Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic, Socio-Economic and Health Information
2.3. Body Composition and Body Fat Distribution Measurements
2.4. Physical Activity, Sedentary Time and Energy Expenditure
2.5. Dietary Intake
2.6. Statistical Analysis
2.7. Power Calculation
3. Results
3.1. Descriptive Characteristics of the Participants
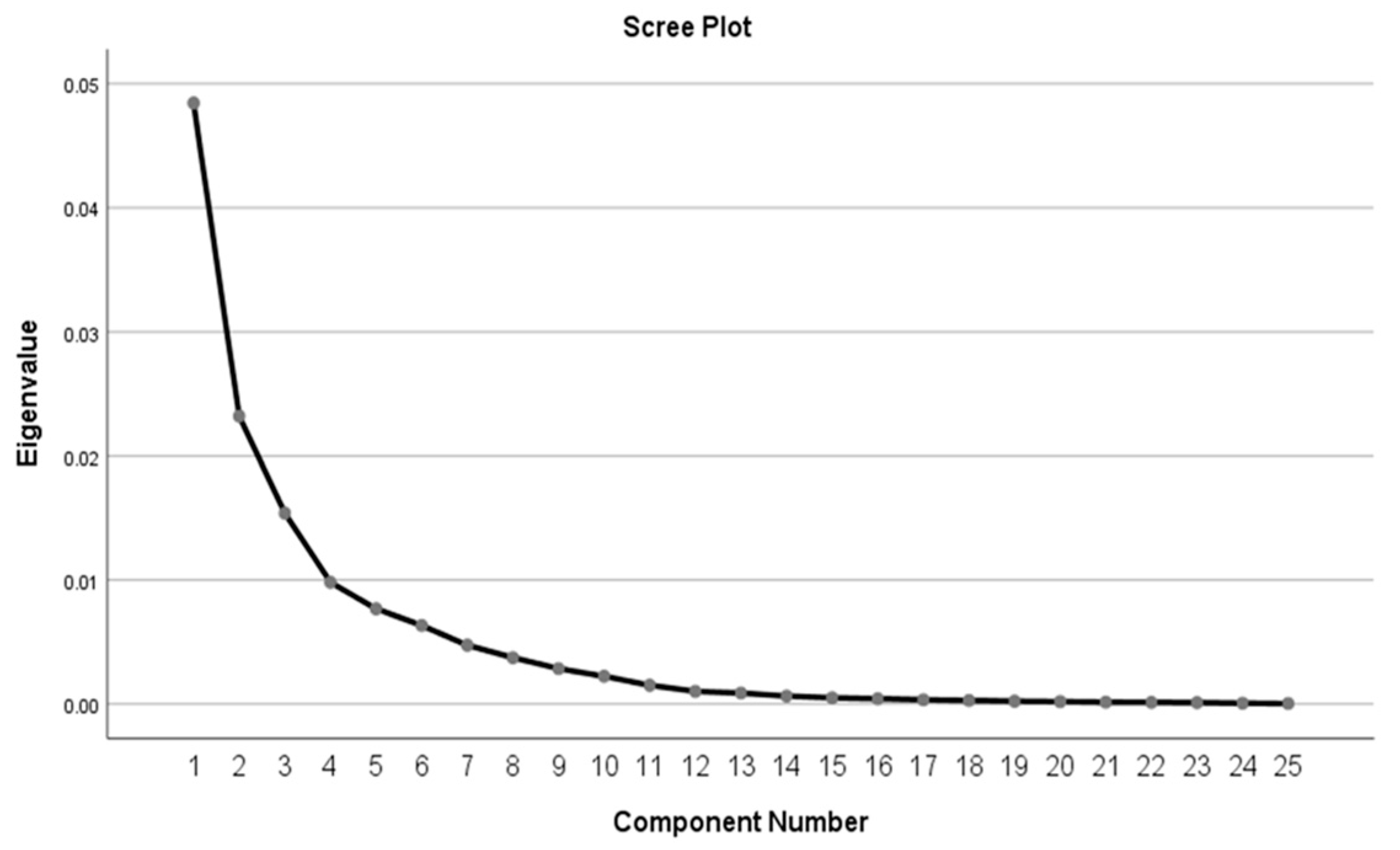
3.2. Nutrient Patterns
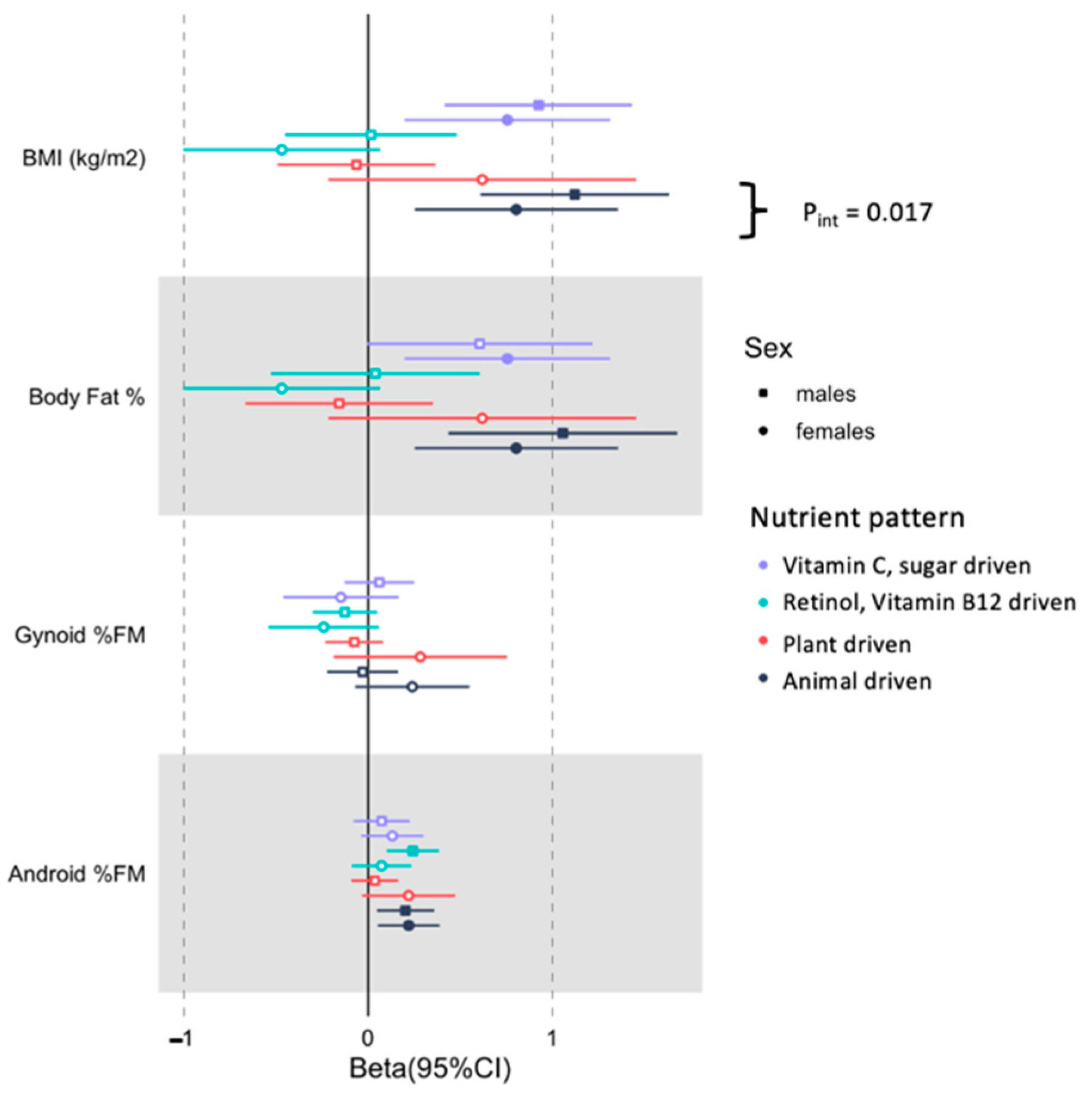
3.3. Associations between Derived Nutrient Patterns with the Selected Body Composition Traits in Men and Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Mchiza, Z.J.; Steyn, N.P.; Hill, J.; Kruger, A.; Schonfeldt, H.; Hettie, C.; Nel, J.; Wentzel-Viljoen, E. A Review of Dietary Surveys in the Adult South African Population from 2000 to 2015. Nutrients 2015, 7, 8227–8250. [Google Scholar] [CrossRef]
- Ajayi, I.O.; Adebamowo, C.; Adami, H.-O.; Dalal, S.; Diamond, M.B.; Bajunirwe, F.; Guwatudde, D.; Njelekela, M.; Nankya-Mutyoba, J.; Chiwanga, F.S.; et al. Urban–rural and geographic differences in overweight and obesity in four sub-Saharan African adult populations: A multi-country cross-sectional study. BMC Public Health 2016, 16, 1126. [Google Scholar] [CrossRef]
- SADHS South Africa Demographic and Health Survey; 2016. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiDibOHuuf0AhVFAd4KHfhGBQUQFnoECAkQAQ&url=https%3A%2F%2Fdhsprogram.com%2Fpubs%2Fpdf%2FSR248%2FSR248.pdf&usg=AOvVaw229kaj36j9x1risqBNKNWa (accessed on 6 December 2021).
- Barroso, T.A.; Marins, L.B.; Alves, R.; Gonçalves, A.C.S.; Barroso, S.G.; Rocha, G.D.S. Association of Central Obesity with The Incidence of Cardiovascular Diseases and Risk Factors. Int. J. Cardiovasc. Sci. 2017. [Google Scholar] [CrossRef]
- World Health Organization. Burden of NCDs and Their Risk Factors in India; Excerpted from Global Status Report on NCDs-2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Centers for Disease Control and Prevention Physical Activity and the Health of Young People. Retrieved February 2013. Available online: https://www.cdc.gov/healthyschools/physicalactivity/facts.htm (accessed on 6 December 2021).
