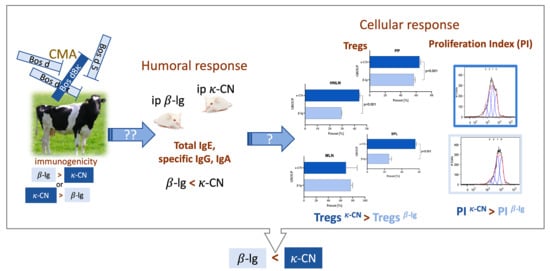
Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies
Abstract
1. Introduction
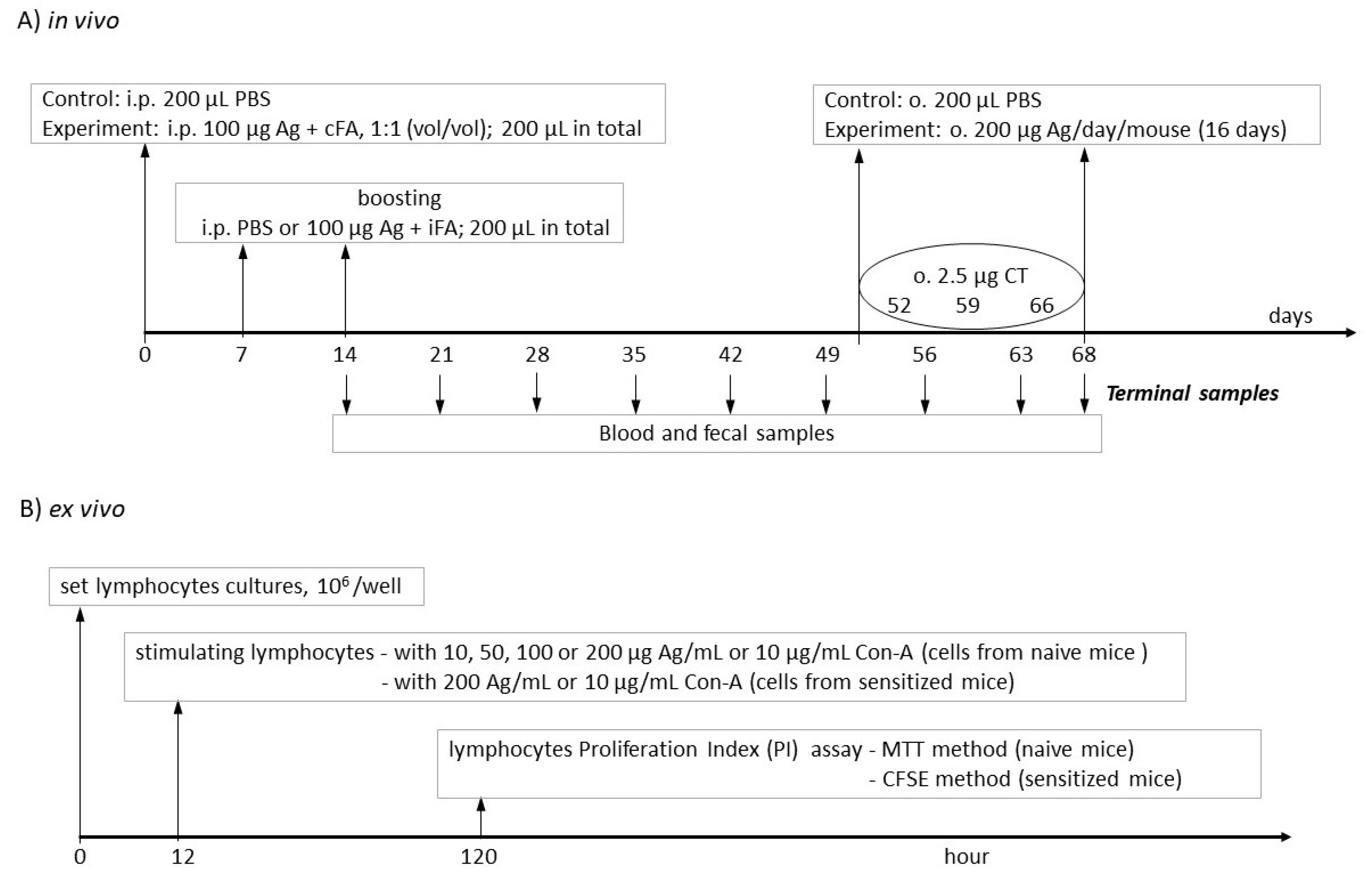
2. Materials and Methods
2.1. Antigens
2.2. Mice and Treatment Groups
2.3. Lymphocyte Isolation
2.4. Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.5. Lymphocyte Proliferation Assay
2.5.1. MTT Assay
2.5.2. Carboxyfluorescein Succinimidyl Ester (CFSE) Assay
2.6. Lymphocyte Phenotyping
2.7. Serum Samples
2.8. Fecal Extracts
2.9. ELISA Assay of Total and Specific Antibodies
2.10. Cytokine Profiles
2.11. Statistical Analysis
3. Results and Discussion
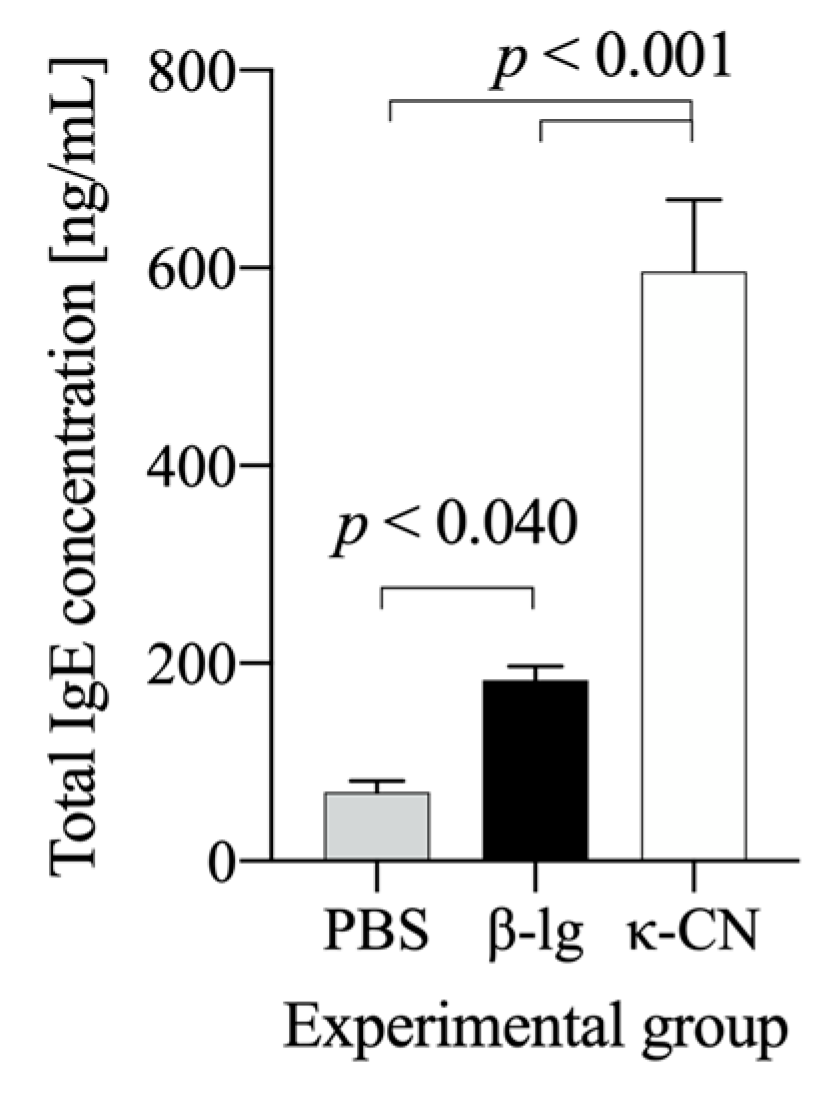
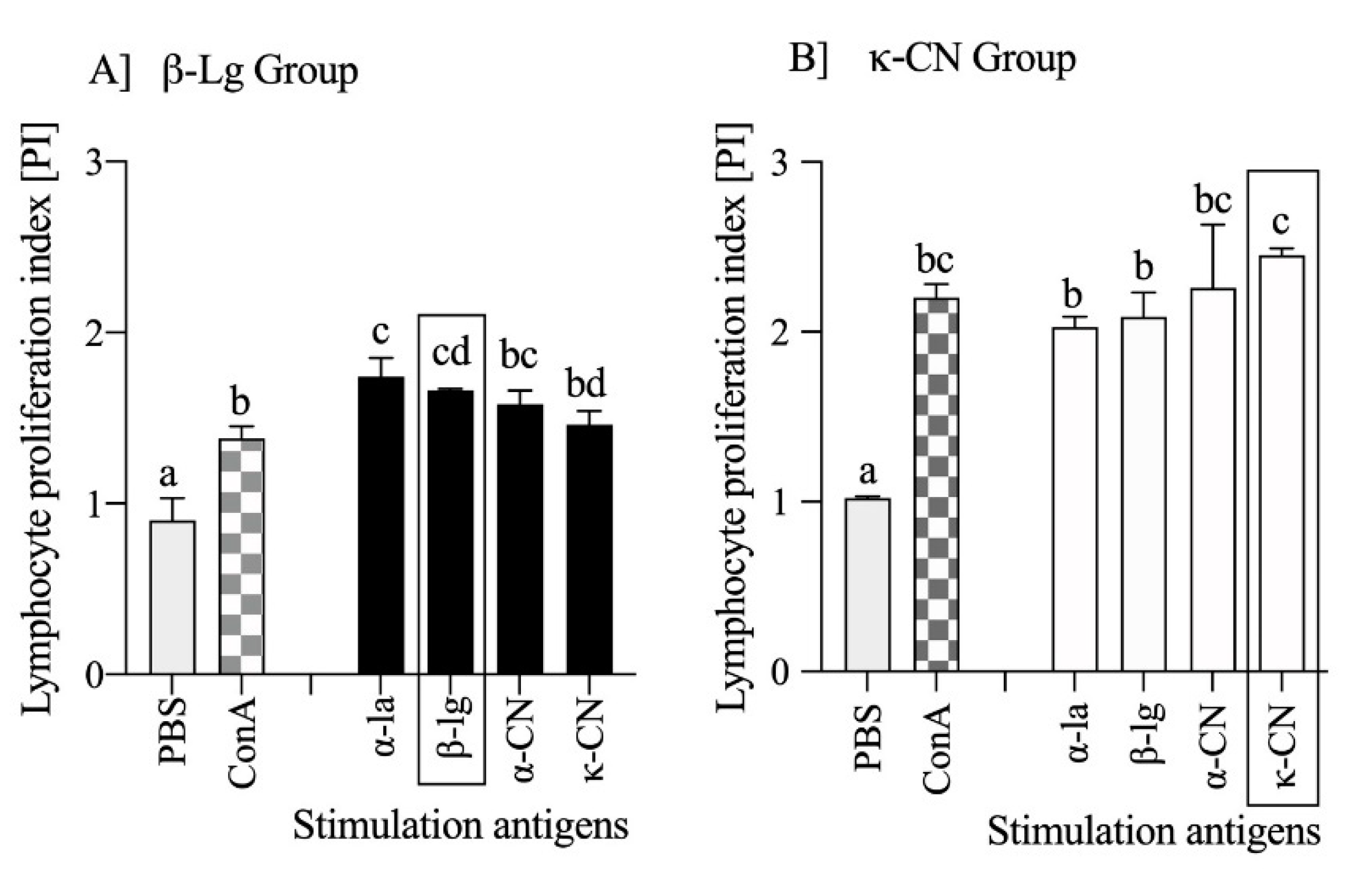
3.1. Preliminary Estimation of β-Lg and κ-CN Immunogenicity Based on the Antigen Dose
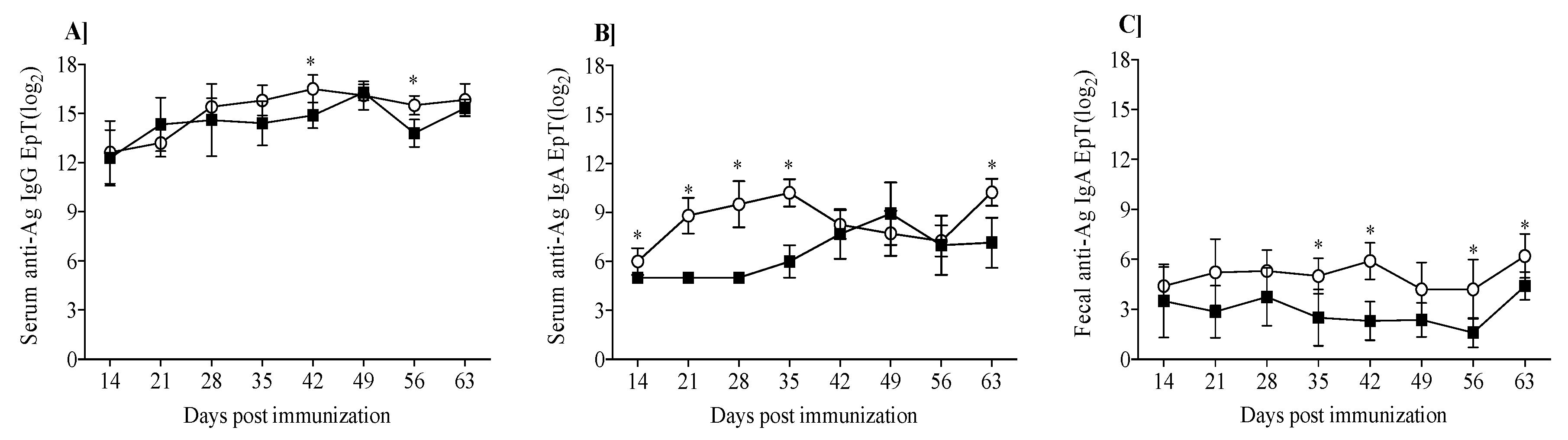
3.2. Mouse Humoral Response to β-Lg and κ-CN
3.3. Milk Antigens Immunoreactivity with β-Lg and κ-CN Activated Splenocytes
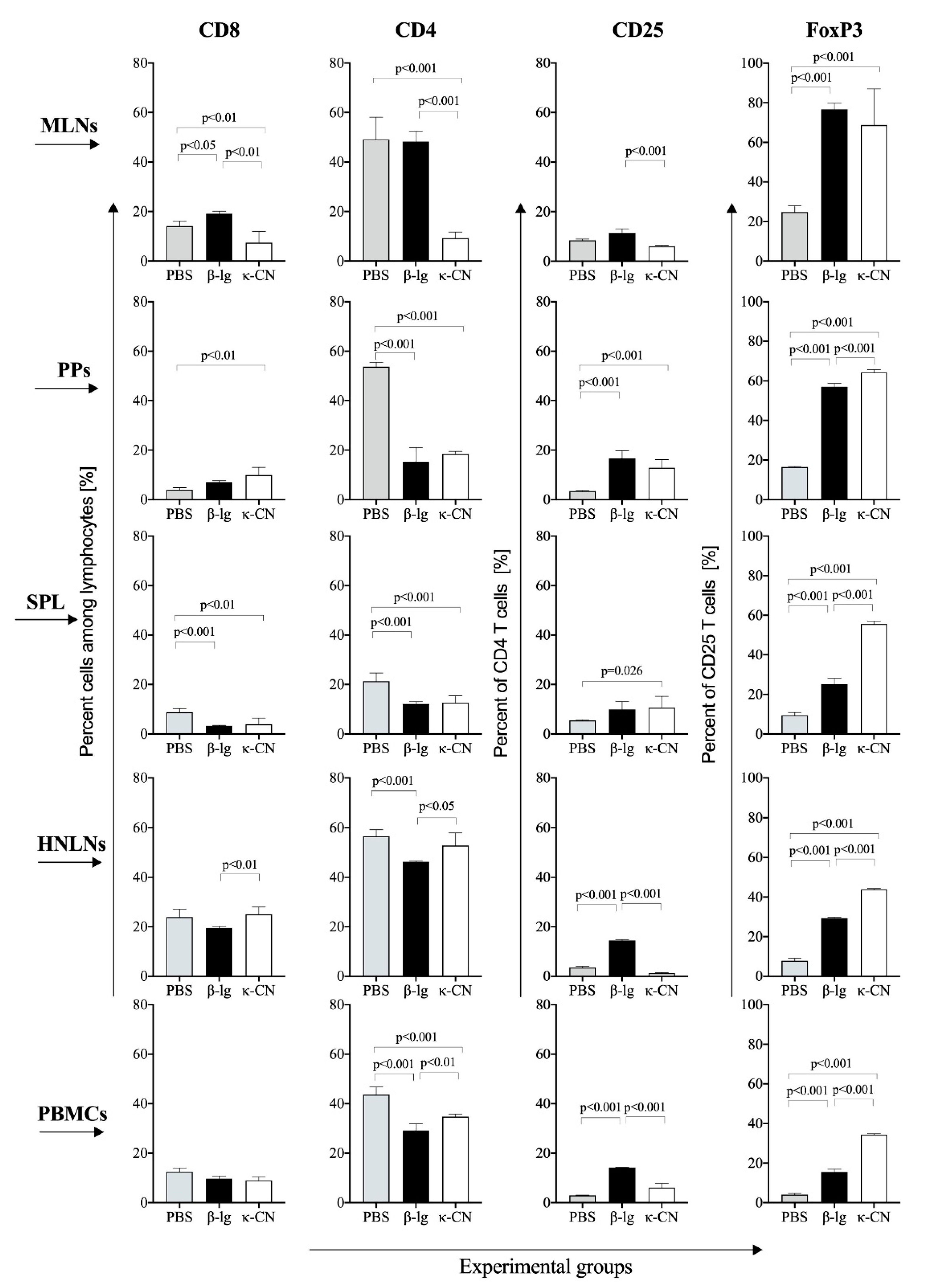
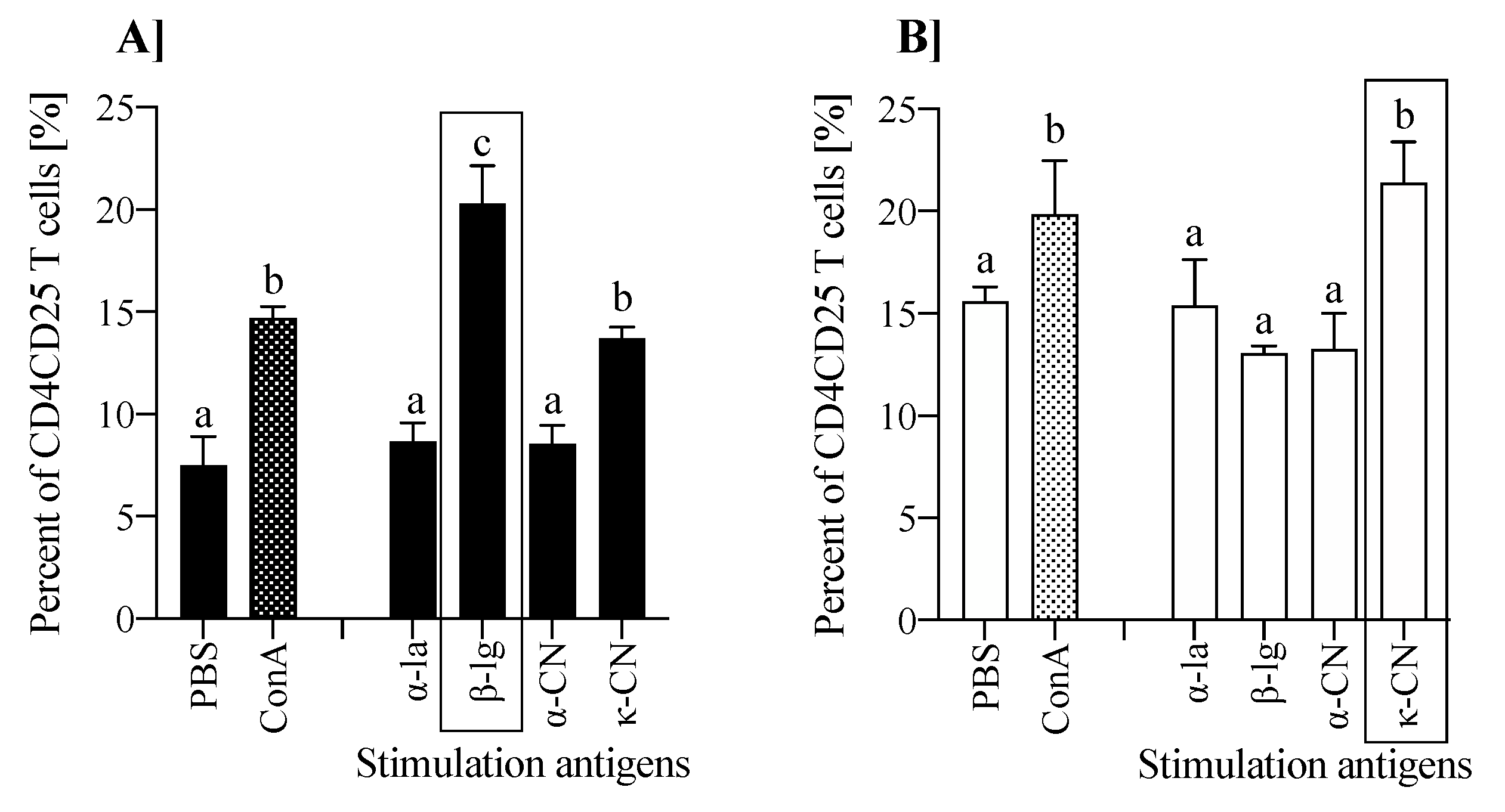
3.4. T Cell Profile of Lymphocytes from Inductive and Peripheral Tissue
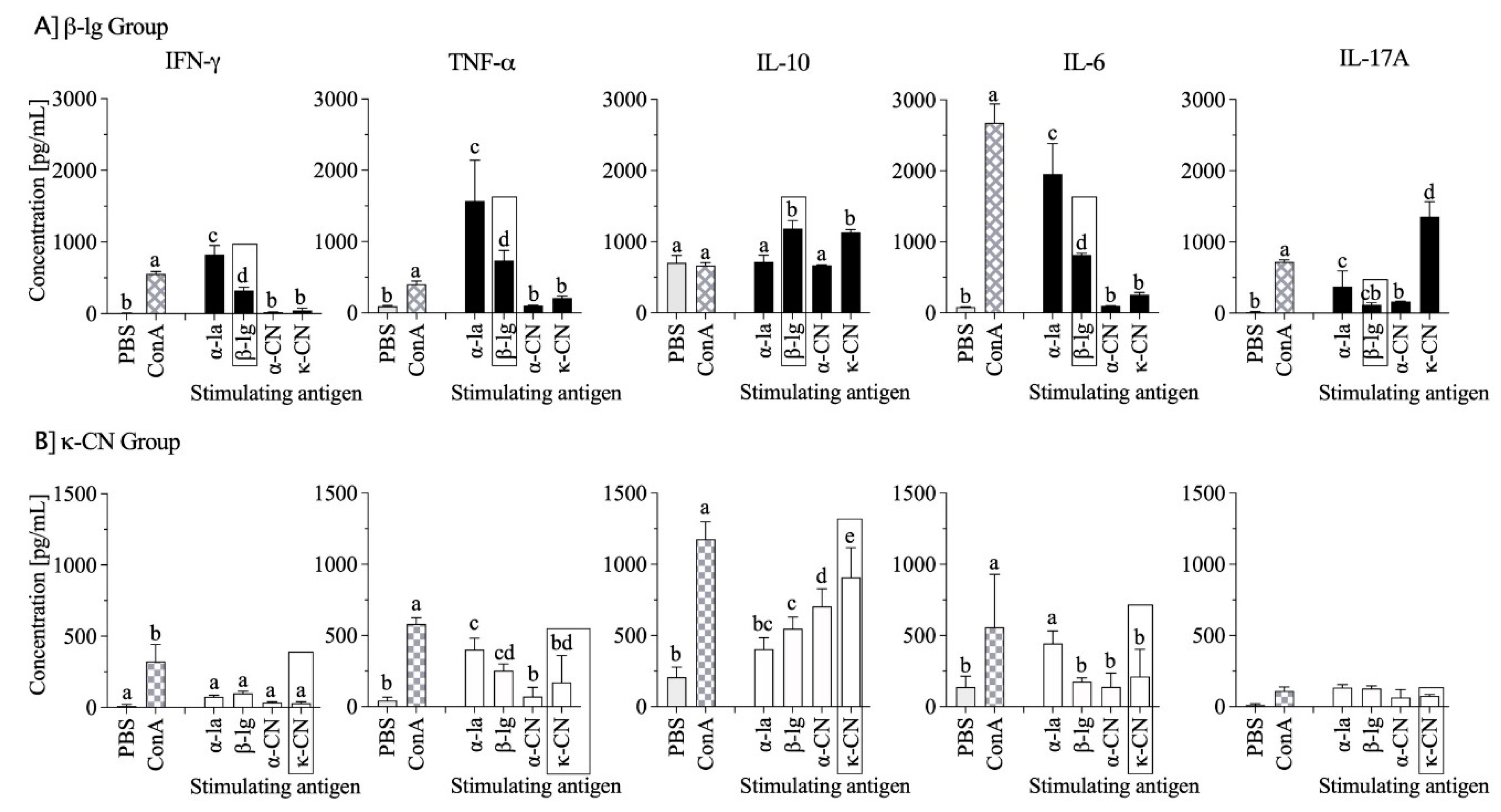
3.5. Cytokine Profiles
