Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial
Abstract
:1. Introduction
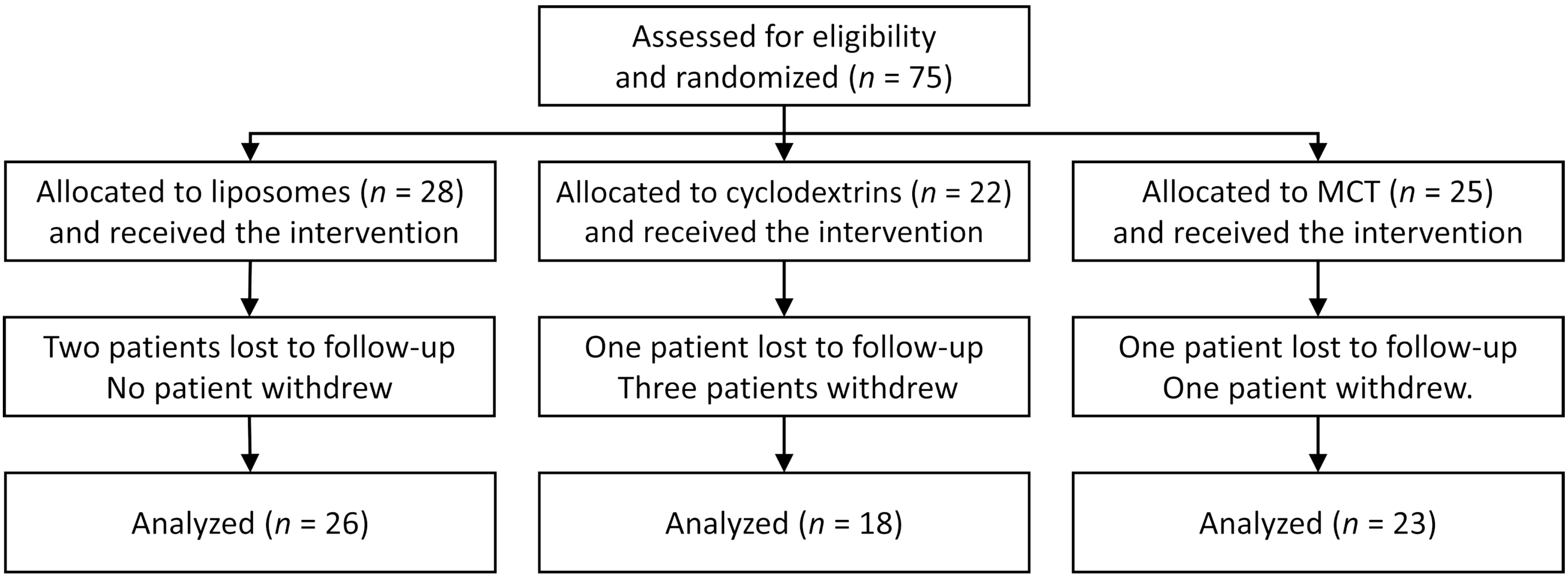
2. Materials and Methods
3. Results
4. Discussion
4.1. Current Challenges in Fat-Soluble Supplementation
4.2. New Formulations: Liposomes and Cyclodextrins
4.3. Outcomes: Vitamins A, D, E, and K
4.4. Outcomes Underscore the Need for Vitamin Dose Individualization
4.5. The Role of Vitamin D in CF
4.6. Generalizability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS Guidelines on Nutrition Care for Infants, Children, and Adults with Cystic Fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU) No 468/2012 of 1 June 2012 Amending Regulation (EU) No 28/2012 Laying Down Requirements for the Certification for Imports into and Transit through the Union of Certain Composite Products; Official Journal of the European Union: Luxembourg, 2012.