- Gallagher, D.; Visser, M.; Sepúlveda, D.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. How Useful Is Body Mass Index for Comparison of Body Fatness across Age, Sex, and Ethnic Groups? Am. J. Epidemiol. 1996, 143, 228–239. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic Obesity: Definition, Cause and Consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Vallero, F.; Di Monaco, R.; Tappero, R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch. Gerontol. Geriatr. 2011, 52, 71–74. [Google Scholar] [CrossRef]
- Preis, S.R.; Massaro, J.M.; Robins, S.J.; Hoffmann, U.; Vasan, R.S.; Irlbeck, T.; Meigs, J.B.; Sutherland, P.; D’Agostino, R.B., Sr.; O’Donnell, C.J.; et al. Abdominal Subcutaneous and Visceral Adipose Tissue and Insulin Resistance in the Framingham Heart Study. Obesity 2010, 18, 2191–2198. [Google Scholar] [CrossRef]
- Plank, L. Dual-energy X-ray absorptiometry and body composition. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 305–309. [Google Scholar] [CrossRef]
- Ellegård, L.H.; Åhlén, M.; Körner, U.; Lundholm, K.; Plank, L.; Bosaeus, I.G. Bioelectric impedance spectroscopy underestimates fat-free mass compared to dual energy X-ray absorptiometry in incurable cancer patients. Eur. J. Clin. Nutr. 2008, 63, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Micklesfield, L.; Goedecke, J.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L. Dual-Energy X-Ray Performs as Well as Clinical Computed Tomography for the Measurement of Visceral Fat. Obesity 2012, 20, 1109–1114. [Google Scholar] [CrossRef]
- Pisa, P.T.; Micklesfield, L.K.; Kagura, J.; Ramsay, M.; Crowther, N.J.; Norris, S.A. Different adiposity indices and their association with blood pressure and hypertension in middle-aged urban black South African men and women: Findings from the AWI-GEN South African Soweto Site. BMC Public Health 2018, 18, 524. [Google Scholar] [CrossRef] [PubMed]