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foegeding, E.; Davis, J.P.; Doucet, D.; McGuffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Wróblewska, B.; Juśkiewicz, J.; Kroplewski, B.; Jurgoński, A.; Wasilewska, E.; Złotkowska, D.; Markiewicz, L. The effects of whey and soy proteins on growth performance, gastrointestinal digestion, and selected physiological responses in rats. Food Funct. 2018, 9, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Wal, J.-M. Cow’s milk allergens. Allergy 1998, 53, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited Review: β-Lactoglobulin: Binding Properties, Structure, and Function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef]
- Fiocchi, A.G.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12. [Google Scholar] [CrossRef]
- Järvinen, K.-M.; Chatchatee, P.; Bardina, L.; Beyer, K.; Sampson, H.A. IgE and IgG Binding Epitopes on α-Lactalbumin and β-Lactoglobulin in Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2001, 126, 111–118. [Google Scholar] [CrossRef]
- Han, N.; Jarvinen, K.M.; Cocco, R.R.; Busse, P.J.; Sampson, H.A.; Beyer, K. Identification of amino acids critical for IgE-binding to sequential epitopes of bovine κ-casein and the similarity of these epitopes to the corresponding human κ-casein sequence. Allergy 2008, 63, 198–204. [Google Scholar] [CrossRef]