- Karl, J.P.; Fu, X.; Wang, X.; Zhao, Y.; Shen, J.; Zhang, C.; Wolfe, B.E.; Saltzman, E.; Zhao, L.; Booth, S.L. Fecal Menaquinone Profiles of Overweight Adults Are Associated with Gut Microbiota Composition during a Gut Microbiota-Targeted Dietary Intervention. Am. J. Clin. Nutr. 2015, 102, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Effect of Food Matrix on Circulating Vitamin K Concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-Year Low-Dose Menaquinone-7 Supplementation Helps Decrease Bone Loss in Healthy Postmenopausal Women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Rønn, S.H.; Harsløf, T.; Pedersen, S.B.; Langdahl, B.L. Vitamin K2 (Menaquinone-7) Prevents Age-Related Deterioration of Trabecular Bone Microarchitecture at the Tibia in Postmenopausal Women. Eur. J. Endocrinol. 2016, 175, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkowiak, J. Assessment of Maldigestion in Cystic Fibrosis. J. Pediatr. 2004, 145, 285–287. [Google Scholar] [CrossRef]
- Walkowiak, J.; Nousia-Arvanitakis, S.; Cade, A.; Kashirskaya, N.; Piotrowski, R.; Strzykala, K.; Kouniou, M.; Pogorzelski, A.; Sands, D.; Kapranov, N. Fecal Elastase-1 Cut-off Levels in the Assessment of Exocrine Pancreatic Function in Cystic Fibrosis. J. Cyst. Fibros. 2002, 1, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Debray, D.; Kelly, D.; Houwen, R.; Strandvik, B.; Colombo, C. Best Practice Guidance for the Diagnosis and Management of Cystic Fibrosis-Associated Liver Disease. J. Cyst. Fibros. 2011, 10, S29–S36. [Google Scholar] [CrossRef] [Green Version]
- Sapiejka, E.; Krzyżanowska, P.; Walkowiak, D.; Wenska-Chyży, E.; Szczepanik, M.; Cofta, S.; Pogorzelski, A.; Skorupa, W.; Walkowiak, J. Vitamin A Status and Its Determinants in Patients with Cystic Fibrosis. Acta Sci. Pol. Technol. Aliment. 2017, 16, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D Deficiency and Its Treatment in Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, S66–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapiejka, E.; Krzyżanowska-Jankowska, P.; Wenska-Chyży, E.; Szczepanik, M.; Walkowiak, D.; Cofta, S.; Pogorzelski, A.; Skorupa, W.; Walkowiak, J. Vitamin E Status and Its Determinants in Patients with Cystic Fibrosis. Adv. Med. Sci. 2018, 63, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, P.; Pogorzelski, A.; Skorupa, W.; Moczko, J.; Grebowiec, P.; Walkowiak, J. Exogenous and Endogenous Determinants of Vitamin K Status in Cystic Fibrosis. Sci. Rep. 2015, 5, 12000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.A.; Rolles, C.J. Vitamin Therapy in Cystic Fibrosis--a Review and Rationale. J. Clin. Pharm. Ther. 1993, 18, 33–38. [Google Scholar] [CrossRef]