- Mtintsilana, A.; Micklesfield, L.K.; Chorell, E.; Olsson, T.; Shivappa, N.; Hebert, J.R.; Kengne, A.P.; Goedecke, J.H. Adiposity Mediates the Association between the Dietary Inflammatory Index and Markers of Type 2 Diabetes Risk in Middle-Aged Black South African Women. Nutrients 2019, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.A.; Comeau, D.L.; Pisa, P.T.; Stein, A.D.; Norris, S.A. Perceptions of diet, physical activity, and obesity-related health among black daughter-mother pairs in Soweto, South Africa: A qualitative study. BMC Public Health 2016, 16, 750. [Google Scholar] [CrossRef]
- Yeom, J.; Kim, J.K.; Crimmins, E.M. Factors Associated with Body Mass Index(BMI) Among Older Adults: A Comparison Study of the U.S., Japan, and Korea. Korea Gerontol. Soc. 2009, 29, 1479–1500. [Google Scholar]
- Micklesfield, L.K.; Lambert, E.; Hume, D.J.; Chantler, S.; Pienaar, P.R.; Dickie, K.; Puoane, T.; Goedecke, J.H. Socio-cultural, environmental and behavioural determinants of obesity in black South African women: Review articles. Cardiovasc. J. Afr. 2013, 24, 369–375. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Song, Y.-M.; Ha, M.; Sung, J. Body Mass Index and Mortality in Middle-Aged Korean Women. Ann. Epidemiol. 2007, 17, 556–563. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight: Fact Sheet; WHO Media Centre: Geneva, Switzerland, 2016. [Google Scholar]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; de Deus Mendonça, R.; De La Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef]
- Otang-Mbeng, W.; Otunola, G.A.; Afolayan, A.J. Lifestyle factors and co-morbidities associated with obesity and overweight in Nkonkobe Municipality of the Eastern Cape, South Africa. J. Health Popul. Nutr. 2017, 36, 22. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Pisa, P.T.; Pedro, T.M.; Kahn, K.; Tollman, S.M.; Pettifor, J.M.; Norris, S.A. Nutrient Patterns and Their Association with Socio-Demographic, Lifestyle Factors and Obesity Risk in Rural South African Adolescents. Nutrients 2015, 7, 3464–3482. [Google Scholar] [CrossRef]