- Chudzik-Kozłowska, J.P.; Wasilewska, E.; Złotkowska, D. Evaluation of Immunoreactivity of Pea (Pisum sativum) Albumins in BALB/c and C57BL/6 Mice. J. Agric. Food Chem. 2020, 68, 3891–3902. [Google Scholar] [CrossRef]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef]
- Fuc, E.; Złotkowska, D.; Wróblewska, B. Milk and Meat Allergens from Bos taurus β-Lactoglobulin, α-Casein, and Bovine Serum Albumin: An In-Vivo Study of the Immune Response in Mice. Nutrients 2019, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, E.; Złotkowska, D.; Wróblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Markiewicz, L.H.; Szyc, A.; Wasilewska, E. Whey prefermented with beneficial microbes modulates immune response and lowers responsiveness to milk allergens in mouse model. J. Funct. Foods 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ohshima, K.; Karube, K.; Yamaguchi, T.; Suefuji, H.; Hamasaki, M.; Kawasaki, C.; Suzumiya, J.; Tomonaga, M.; Kikuchi, M. Th1, Th2, and activated T-cell marker and clinical prognosis in peripheral T-cell lymphoma, unspecified: Comparison with AILD, ALCL, lymphoblastic lymphoma, and ATLL. Blood 2004, 103, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Boirivant, M.; Fuss, I.J.; Chu, A.; Strober, W. Oxazolone Colitis: A Murine Model of T Helper Cell Type 2 Colitis Treatable with Antibodies to Interleukin 4. J. Exp. Med. 1998, 188, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, D.; Li, Y.; Sang, L.