- Papas, K.; Kalbfleisch, J.; Mohon, R. Bioavailability of a Novel, Water-Soluble Vitamin E Formulation in Malabsorbing Patients. Dig. Dis. Sci. 2007, 52, 347–352. [Google Scholar] [CrossRef]
- Krzyżanowska, P.; Drzymała-Czyż, S.; Pogorzelski, A.; Duś-Żuchowska, M.; Skorupa, W.; Bober, L.; Sapiejka, E.; Oralewska, B.; Rohovyk, N.; Moczko, J.; et al. Vitamin K Status in Cystic Fibrosis Patients with Liver Cirrhosis. Dig. Liver Dis. 2017, 49, 672–675. [Google Scholar] [CrossRef]
- Krzyżanowska, P.; Drzymala-Czyż, S.; Rohovyk, N.; Bober, L.; Moczkco, J.; Rachel, M.; Walkowiak, J. Prevalence of Vitamin K Deficiency and Associated Factors in Non-Supplemented Cystic Fibrosis Patients. Arch. Argent. Pediatr. 2018, 116, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska-Jankowska, P.; Sobkowiak, P.; Thalmann, O.; Drzymała-Czyż, S.; Glapa, A.; Hołysz, M.; Walkowiak, D.; Rohovyk, N.; Bober, L.; Pogorzelski, A.; et al. Apolipoprotein E Polymorphism Determines Vitamin K Supplementation Effectiveness in Cystic Fibrosis Patients. J. Cyst. Fibros. 2018, 17, e39–e40. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska-Jankowska, P.; Walkowiak, D.; Drzymała-Czyż, S.; Rohovyk, N.; Bober, L.; Walkowiak, J. Apolipoprotein E Polymorphism and Vitamin K Status in Cystic Fibrosis Patients Not Supplemented with Vitamin K. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7077–7082. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of Cyclodextrins in Food Science. A Review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Lemma, S.M.; Scampicchio, M.; Mahon, P.J.; Sbarski, I.; Wang, J.; Kingshott, P. Controlled Release of Retinyl Acetate from β-Cyclodextrin Functionalized Poly(Vinyl Alcohol) Electrospun Nanofibers. J. Agric. Food Chem. 2015, 63, 3481–3488. [Google Scholar] [CrossRef]
- Lee, S.-C.; Yuk, H.-G.; Lee, D.-H.; Lee, K.-E.; Hwang, Y.-I.; Ludescher, R.D. Stabilization of Retinol through Incorporation into Liposomes. J. Biochem. Mol. Biol. 2002, 35, 358–363. [Google Scholar]
- Szejtli, J.; Bolla-Pusztai, E.; Tardy-Lengyel, M.; Szabó, P.; Ferenczy, T. Preparation, Properties and Biological Activity of Beta-Cyclodextrin Inclusion Complex of Menadione. Pharmazie 1983, 38, 189–193. [Google Scholar]
