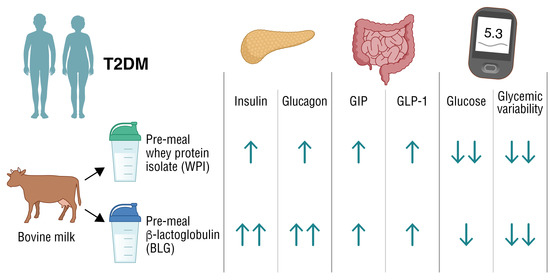
β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein—A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Participants
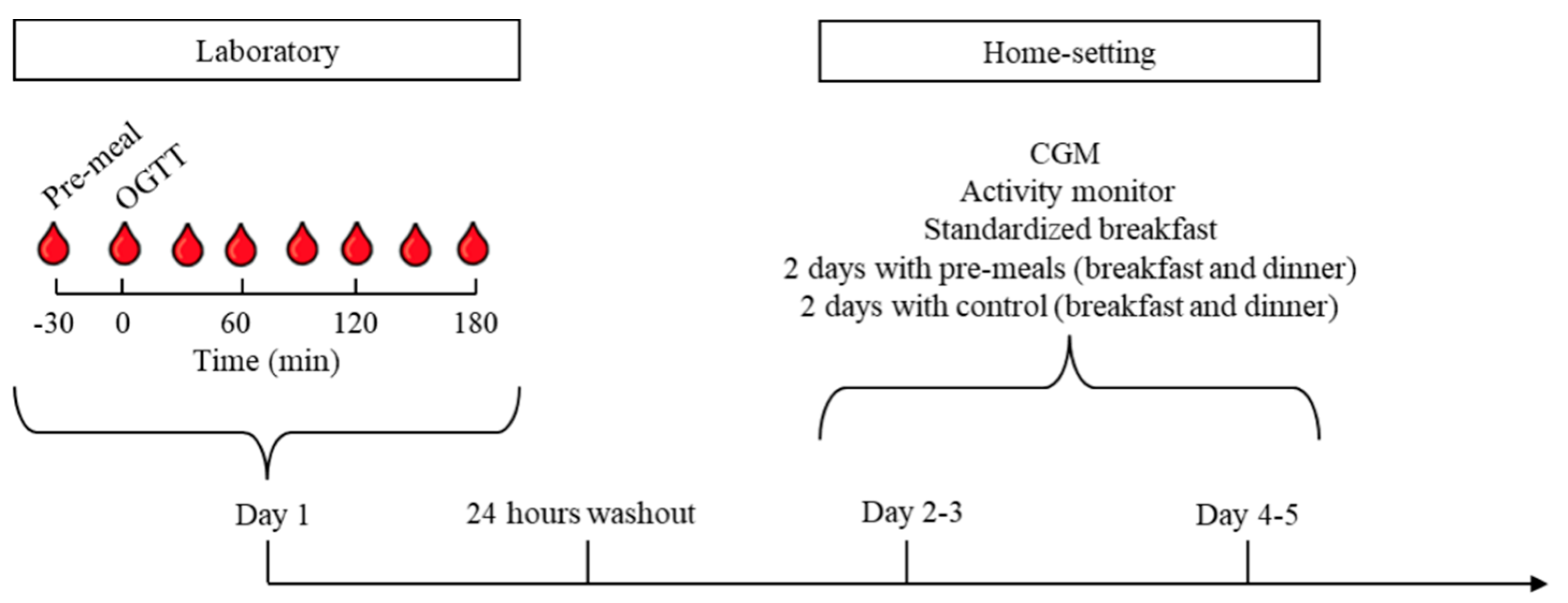
2.3. Design and Protocol
2.4. Interventions and Meals
2.5. Blood Analysis
2.6. Continuous Glucose Monitoring