-X.; Zhu, J.-F.; Wang, J.; Wei, B.; Lu, C.; Sun, X. Th1/Th2 Balance and Th17/Treg-Mediated Immunity in relation to Murine Resistance to Dextran Sulfate-Induced Colitis. J. Immunol. Res. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Issazadeh-Navikas, S.; Teimer, R.; Bockermann, R. Influence of Dietary Components on Regulatory T Cells. Mol. Med. 2011, 18, 95–110. [Google Scholar] [CrossRef]
- Fuc, E.; Złotkowska, D.; Stachurska, E.; Wróblewska, B. Immunoreactive properties of α-casein and κ-casein: Ex vivo and in vivo studies. J. Dairy Sci. 2018, 101, 10703–10713. [Google Scholar] [CrossRef]
- Rynda, A.; Maddaloni, M.; Mierzejewska, D.; Ochoa-Repáraz, J.; Maślanka, T.; Crist, K.; Riccardi, C.; Barszczewska, B.; Fujihashi, K.; McGhee, J.R.; et al. Low-Dose Tolerance Is Mediated by the Microfold Cell Ligand, Reovirus Protein σ1. J. Immunol. 2008, 180, 5187–5200. [Google Scholar] [CrossRef]
- Mierzejewska, D.; Mitrowska, P.; Rudnicka, B.; Kubicka, E.; Kostyra, H. Effect of non-enzymatic glycosylation of pea albumins on their immunoreactive properties. Food Chem. 2008, 111, 127–131. [Google Scholar] [CrossRef]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.R.; Johansen, F.