- Makura-Kankwende, C.B.T.; Gradidge, P.J.; Crowther, N.J.; Norris, S.A.; Chikowore, T. Nutrient Patterns and Body Composition Parameters of Black South African Women. Nutrients 2020, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.; Crowther, N.; Tambo, E.; Agongo, G.; Baloyi, V.; Dikotope, S.; Gómez-Olivé, X.; Jaff, N.; Sorgho, H.; Wagner, R.; et al. H3Africa AWI-Gen Collaborative Centre: A resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob. Health Epidemiol. Genom. 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Jessri, M.; Lou, W.Y.; L’Abbé, M.R. Evaluation of Different Methods to Handle Misreporting in Obesity Research: Evidence from the Canadian National Nutrition Survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional Epidemiology. 1998. Available online: https://www.worldcat.org/title/nutritional-epidemiology/oclc/938913631 (accessed on 6 December 2021).
- Steyn, N.P.; Senekal, M.; Norris, S.A.; Whati, L.; MacKeown, J.M.; Nel, J.H. How Well Do Adolescents Determine Portion Sizes of Foods and Beverages? Asia Pac. J. Clin. Nutr. 2006, 15, 35–42. [Google Scholar]
- Zingoni, C.; Norris, S.; Griffiths, P.L.; Cameron, N. Studying a Population Undergoing Nutrition Transition: A Practical Case Study of Dietary Assessment in Urban South African Adolescents. Ecol. Food Nutr. 2009, 48, 178–198. [Google Scholar] [CrossRef][Green Version]
- Hausman, D.B.; Johnson, M.A.; Davey, A.; Poon, L.W. Body Mass Index Is Associated with Dietary Patterns and Health Conditions in Georgia Centenarians. J. Aging Res. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Wolmarans, P.; Kunneke, E.; Laubscher, R. Use of the South African Food Composition Database System (SAFOODS) and its products in assessing dietary intake data: Part II. S. Afr. J. Clin. Nutr. 2009, 22, 59–67. [Google Scholar] [CrossRef]
- Chikowore, T.; Pisa, P.T.; Van Zyl, T.; Feskens, E.J.M.; Wentzel-Viljoen, E.; Conradie, K.R. Nutrient Patterns Associated with Fasting Glucose and Glycated Haemoglobin Levels in a Black South African Population. Nutrients 2017, 9, 9. [Google Scholar] [CrossRef]
- Lingard, H.C.; Rowlinson, S. Sample Size in Factor Analysis: Why Size Matters. Factor Analysis 1992. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.511.9840 (accessed on 6 December 2021).