2.7. Activity Monitoring
2.8. Statistical Analysis
3. Results
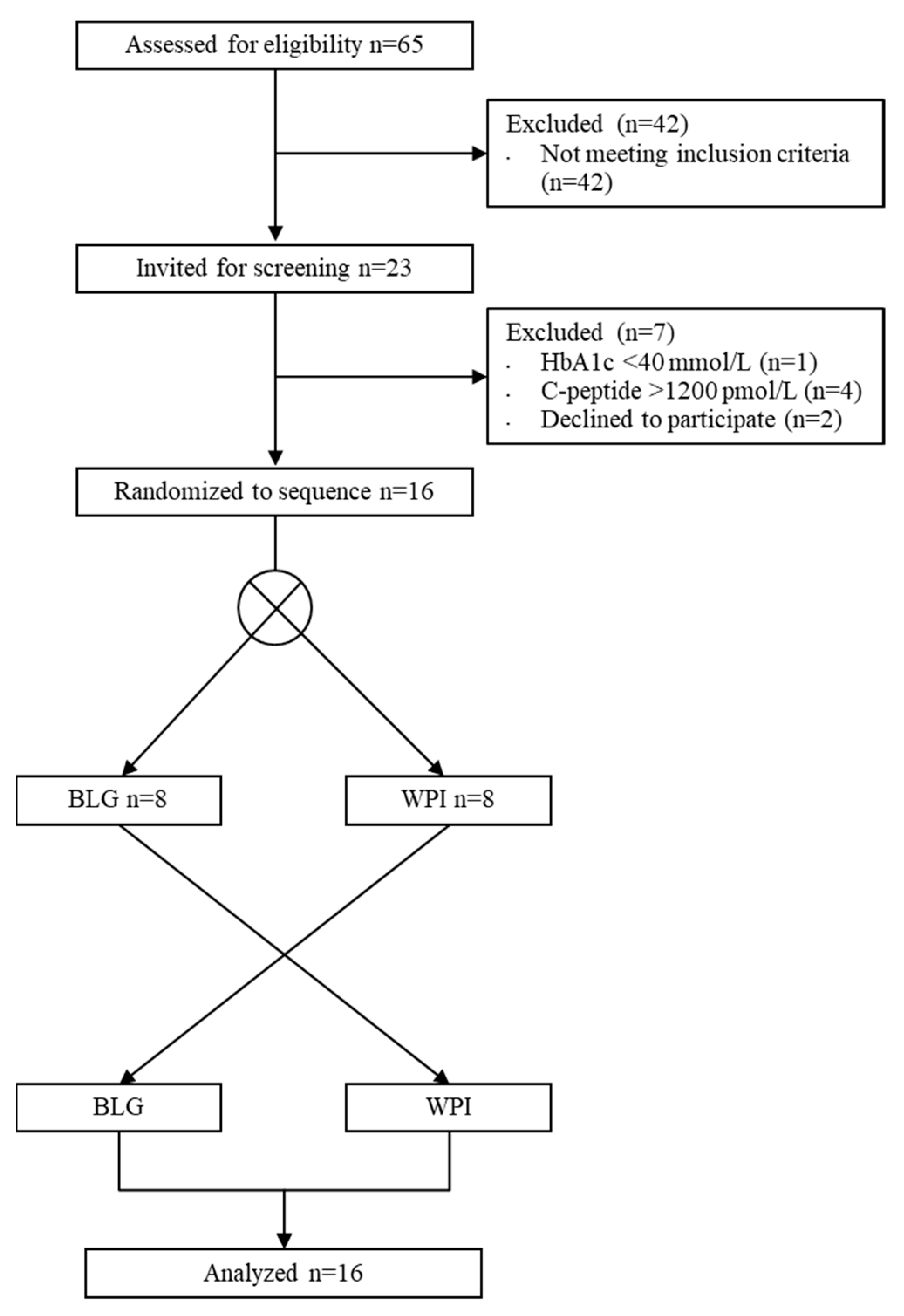
3.1. Participants
3.2. Oral Glucose Tolerance Test (OGTT)
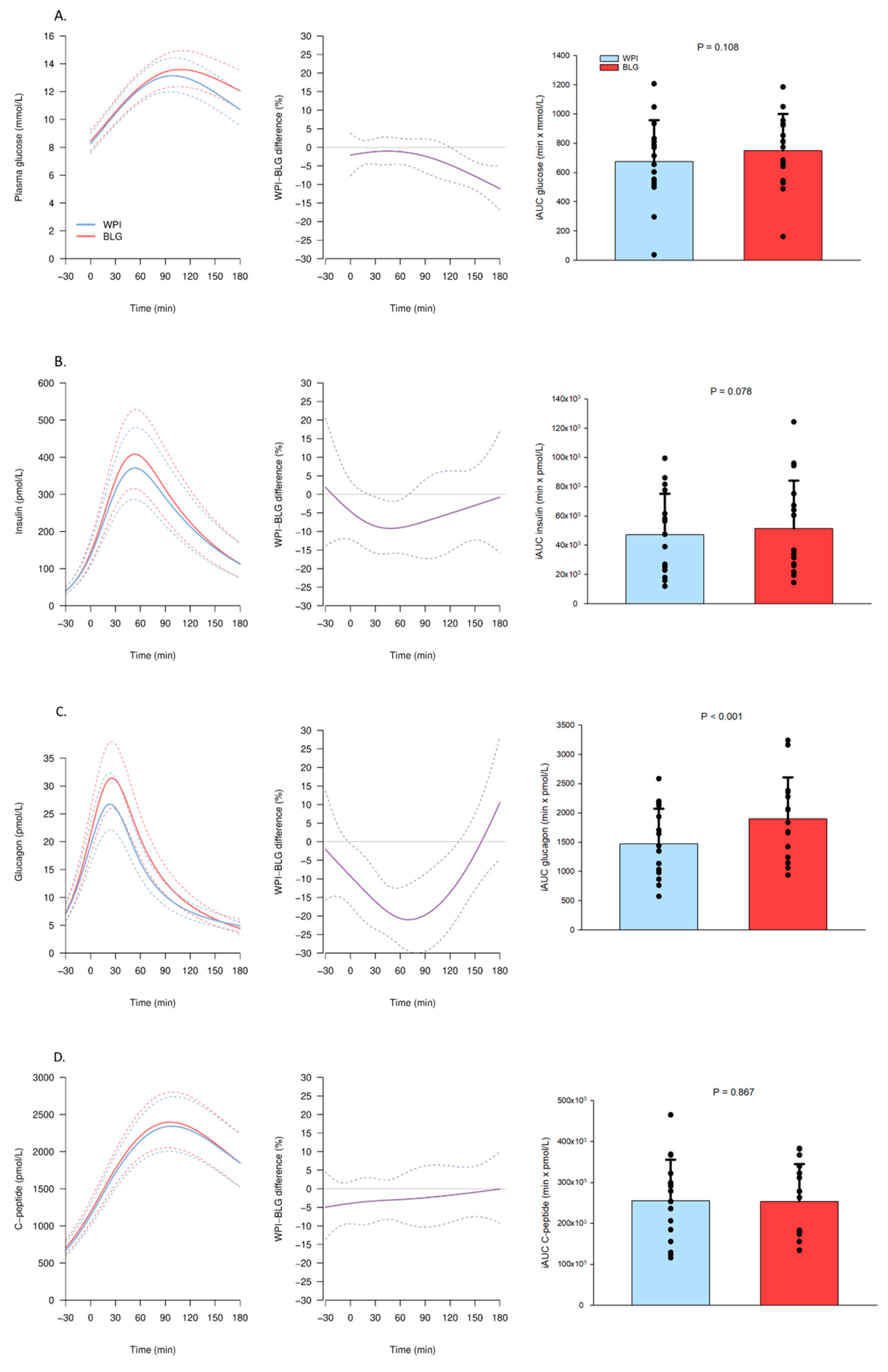
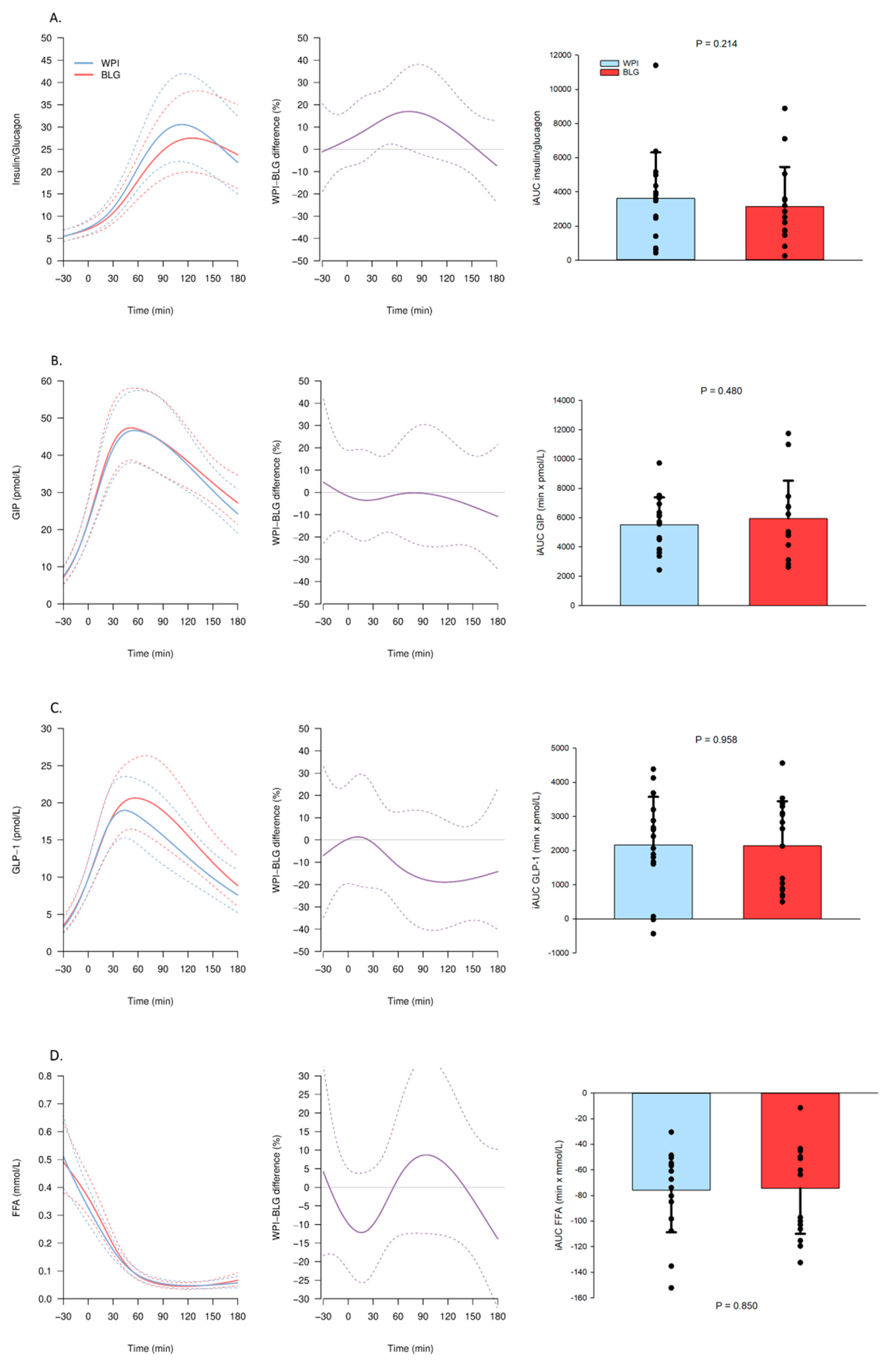
3.2.1. Substrate and Hormone Concentrations
3.2.2. Amino Acids
3.3. Home-Monitoring with Continuous Glucose Monitoring (CGM)