-E.; Kahu, H.; MacPherson, A.; Brandtzaeg, P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 2010, 65, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Dearman, R.J.; Stone, S.; Caddick, H.T.; Basketter, D.; Kimber, I. Evaluation of protein allergenic potential in mice: Dose-response analyses. Clin. Exp. Allergy 2003, 33, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Chudzik-Kozłowska, J.P.; Złotkowska, D.; Kostyra, H. BALB/C mice as a model for immunogenicity testing of food proteins on the example of egg ovalbumin. Central Eur. J. Immunol. 2013, 4, 430–433. [Google Scholar] [CrossRef]
- Baba, Y.; Honjo, A.; Yamazaki, S.; Inage, E.; Mori, M.; Kantake, M.; Ohtsuka, Y.; Shimizu, T. Measurement of Antigen-Specific IgA May be Useful in Children with Food Allergy. J. Allergy Clin. Immunol. 2016, 137, AB171. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef]
- Berin, M.C.; Sampson, H. Mucosal Immunology of Food Allergy. Curr. Biol. 2013, 23, R389–R400. [Google Scholar] [CrossRef]
- Shandilya, A.S.U.K.; Kapila, R.; Kapila, S.; Kansal, V.K. Different stimulating effects of caseins and whey proteins of processed cow and buffalo milk on lymphocyte proliferation in vitro. Open Access Anim. Physiol. 2015, 7, 121. [Google Scholar] [CrossRef][Green Version]