- Mundfrom, D.J.; Shaw, D.G.; Ke, T.L. Minimum Sample Size Recommendations for Conducting Factor Analyses. Int. J. Test. 2005, 5, 159–168. [Google Scholar] [CrossRef]
- Guilford, J.P. The Constant Methods. In Psychometric Methods; 1954. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1301999 (accessed on 9 December 2021).
- Visser, M.; Van Zyl, T.; Hanekom, S.M.; Baumgartner, J.; van der Hoeven, M.; Taljaard-Krugell, C.; Smuts, C.M.; Faber, M. Nutrient patterns and their relation to anemia and iron status in 5- to 12-y-old children in South Africa. Nutrients 2019, 62, 194–200. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Food Balance Sheets; FAO: Rome, Italy, 1949. [Google Scholar]
- McKeown, N.M.; Troy, L.M.; Jacques, P.F.; Hoffmann, U.; O’Donnell, C.J.; Fox, C.S. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: The Framingham Heart Study. Am. J. Clin. Nutr. 2010, 92, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Dave, J.A.; Faulenbach, M.V.; Utzschneider, K.M.; Lambert, E.V.; West, S.; Collins, M.; Olsson, T.; Walker, B.R.; Seckl, J.R.; et al. Insulin Response in Relation to Insulin Sensitivity: An appropriate -cell response in black South African women. Diabetes Care 2009, 32, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Olsson, T. Pathogenesis of type 2 diabetes risk in black Africans: A South African perspective. J. Intern. Med. 2020, 288, 284–294. [Google Scholar] [CrossRef]
- Gower, B.A.; Fowler, L.A. Obesity in African-Americans: The role of physiology. J. Intern. Med. 2020, 288, 288. [Google Scholar] [CrossRef] [PubMed]
- Keswell, D.; Tootla, M.; Goedecke, J.H. Associations between body fat distribution, insulin resistance and dyslipidaemia in black and white South African women. Cardiovasc. J. Afr. 2016, 27, 177–183. [Google Scholar] [CrossRef]
- Puoane, T.; Matwa, P.; Bradley, H.; Hughes, G. Socio-Cultural Factors Influencing Food Consumption Patterns in the Black African Population in an Urban Township in South Africa. 2006. Available online: http://hdl.handle.net/10566/253 (accessed on 6 December 2021).
