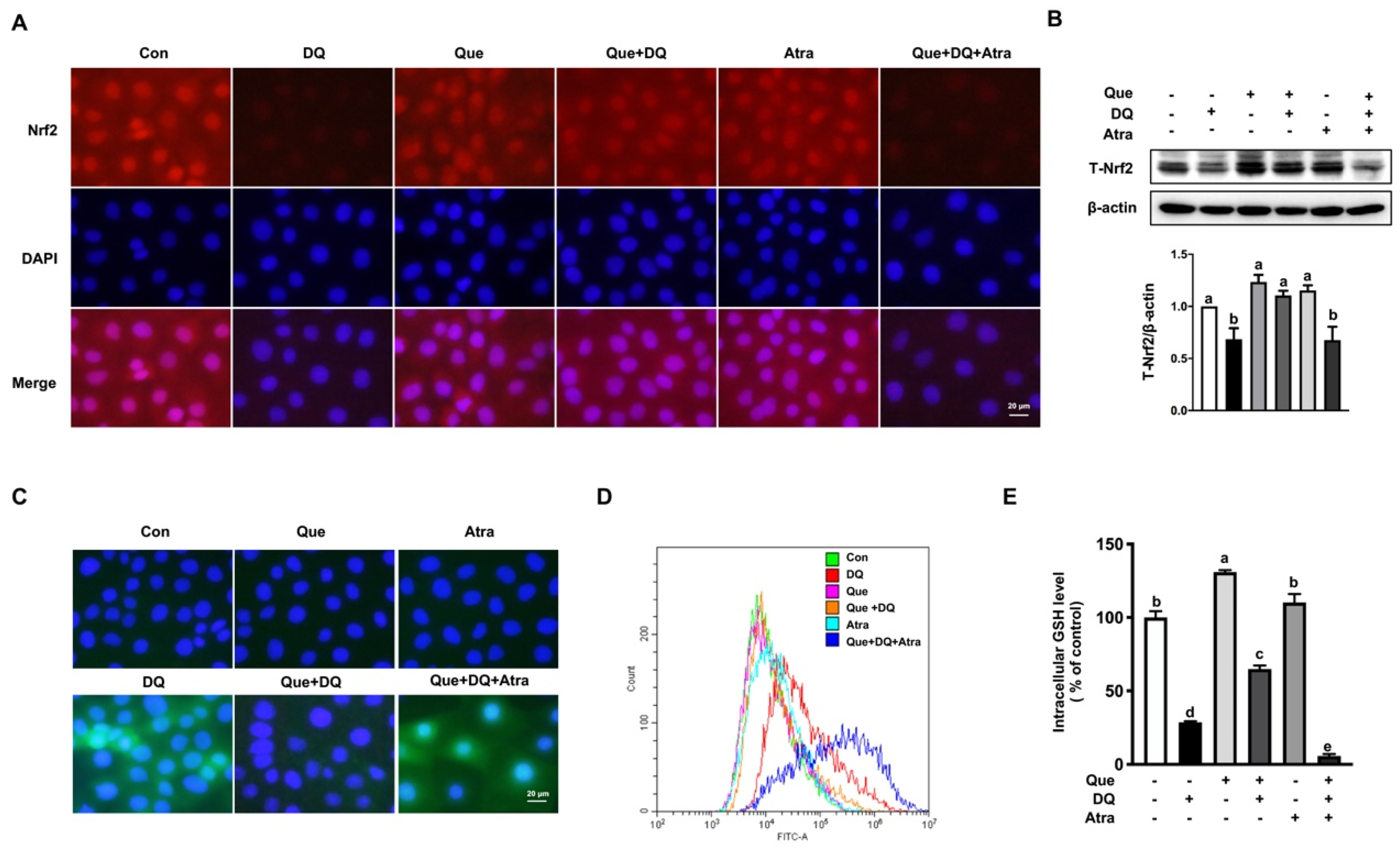
3.5. Quercetin Regulated the Nrf2 Signaling in IPEC-1 Cells
3.6. Quercetin Improved the Antioxidant Capacity of IPEC-1 Cells Challenged by Diquat in a Nrf2-Dependent Manner
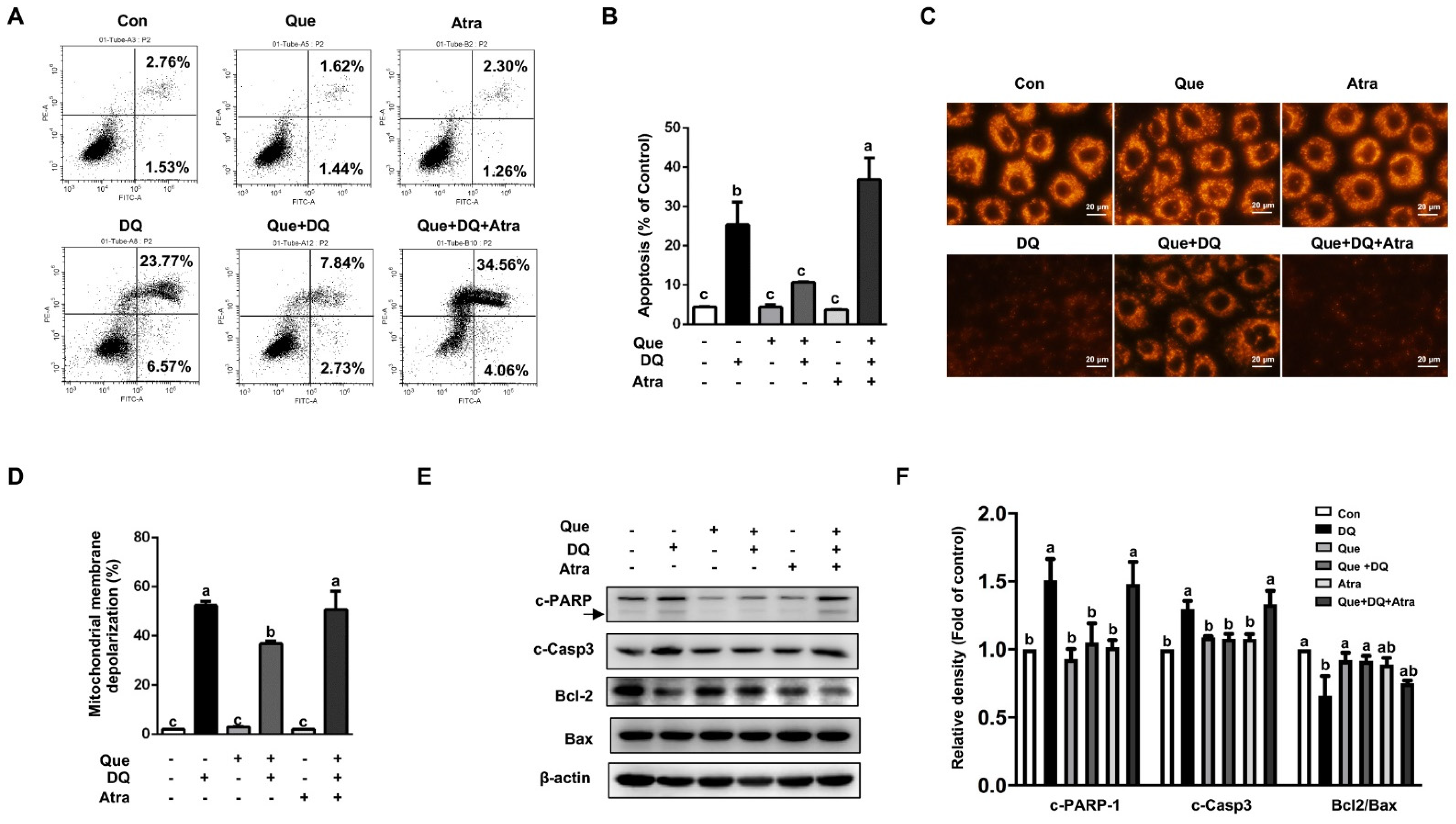
3.7. Quercetin Protected Cells Against Diquat-Induced Apoptosis in a Nrf2-Dependent Manner
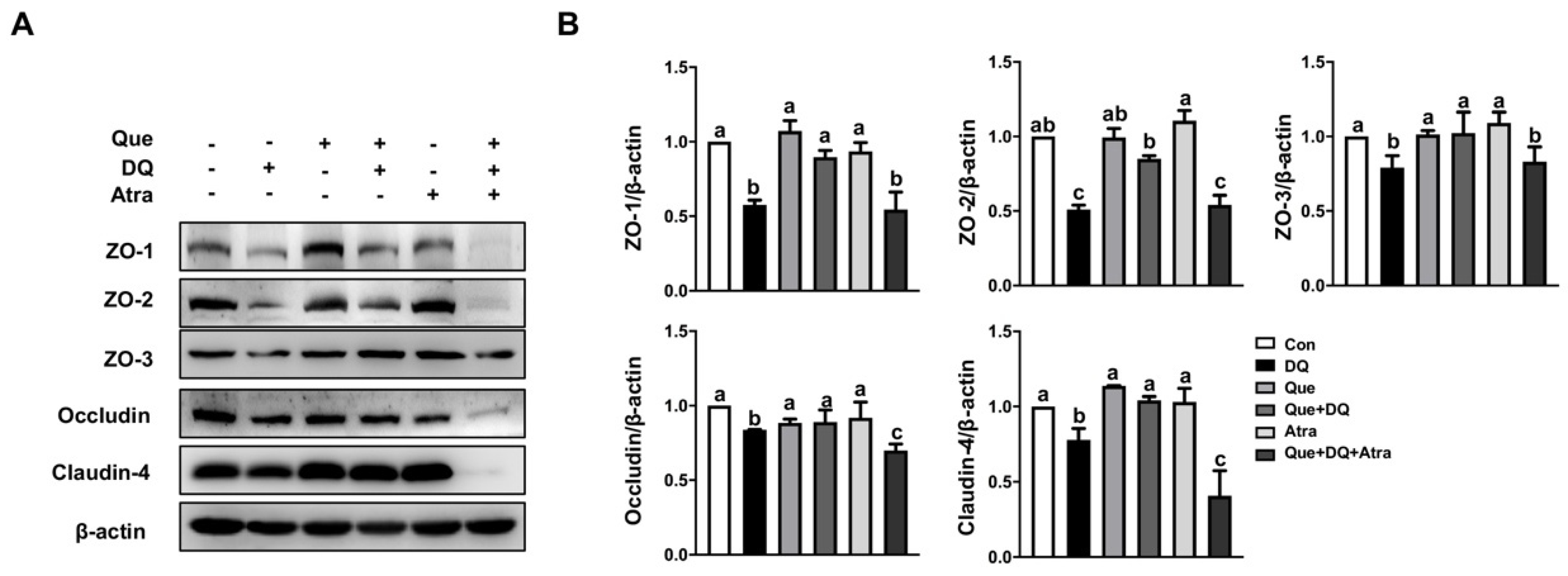
3.8. Quercetin Restored Tight Junction Proteins in Diquat-Treated Cells by Regulating Nrf2 Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hu, J.; Chen, L.; Zheng, W.; Shi, M.; Liu, L.; Xie, C.; Wang, X.; Niu, Y.; Hou, Q.; Xu, X.; et al. Lactobacillus frumenti facilitates intestinal epithelial barrier function maintenance in early-weaned piglets. Front. Microbiol. 2018, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; DeWilde, M.P.; Williams, K.J.; Moeser, A.J. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and Pptential antioxidant therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef]
- Van Le Thanh, B.; Lemay, M.; Bastien, A.; Lapointe, J.; Lessard, M.; Chorfi, Y.; Guay, F. The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with Fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. Mycotoxin Res. 2016, 32, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid. Med. Cell Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.; Hass, R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008, 389, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Ong Tone, S.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583. [Google Scholar] [CrossRef]
- Xie, F.; Sun, S.; Xu, A.; Zheng, S.; Xue, M.; Wu, P.; Zeng, J.H.; Bai, L. Advanced oxidation protein products induce intestine epithelial cell death through a redox-dependent, c-jun N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated pathway. Cell Death Dis. 2014, 5, e1006. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H(2)O(2)-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef]