- Złotkowska, D.; Wasilewska, E.; Kubicka, E.; Wróblewska, B. Experimental immunology The effect of pea albumins on immune response in mice. Central Eur. J. Immunol. 2012, 3, 232–236. [Google Scholar] [CrossRef]
- Harrison, O.J.; Powrie, F. Regulatory T Cells and Immune Tolerance in the Intestine. Cold Spring Harb. Perspect. Biol. 2013, 5, a018341. [Google Scholar] [CrossRef]
- Roncarolo, M.-G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Lim, H.W.; Hillsamer, P.; Banham, A.H.; Kim, C.H.; Banham, A.H. Cutting Edge: Direct Suppression of B Cells by CD4+CD25+ Regulatory T Cells. J. Immunol. 2005, 175, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Bergmann, C.; Whiteside, T.L. Human Circulating CD4+CD25highFoxp3+Regulatory T Cells Kill Autologous CD8+but Not CD4+Responder Cells by Fas-Mediated Apoptosis1. J. Immunol. 2009, 182, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunology 2013, 39, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunology 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Guo, Z.; Jang, M.H.; Otani, K.; Bai, Z.; Umemoto, E.; Matsumoto, M.; Nishiyama, M.; Yamasaki, M.; Ueha, S.; Matsushima, K.; et al. CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int. Immunol. 2008, 20, 307–315. [Google Scholar] [CrossRef]