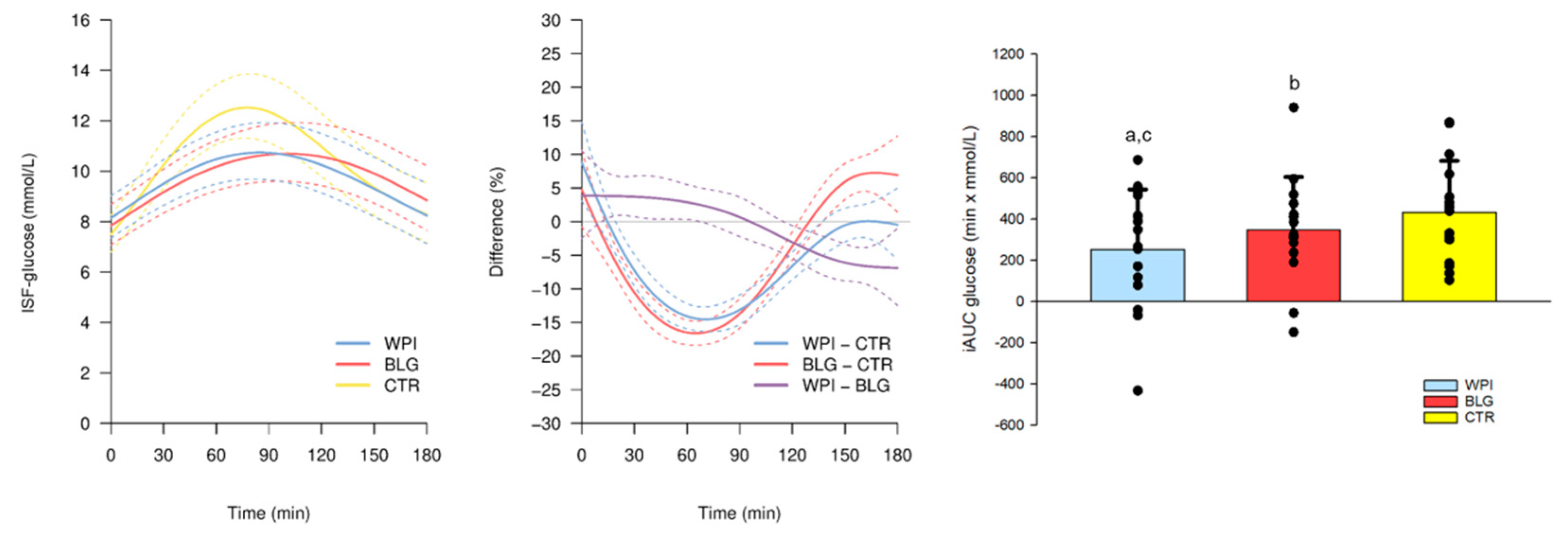
3.3.1. CGM Glucose Trajectories Following Breakfast
3.3.2. CGM and Summary Statistics
3.4. Energy Expenditure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Bjørnshave, A.; Holst, J.J.; Hermansen, K. Pre-Meal Effect of Whey Proteins on Metabolic Parameters in Subjects with and without Type 2 Diabetes: A Randomized, Crossover Trial. Nutrients 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Ostman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Holst, J.J.; Björck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [CrossRef]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Froy, O.; Ahrén, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef]
- Zheng, H.; Clausen, M.R.; Dalsgaard, T.K.; Bertram, H.C. Metabolomics to Explore Impact of Dairy Intake. Nutrients 2015, 7, 4875–4896. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: http://www.randomizer.org/ (accessed on 9 January 2020).