- Vorster, H.H.; Kruger, A.; Wentzel-Viljoen, E.; Kruger, H.S.; Margetts, B.M. Added sugar intake in South Africa: Findings from the Adult Prospective Urban and Rural Epidemiology cohort study. Am. J. Clin. Nutr. 2014, 99, 1479–1486. [Google Scholar] [CrossRef]
- Steyn, N.P.; Jaffer, N.; Nel, J.; Levitt, N.; Steyn, K.; Lombard, C.; Peer, N. Dietary Intake of the Urban Black Population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) Study. Nutrients 2016, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Yagan, F.C.C.; Olcum, G.G.; Kurtulus, D.; Tugtekin, H.B. The relationship between obesity and serum Vitamin B12, folic acid, vitamin D concentrations in obes adults: A retrospective study. Endocr. Abstr. 2016. [Google Scholar] [CrossRef]
- Allin, K.H.; Friedrich, N.; Pietzner, M.; Grarup, N.; Thuesen, B.H.; Linneberg, A.; Pisinger, C.; Hansen, T.; Pedersen, O.; Sandholt, C.H. Genetic determinants of serum vitamin B12 and their relation to body mass index. Eur. J. Epidemiol. 2016, 32, 125–134. [Google Scholar] [CrossRef] [PubMed]

| Variables | Men (n = 414) | Women (n = 346) | p-Value |
|---|---|---|---|
| Age (yrs) | 54 ± 6 | 54 ± 6 | 0.817 |
| Measure of Ses | |||
| Education (n (%)) | |||
| Primary | 101 (24.5) | 69 (20.1) | <0.001 |
| Secondary | 231 (56.1) | 239 (68.3) | |
| Tertiary | 80 (19.4) | 40 (11.6) | |
| % Employed (n (%)) | 255 (61.7) | 212 (61.4) | 0.934 |
| % Married (n (%)) | 208 (50.4) | 147 (42.6) | 0.033 |
| BMI (kg/m2) | 25.5 ± 5.9 | 33.2 ± 6.5 | <0.001 |
| BMI categories (n (%)) | |||
| Underweight | 38 (9.2) | 2 (0.6) | <0.001 |
| Normal weight | 167 (40.4) | 32 (9.2) | |
| Overweight | 119 (28.8) | 82 (23.7) | |
| Obese | 89 (21.5) | 230 (66.5) | |
| Total and Regional Adiposity | |||
| Fat mass (kg) | 18.9 ± 8.9 | 35.5 ± 10.2 | <0.001 |
| Body fat (%) | 26.0 ± 6.8 | 44.6 ± 5.2 | <0.001 |
| Gynoid (% FM) | 17.0 ± 1.9 | 17.7 ± 2.7 | <0.001 |
| Android (% FM) | 8.5 ± 1.6 | 7.4 ± 1.5 | <0.001 |
| VAT (cm2) | 87.4 ± 46.0 | 104.1 ± 44.3 | <0.001 |
| SAT (cm2) | 311 ± 192 | 460 ± 155 | <0.001 |
| VAT/SAT ratio | 1 ± 0 | 0 ± 0 | <0.001 |
| Dietary Intake | |||
| Energy intake (kj) | 8691 ± 4192 | 6960 ± 2923 | <0.001 |
| Carbohydrates (% EI) | 53.8 ± 9.3 | 56.3 ± 8.4 | <0.001 |
| Protein (% EI) | 12.1 ± 3.0 | 11.5 ± 2.6 | 0.009 |
| Fat (% EI) | 28.9 ± 7.2 | 30.9 ± 7.0 | <0.001 |
| Fibre (g) | 19.9 ± 9.4 | 17.6 ± 8.5 | 0.001 |
| Lifestyle Factors | |||
| Number of steps (×1000) | 10.6 ± 4.7 | 9.2 ± 3.7 | <0.001 |
| Sitting time (hours) | 7.8 ± 1.9 | 7.1 ± 1.9 | <0.001 |
| % Smokers (n (%)) | 185 (44.8) | 21 (6.1) | <0.001 |
| % HIV Positive (n (%)) | 86 (20.9) | 66 (19.1) | 0.527 |
| % ARVs | 75 (92.8) | 53 (93.0) | 0.931 |
| Dietary energy reporting (n (%)) | |||
| Underreporting | 176 (42.5) | 244 (70.5) | <0.001 |
| Over reporting | 34 (8.2) | 7 (2.0) | |