- Liu, N.; Ma, X.; Luo, X.; Zhang, Y.; He, Y.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. L-Glutamine attenuates apoptosis in porcine enterocytes by regulating glutathione-related redox homeostasis. J. Nutr. 2018, 148, 526–534. [Google Scholar] [CrossRef]
- Wang, G.; Yao, J.; Li, Z.; Zu, G.; Feng, D.; Shan, W.; Li, Y.; Hu, Y.; Zhao, Y.; Tian, X. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid. Redox Signal 2016, 24, 961–973. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Zizkova, P.; Stefek, M.; Rackova, L.; Prnova, M.; Horakova, L. Novel quercetin derivatives: From redox properties to promising treatment of oxidative stress related diseases. Chem. Biol. Interact. 2017, 265, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Liu, Z.; Zhang, W.; Li, W.; Wu, Z.; Wang, W.; Ren, R.; Su, Y.; Wang, P.; Sun, L.; et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef]
- Yazıcı, S.; Ozcan, C.U.; Hismiogullari, A.A.; Sunay, F.B.; Ozcan, T.; Berksoy, E.A.; Aksoz, E. Protective effects of quercetin on necrotizing enterocolitis in a neonatal rat model. Am. J. Perinatol. 2018, 35, 434–440. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of quercetin on proliferation and H₂O₂-induced apoptosis of intestinal porcine enterocyte cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef]
- Tian, R.; Yang, Z.; Lu, N.; Peng, Y.Y. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch Biochem. Biophys. 2019, 676, 108157. [Google Scholar] [CrossRef]
- Hu, X.T.; Ding, C.; Zhou, N.; Xu, C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur. J. Pharmacol. 2015, 754, 115–124. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr. Cancer 1999, 34, 160–166. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Zhang, Y.; Klaassen, C.D. Nrf2 protects against diquat-induced liver and lung injury. Free Radic. Res. 2012, 46, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Wakabayashi, N.; Misra, V.; Nilles, T.; Biswal, S.; Trush, M.A.; Kensler, T.W. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem. Biophys. 2006, 454, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, Y.; Kijima, A.; Ishii, Y.; Takasu, S.; Tsuchiya, T.; Umemura, T. Effects of Nrf2 silencing on oxidative stress-associated intestinal carcinogenesis in mice. Cancer Med. 2016, 5, 1228–1238. [Google Scholar] [CrossRef]
- Saw, C.L.; Yang, A.Y.; Guo, Y.; Kong, A.N. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef]
- Lázaro, I.; Ferré, R.; Masana, L.; Cabré, A. Akt and ERK/Nrf2 activation by PUFA oxidation-derived aldehydes upregulates FABP4 expression in human macrophages. Atherosclerosis 2013, 230, 216–222. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, D.; Tan, B.; Wang, J.; Liu, Y.; Tan, B. The Role of Nrf2 Signaling Pathway in Eucommia ulmoides Flavones Regulating Oxidative Stress in the Intestine of Piglets. Oxid. Med. Cell Longev. 2019, 2019, 9719618. [Google Scholar] [CrossRef]
- Adam, A.; Smith, L.L.; Cohen, G.M. An assessment of the role of redox cycling in mediating the toxicity of paraquat and nitrofurantoin. Environ. Health Perspect. 1990, 85, 113–117. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Li, Y.; Yan, H.; Zhang, H. L-tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals 2019, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-B.; Chen, D.-W.; Zhang, K.-Y.; Yu, B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian Australas. J. Anim. Sci. 2007, 20, 1600–1605. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, B.; Lv, M.; Chen, D. Effects of oxidative stress induced by diquat on arginine metabolism of postweaning pigs. Asian Australas. J. Anim. Sci. 2010, 23, 98–105. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary tributyrin attenuates intestinal inflammation, enhances mitochondrial function, and induces mitophagy in piglets challenged with diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.J. Quercetin Prevents Escherichia coli O157:H7 Adhesion to Epithelial Cells via Suppressing Focal Adhesions. Front. Microbiol. 2018, 9, 3278. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Moine, L.; Rivoira, M.; Díaz de Barboza, G.; Pérez, A.; Tolosa de Talamoni, N. Glutathione depleting drugs, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2018, 24, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Takahashi, Y.; Akimoto, Y.; Sakurai, M.; Matsunaga, I.; Nishimuro, H.; Ippoushi, K.; Oike, H.; Ohnishi-Kameyama, M. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods 2015, 15, 551–560. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Li, X.L.; Jiang, M.; Ruan, Z.; Mi, S.M.; Wu, X.; Yao, K.; Xiong, X.; Zhou, Y.; Yin, Y.L. Tryptophan increases intestinal permeability and decreases intestinal tight junction protein expression in weanling piglets. J. Anim. Sci. 2016. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef]
- Cao, S.; Wu, H.; Wang, C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. https://doi.org/10.3390/nu13020375
Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z, Wu Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients. 2021; 13(2):375. https://doi.org/10.3390/nu13020375
Chicago/Turabian StyleJia, Hai, Yunchang Zhang, Xuemeng Si, Yuhang Jin, Da Jiang, Zhaolai Dai, and Zhenlong Wu. 2021. "Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes" Nutrients 13, no. 2: 375. https://doi.org/10.3390/nu13020375
APA StyleJia, H., Zhang, Y., Si, X., Jin, Y., Jiang, D., Dai, Z., & Wu, Z. (2021). Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients, 13(2), 375. https://doi.org/10.3390/nu13020375