- Horiuchi, Y.; Kikuchi, M.; Hirayama, F.; Uekama, K.; Ueno, M.; Ijitsu, T. Improvement of bioavailability of menaquinone-4 by dimethyl-beta-cyclodextrin complexation following oral administration]. Yakugaku Zasshi 1988, 108, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovoli, M.; Pappas, I.; Lalas, S.; Gortzi, O.; Kontopidis, G. In Vitro and in Vivo Assessment of Vitamin A Encapsulation in a Liposome-Protein Delivery System. J. Liposome Res. 2019, 29, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Turanek Knotigova, P.; Masek, J.; Prochazka, L.; Lukac, R.; Miller, A.D.; Neuzil, J.; Turanek, J. Liposomal Delivery Systems for Anti-Cancer Analogues of Vitamin E. J. Control. Release 2015, 207, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hatziparasides, G.; Loukou, I.; Moustaki, M.; Douros, K. Vitamin K and Cystic Fibrosis: A Gordian Knot That Deserves Our Attention. Respir. Med. 2019, 155, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Regalado Lam Chew Tun, R.; Porhownik, N.; Taback, S.; Oleschuk, C. Effect of High Dose Vitamin D3 Therapy on Serum Vitamin D3 Levels in Vitamin D Insufficient Adults with Cystic Fibrosis. Clin. Nutr. ESPEN 2018, 23, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]

| Vitamin | Liposomes | Cyclodextrins | MCT |
|---|---|---|---|
| A, retinyl palmitate | 2000 IU | 2000 IU | 2000 IU |
| Vitamin D3 | 4000 IU | 4000 IU | 4000 IU |
| E, RRR-α-tocopherol | - | 200 IU | 200 IU |
| K2, menaquinone-7 | 200 µg | 200 µg | 200 µg |
| Vitamins in standard form | |||
| A, β-carotene (4 mg = 6667 IU) | 4 mg | 4 mg | 4 mg |
| E, RRR-α-tocopherol | 200 IU | - | - |
| K1, phylloquinone (2.5 mg thrice per week) | 1.07 mg | 1.07 mg | 1.07 mg |
| Parameter | Liposomes (n = 28) | Cyclodextrins (n = 22) | MCT (n = 25) | p |
|---|---|---|---|---|
| Age, years | 23.1 (21.2–30.2) | 23.2 (18.7–28.8) | 20.4 (17.5–22.9) | 0.035 |
| Sex: female | 53.6% | 63.6% | 72.0% | 0.381 |
| Mass, kg | 58.5 (52.0–64.0) | 54 (48.5–60.0) | 57.0 (48.0–65.0) | 0.340 |
| Height, cm | 167 (161–175.5) | 165 (158–173) | 168 (160–173) | 0.551 |
| BMI, kg/m2 | 20.9 (19.5–22.4) | 20.4 (18.3–21.5) | 20.5 (18.4–21.7) | 0.494 |
| FEV1% | 58.6% | 66.5% | 70.2% | 0.256 |
| CF liver disease [10] | 42.9% | 36.4% | 56.0% | 0.381 |
| Nasal polyps | 25.0% | 27.3% | 40.0% | 0.457 |
| GERD | 25.0% | 27.3% | 36.0% | 0.659 |
| Diabetes | 21.4% | 9.1% | 24.0% | 0.379 |
| P. aeruginosa colonization | 78.6% | 72.7% | 48.0% | 0.048 |