| Plausible reporters | 204 (49.3) | 95 (27.5) | |
| Nutrients | Plant Driven Nutrient Pattern | Animal Protein and Fat Driven Nutrient Pattern | Vitamin C, Sugar and Potassium Driven Nutrient Pattern | Retinol and Vitamin B12 Driven Nutrient Pattern |
|---|---|---|---|---|
| Plant protein | 0.821 | 0.116 | 0.122 | −0.056 |
| Animal protein | 0.131 | 0.725 | 0.175 | 0.243 |
| Saturated fat | 0.315 | 0.661 | 0.206 | 0.077 |
| Monounsaturated fat | 0.296 | 0.712 | 0.156 | −0.017 |
| Polyunsaturated fat | 0.613 | 0.565 | 0.019 | −0.064 |
| Cholesterol | 0.095 | 0.769 | −0.020 | 0.463 |
| Starch | 0.799 | 0.092 | −0.167 | −0.042 |
| Sugar | 0.021 | −0.046 | 0.726 | 0.033 |
| Dietary Fibre | 0.632 | 0.063 | 0.477 | −0.047 |
| Calcium | 0.220 | 0.224 | 0.555 | 0.287 |
| Iron | 0.856 | 0.295 | 0.241 | 0.120 |
| Magnesium | 0.795 | 0.135 | 0.259 | 0.056 |
| Phosphorus | 0.739 | 0.301 | 0.147 | 0.142 |
| Potassium | 0.318 | 0.079 | 0.653 | 0.075 |
| Zinc | 0.852 | 0.350 | 0.173 | 0.073 |
| Retinol | 0.080 | 0.206 | 0.130 | 0.960 |
| Beta carotene | 0.008 | 0.058 | 0.279 | −0.017 |
| Thiamine | 0.901 | 0.287 | 0.221 | 0.012 |
| Riboflavin | 0.754 | 0.408 | 0.252 | 0.307 |
| Vitamin B6 | 0.674 | 0.082 | 0.033 | 0.014 |
| Folate | 0.748 | 0.025 | 0.067 | 0.402 |
| Vitamin B12 | 0.069 | 0.496 | 0.073 | 0.636 |
| Vitamin C | 0.094 | 0.181 | 0.888 | −0.019 |
| Vitamin D | 0.064 | 0.753 | −0.048 | 0.205 |
| Vitamin E | 0.256 | 0.610 | 0.030 | −0.036 |
| Explained variance % | 30.287 | 17.202 | 11.263 | 8.199 |
| Cumulative explained variance % | 30.287 | 47.490 | 58.753 | 66.952 |
| BMI | Body Fat % | Gynoid Fat % | Android Fat % | VAT (cm2) | SAT(cm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| Plant Driven Nutrient pattern | 0.39 (−0.02; 0.80) | 0.065 | 0.05(−0.37; 0.46) | 0.831 | −0.02(−0.1; 0.149) | 0.785 | 0.08(−0.03; 0.19) | 0.153 | 1.12 (−1.28; 3.53) | 0.360 | −1.10(−5.18; 2.99) | 0.598 |
| Animal protein and Fat Driven Nutrient pattern | 0.80 (0.40; 1.20) | <0.001 | 0.91(0.50; 1.32) | <0.001 | 0.08(−0.10; 0.25) | 0.382 | 0.21(0.10; 0.32) | <0.001 | 1.519 (−0.90; 3.94) | 0.218 | 2.37(−1.73; 6.47) | 0.257 |
| Vitamin C, sugar and potassium Driven Nutrient pattern | 0.99 (0.59; 1.39) | <0.001 | 0.74(0.32; 1.15) | <0.001 | −0.02(−0.19; 0.16) | 0.866 | 0.99 (−0.01; 0.21) | 0.081 | 0.79(−1.63; 3.21) | 0.522 | 0.83(−3.27; 4.93) | 0.692 |
| Retinol and Vitamin B12 Driven Nutrient pattern | 0.44 (−0.34; 0.43) | 0.819 | −0.09 (−0.48; 0.31) | 0.672 | −0.16(−0.32; 0.003) | 0.054 | 0.19(0.08; 0.30) | <0.001 | 4.15 (1.86; 6.44) | <0.001 | 3.82(−0.07; 7.70) | 0.054 |
| Dietary energy intake reporting | ||||||||||||
| Underreporting | 5.65 (4.75; 6.54) | <0.001 | 4.35(3.44; 5.27) | <0.001 | −0.87(−1.25; −0.50) | <0.001 | 0.76(0.51; 1.00) | <0.001 | −4.97 (−10.81; 7.46) | 0.095 | 1.62(−17.99; 21.22) | 0.872 |