- Savilahti, E.M.; Karinen, S.; Salo, H.M.; Klemetti, P.; Saarinen, K.M.; Klemola, T.; Kuitunen, M.; Hautaniemi, S.; Savilahti, E.; Vaarala, O. Combined T regulatory cell and Th2 expression profile identifies children with cow’s milk allergy. Clin. Immunol. 2010, 136, 16–20. [Google Scholar] [CrossRef]
- Weiberg, D.; Basic, M.; Smoczek, M.; Bode, U.; Bornemann, M.; Buettner, M. Participation of the spleen in the IgA immune response in the gut. PLoS ONE 2018, 13, e0205247. [Google Scholar] [CrossRef]
- Bellinghausen, I.; Knop, J.; Saloga, J. The role of interleukin 10 in the regulation of allergic immune responses. Int. Arch. Allergy Immunol. 2001, 126, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, E.M.; Williams, C.B. The Treg/Th17 Cell Balance: A New Paradigm for Autoimmunity. Pediatric Res. 2009, 65, 26R–31R. [Google Scholar] [CrossRef]
- Bin Dhuban, K.; D’Hennezel, E.; Ben-Shoshan, M.; McCusker, C.; Clarke, A.; Fiset, P.; Mazer, B.; Piccirillo, C.A. Altered T Helper 17 Responses in Children with Food Allergy. Int. Arch. Allergy Immunol. 2013, 162, 318–322. [Google Scholar] [CrossRef]