- Liebenberg, N.; Jensen, E.; Larsen, E.R.; Kousholt, B.S.; Pereira, V.S.; Fischer, C.W.; Wegener, G. A Preclinical Study of Casein Glycomacropeptide as a Dietary Intervention for Acute Mania. Int. J. Neuropsychopharmacol. 2018, 21, 473–484. [Google Scholar] [CrossRef]
- Orskov, C.; Rabenhøj, L.; Wettergren, A.; Kofod, H.; Holst, J.J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994, 43, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Carr, R.D.; Deacon, C.F.; Holst, J.J.; Pacini, G.; Mari, A.; Ahrén, B. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J. Clin. Endocrinol. Metab. 2011, 96, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- CamNtech Ltd. Actiheart User Manual 5.1.14; CamNtech Ltd.: Fenstanton, UK, 2020. [Google Scholar]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; MacLean, D.A. Plasma Glucagon and Insulin Responses Depend on the Rate of Appearance of Amino Acids after Ingestion of Different Protein Solutions in Humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef]
- Rocha, D.M.; Faloona, G.R.; Unger, R.H. Glucagon-stimulating activity of 20 amino acids in dogs. J. Clin. Investig. 1972, 51, 2346–2351. [Google Scholar] [CrossRef]
- Galsgaard, K.D.; Jepsen, S.L.; Kjeldsen, S.A.S.; Pedersen, J.; Wewer Albrechtsen, N.J.; Holst, J.J. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E920–E929. [Google Scholar] [CrossRef]
- Lund, A.; Vilsbøll, T.; Bagger, J.I.; Holst, J.J.; Knop, F.K. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1038–E1046. [Google Scholar] [CrossRef]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.L.; Grivell, J.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. A whey/guar "preload" improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: A 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 930–938. [Google Scholar] [CrossRef]
- Almario, R.U.; Buchan, W.M.; Rocke, D.M.; Karakas, S.E. Glucose-lowering effect of whey protein depends upon clinical characteristics of patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000420. [Google Scholar] [CrossRef]
- Staub, H. Untersuchungen über den Zuckerstoffwechsel des Menschen (Studies on sugar metabolism of humans). Z. Klin. Med. 1921, 91, 44–60. [Google Scholar]
- Traugott, K. Über das Verhalten des Blutzuckerspiegels bei Wiederholter und Verschiedener Art Enteraler Zuckerzufuhr und Dessen Bedeutung für die Leberfunktion (About the behavior of blood sugar level in repeated and different types of enteral sugar intake and their impact on liverfunction). Klin. Wochenschr. 2005, 1, 892–894. [Google Scholar]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Pagliarino, A.; Valle, M.; Di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef]
- Xia, W.; Luo, Y.; Chen, Y.C.; Chen, H.; Ma, J.; Yin, X. Glucose Fluctuations Are Linked to Disrupted Brain Functional Architecture and Cognitive Impairment. J. Alzheimer’s Dis. 2020, 74, 603–613. [Google Scholar] [CrossRef] [PubMed]
- King, D.G.; Walker, M.; Campbell, M.D.; Breen, L.; Stevenson, E.J.; West, D.J. A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 550–557. [Google Scholar] [CrossRef]
| Nutritional Content | BLG /100 g Product | WPI /100 g Product |
| Total energy, kCal | 375 | 355 |
| Fat, g | 0.1 | 0.1 |
| Carbohydrate, g | 0.1 | 0.1 |
| Protein, g | 93.5 | 88.3 |
| No-calorie flavor, % | 0.56 | 0.56 |
| Amino Acids | BLG g/100 g Protein | WPI g/100 g Protein |
| Alanine | 7.0 | 6.0 |
| Arginine | 2.8 | 2.3 |
| Aspartic acid | 12.1 | 11.8 |
| Cysteine | 3.1 | 2.6 |
| Glutamic acid | 20.1 | 19.7 |
| Glycine | 1.3 | 1.6 |