| Over reporting | −2.89 (−4.67; −1.12) | <0.001 | −3.46(−5.28;−1.65) | <0.001 | 0.07(−0.68; 0.82) | 0.855 | −0.36(−0.85; 0.13) | 0.154 | −8.93 (−20.49; 2.64) | 0.130 | 3.83(−6.08; 13.73) | 0.449 |
| Plausible reporting (reference) | ||||||||||||
| Age | 0.02 (−0.05; 0.08) | 0.648 | 0.10(0.03; 0.17) | 0.005 | −0.01(−0.04; 0.01) | 0.318 | 0.03(0.01; 0.04) | 0.006 | 0.92 (0.52; 1.31) | 6.85 × 10−6 | 0.12(−0.55; 0.79) | 0.731 |
| Sex (Male; female reference) | −5.80 (−6.65; −4.94) | <0.001 | −16.97(−17.85;−16.09) | <0.001 | −1.07(−1.43;−0.70) | <0.001 | 1.35(1.12; 1.16) | <0.001 | −39.02(−45.47; −32.58) | <0.001 | −35.19(−46.12; −24.26) | <0.001 |
| Number of steps (×1000) | −0.25 (−0.35; −0.16) | <0.001 | −0.27(-0.37; −0.17) | <0.001 | 0.08(0.04; 0.12) | <0.001 | −0.06(−0.08; −0.03) | <0.001 | −0.70 (−1.30; −0.10) | 0.021 | −0.05(−1.07; 0.96) | 0.917 |
| Sitting time (h) | 0.28(0.07; 0.50) | 0.011 | 0.12(−0.11; 0.34) | 0.303 | 0.05(−0.04; 0.15) | 0.274 | −0.002(−0.06; 0.06) | 0.955 | −1.13 (−2.44; −0.19) | 0.092 | −1.89(−4.11; 0.34) | 0.098 |
| Education | ||||||||||||
| Primary | 0.88 (−0.41; 2.18) | 0.181 | 0.04(−1.30; 1.38) | 0.952 | −0.29(−0.85; 0.26) | 0.301 | 0.002(−0.36; 0.36) | 0.989 | 1.80 (−3.97; 7.58) | 0.541 | −7.18(−17.54; 9.05) | 0.151 |
| Secondary | −0.02 (−1.11; 1.06) | 0.967 | −0.07(−1.19; 1.06) | 0.904 | 0.01(−0.45; 0.48) | 0.962 | 0.06(−0.24; 0.37) | 0.677 | −0.38 (−8.22; 7.46) | 0.924 | −4.25(−16.97; 2.72) | 0.531 |
| Tertiary (reference) | ||||||||||||
| Body fat (kg) | 3.18 (2.89; 3.46) | <0.001 | 13.24(12.76; 13.72) | <0.001 | ||||||||
| Unadjusted R2 | 0.478 | 0.771 | 0.089 | 0.212 | 0.532 | 0.871 | ||||||
| Adjusted R2 | 0.469 | 0.767 | 0.074 | 0.199 | 0.501 | 0.863 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratshikombo, T.; Goedecke, J.H.; Soboyisi, M.; Kufe, C.; Makura-Kankwende, C.B.T.; Masemola, M.; Micklesfield, L.K.; Chikowore, T. Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women. Nutrients 2021, 13, 4558. https://doi.org/10.3390/nu13124558
Ratshikombo T, Goedecke JH, Soboyisi M, Kufe C, Makura-Kankwende CBT, Masemola M, Micklesfield LK, Chikowore T. Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women. Nutrients. 2021; 13(12):4558. https://doi.org/10.3390/nu13124558
Chicago/Turabian StyleRatshikombo, Tshifhiwa, Julia H. Goedecke, Melikhaya Soboyisi, Clement Kufe, Caroline B. T. Makura-Kankwende, Maphoko Masemola, Lisa K. Micklesfield, and Tinashe Chikowore. 2021. "Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women" Nutrients 13, no. 12: 4558. https://doi.org/10.3390/nu13124558
APA StyleRatshikombo, T., Goedecke, J. H., Soboyisi, M., Kufe, C., Makura-Kankwende, C. B. T., Masemola, M., Micklesfield, L. K., & Chikowore, T. (2021). Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women. Nutrients, 13(12), 4558. https://doi.org/10.3390/nu13124558