- Zhou, X.; Bailey-Bucktrout, S.L.; Jeker, L.T.; Penaranda, C.; Martínez-Llordella, M.; Ashby, M.; Nakayama, M.; Rosenthal, W.; Bluestone, J.A. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009, 10, 1000–1007. [Google Scholar] [CrossRef]
- Wal, J.-M. Bovine milk allergenicity. Ann. Allergy Asthma Immunol. 2004, 93, S2–S11. [Google Scholar] [CrossRef]
- Monaci, L.; Tregoat, V.; Van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Złotkowska, D.; Stachurska, E.; Fuc, E.; Wróblewska, B.; Mikołajczyk, A.; Wasilewska, E. Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies. Nutrients 2021, 13, 349. https://doi.org/10.3390/nu13020349
Złotkowska D, Stachurska E, Fuc E, Wróblewska B, Mikołajczyk A, Wasilewska E. Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies. Nutrients. 2021; 13(2):349. https://doi.org/10.3390/nu13020349
Chicago/Turabian StyleZłotkowska, Dagmara, Emilia Stachurska, Ewa Fuc, Barbara Wróblewska, Anita Mikołajczyk, and Ewa Wasilewska. 2021. "Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies" Nutrients 13, no. 2: 349. https://doi.org/10.3390/nu13020349
APA StyleZłotkowska, D., Stachurska, E., Fuc, E., Wróblewska, B., Mikołajczyk, A., & Wasilewska, E. (2021). Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies. Nutrients, 13(2), 349. https://doi.org/10.3390/nu13020349