| Histidine | 1.7 | 1.7 |
| Hydroxyproline | <0.1 | <0.1 |
| Isoleucine | 6.3 | 7.0 |
| Leucine | 16.1 | 11.7 |
| Lysine | 12.3 | 10.6 |
| Methionine | 2.8 | 2.4 |
| Ornithine | <0.1 | <0.1 |
| Phenylalanine | 3.6 | 3.1 |
| Proline | 5.4 | 6.8 |
| Serine | 3.9 | 5.2 |
| Threonine | 5.3 | 8.0 |
| Tryptophan | 2.2 | 1.9 |
| Tyrosine | 3.7 | 2.9 |
| Valine | 6.1 | 6.4 |
| Sum | 115.9 | 111.8 |
| Characteristics | n = 16 |
|---|---|
| Age, years | 67.5 (40–78) |
| BMI, kg/m2 | 27.0 (21.2–32.9) |
| Women, % | 56.2 |
| Metformin treatment, n | 14 |
| HbA1c, mmol/mol | 50 (43–55) |
| Fasting c-peptide, nmol/L | 932 (499–1155) |
| Fasting insulin, pmol/L | 40 (20–94) |
| n = 15 | Mean Glucose, mmol/L | SD, mmol/L | CV, % |
| CTR | 8.7 (7.9; 9.5) | 2.0 (1.8; 2.2) | 22.7 (21.3; 24.1) |
| WPI | 8.8 (8.0; 9.7) | 1.8 (1.6; 2.0) | 20.6 (18.9; 22.2) |
| BLG | 8.6 (8.0; 9.7) | 1.7 (1.5; 1.9) | 19.2 (17.5; 20.9) |
| WPI–CTR | 0.1 (−0.2; 0.4) | −0.2 (−0.3; −0.0) ** | −2.2 (−3.9; −0.5) * |
| BLG–CTR | 0.2 (−0.1; 0.5) | −0.3 (−0.4; −0.1) ** | −3.5 (−5.2; −1.8) ** |
| WPI–BLG | −0.1 (−0.4; 0.3) | 0.1 (−0.1; 0.3) | 1.3 (−0.6; 3.3) |
| n = 15 | Max after Breakfast, mmol/L | Daily max, mmol/L | Max after Dinner, mmol/L |
| CTR | 14.4 (13.1; 15.6) | 14.5 (13.2; 15.9) | 10.8 (9.8; 11.8) |
| WPI | 12.5 (11.2; 13.9) | 13.5 (12.2; 14.9) | 10.9 (9.7; 12.0) |
| BLG | 12.7 (11.4; 14.0) | 13.8 (12.5; 15.2) | 11.0 (9.8; 12.1) |
| WPI–CTR | −1.8 (−2.4; −1.3) * | −1.0 (−1.6; −0.4) ** | 0.1 (−0.8; 1.0) |
| BLG–CTR | −1.7 (−2.2; −1.1) ** | −0.7 (−1.3; −0.1) * | 0.2 (−0.7; 1.1) |
| WPI–BLG | −0.2 (−0.8; 0.5) | −0.3 (−1.0; 0.4) | −0.1 (−1.1; 0.9) |
| n = 15 | TEE, kCal | AEE, kCal | Activity, counts/min | Mean HR, BPM | Maximum HR, BPM |
|---|---|---|---|---|---|
| CTR | 2446 (2234; 2658) | 659 (514; 804) | 34 (26; 41) | 74 (70; 79) | 104 (97; 110) |
| WPI | 2338 (2110; 2565) | 559 (396; 721) | 28 (19; 36) | 75 (70; 79) | 103 (96; 111) |
| BLG | 2499 (2277; 2722) | 708 (551; 865) | 34 (25; 42) | 76 (71; 80) | 105 (98; 112) |
| WPI–CTR | −109 (−243; 26) | −100 (−221; 20) | −7 (−13; 0) * | 0 (−2; 3) | 0 (−5; 5) |
| BLG–CTR | 53 (−74; 180) | 49 (−66; 163) | 0 (−6; 6) | 2 (−1; 4) | 2 (−3; 7) |
| WPI–BLG | −161 (−313; −10) * | −149 (−286; −13) * | −6 (−14; 1) | −1 (−4; 1) | −2 (−8; 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smedegaard, S.B.; Mose, M.; Hulman, A.; Mikkelsen, U.R.; Møller, N.; Wegener, G.; Jessen, N.; Rittig, N. β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein—A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus. Nutrients 2021, 13, 308. https://doi.org/10.3390/nu13020308
Smedegaard SB, Mose M, Hulman A, Mikkelsen UR, Møller N, Wegener G, Jessen N, Rittig N. β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein—A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus. Nutrients. 2021; 13(2):308. https://doi.org/10.3390/nu13020308
Chicago/Turabian StyleSmedegaard, Stine B., Maike Mose, Adam Hulman, Ulla R. Mikkelsen, Niels Møller, Gregers Wegener, Niels Jessen, and Nikolaj Rittig. 2021. "β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein—A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus" Nutrients 13, no. 2: 308. https://doi.org/10.3390/nu13020308
APA StyleSmedegaard, S. B., Mose, M., Hulman, A., Mikkelsen, U. R., Møller, N., Wegener, G., Jessen, N., & Rittig, N. (2021). β-Lactoglobulin Elevates Insulin and Glucagon Concentrations Compared with Whey Protein—A Randomized Double-Blinded Crossover Trial in Patients with Type Two Diabetes Mellitus. Nutrients, 13(2), 308. https://doi.org/10.3390/nu13020308