| Vitamin | Liposomes (n = 28) | Cyclodextrins (n = 22) | MCT (n = 25) | p |
|---|---|---|---|---|
| Vitamin A, IU/d, incl. β-carotene | 5000 (0–7492) | 6662 (0–10,500) | 5000 (2000–10,000) | 0.30 |
| Vitamin D, IU/d | 3000 (2000–4100) | 3000 (2000–5000) | 3000 (2000–5000) | 0.92 |
| Vitamin E, mg/d | 270 (135–400) | 236 (135–300) | 236 (100–400) | 0.99 |
| Vitamin K1, mean daily mg/d | 1.43 (0.10–4.64) | 1.71 (0.20–4.14) | 1.63 (1.43–2.86) | 0.91 |
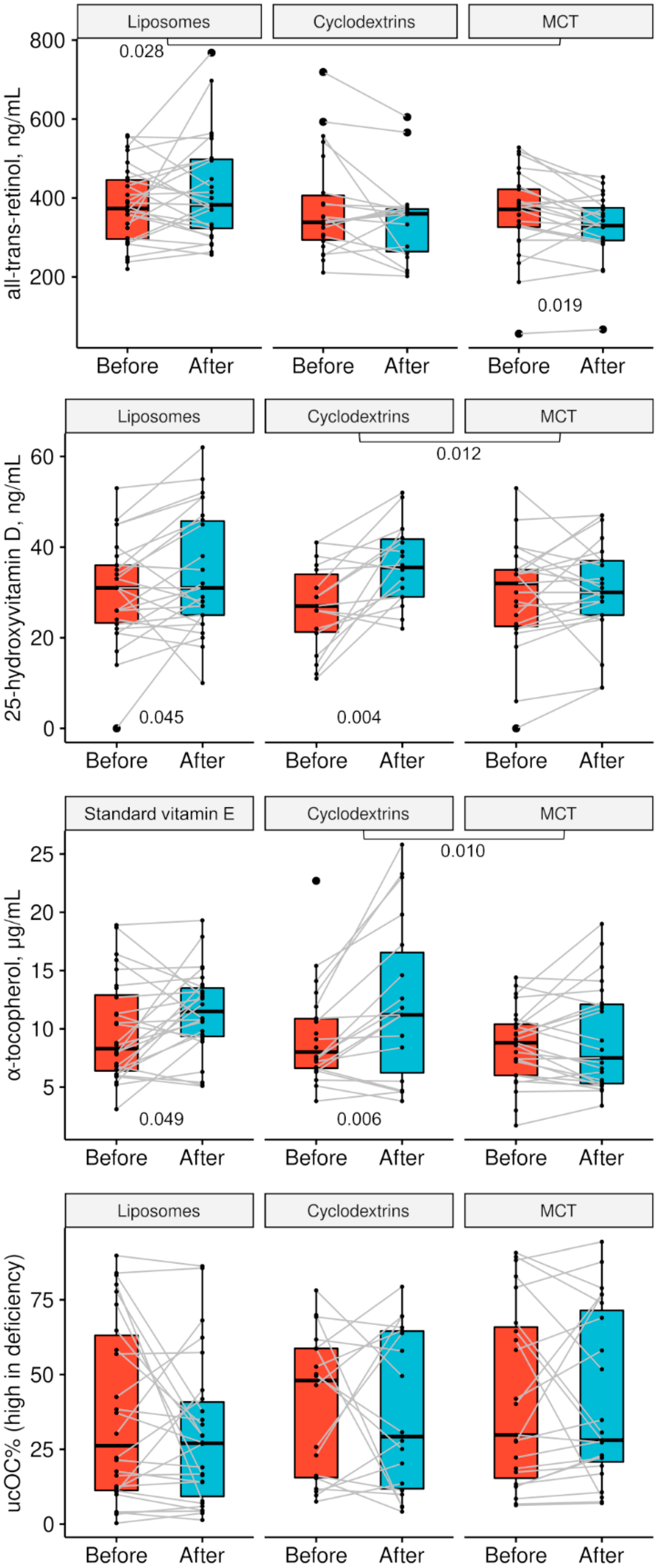
| Parameter | Liposomes (n = 26–28) | Cyclodextrins (n = 18–22) | MCT (n = 23–25) | pLIPvsMCT | pCYKvsMCT | pLIPvsCYK |
|---|---|---|---|---|---|---|
| Start: all-trans-retinol, ng/mL | 374 (294–448) 381 ± 98 | 338 (291–413) 376 ± 129 | 370 (325–422) 360 ± 107 | 0.281 | 0.509 | 0.869 |
| End: all-trans-retinol, ng/mL | 382 (322–499) 418 ± 131 | 360 (260–373) 346 ± 107 | 330 (292–379) 327 ± 83 | 0.005 | 0.541 | 0.052 |
| Start: 25-OHD3, ng/mL | 31.0 (23.0–36.0) 30.2 ± 11.3 | 27.0 (21.0–35.0) 26.7 ± 9.4 | 32.0 (22.0–35.0) 29.4 ± 11.8 | 0.802 | 0.428 | 0.274 |
| End: 25-OHD3, ng/mL | 31.0 (25.0–46.0) 34.1 ± 13.2 | 35.5 (29.0–42.0) 35.9 ± 8.7 | 30.0 (25.0–37.0) 30.5 ± 10.6 | 0.296 | 0.083 | 0.594 |
| Start: α-tocopherol, µg/mL | 8.32 (6.28–13.11) (Tokovit) 9.80 ± 4.33 | 7.99 (6.55–10.86) 9.39 ± 4.22 | 8.81 (6.03–10.44) 8.57 ± 3.24 | 0.329 | 0.937 | 0.330 |
| End: α-tocopherol, µg/mL | 11.52 (9.32–13.58) (Tokovit) 11.44 ± 3.63 | 11.22 (5.47–17.16) 12.4 ± 7.0 | 7.47 (5.24–12.21) 9.14 ± 4.48 | 0.057 | 0.099 | 0.611 |
| Start: %ucOC | 26.2 (11.1–64.7) 37.0 ± 30.0 | 48.0 (15.3–58.8) 39.7 ± 24.3 | 29.8 (13.3–67.3) 42.1 ± 30.1 | 0.559 | 0.785 | 0.740 |
| End: %ucOC | 27.0 (7.7–41.8) 30.0 ± 24.8 | 29.3 (11.2–64.8) 37.5 ± 26.9 | 28.1 (19.5–73.9) 41.4 ± 28.8 | 0.146 | 0.650 | 0.357 |
| Parameter | Liposomes | pLIPvsMCT | Cyclodextrins | pCYKvsMCT | MCT | pLIPvsCYK |
|---|---|---|---|---|---|---|
| Δ all-trans-retinol, ng/mL | 1.7 (−44.3–86.1) 26.2 ± 114.4 | 0.028 | −22.5 (−81.2–20.6) −39.7 ± 96.3 | 0.787 | −38.8 (−71.2–6.8) −32.6 ± 61.4 | 0.045 |
| Δ 25-OHD3, ng/mL | 2.0 (−1.0–1.0) 3.7 ± 10.0 | 0.330 | 9.0 (1.0–17.0) 9.1 ± 10.2 | 0.012 | 3.0 (−4.0–7.0) 1.1 ± 8.8 | 0.095 |
| Δ α-tocopherol, µg/mL | 0.92 (−0.73–4.73) 1.68 ± 3.94 | 0.206 | 4.34 (0.33–6.52) 3.75 ± 4.44 | 0.010 | −0.34 (−1.71–2.15) 0.44 ± 2.73 | 0.121 |
| Δ %ucOC (increased in deficiency) | −2.5 (−9.9–2.6) −7.0 ± 26.3 | 0.367 | −2.7 (−18.8–18.3) −2.2 ± 28.7 | 0.842 | 3.5 (−11.8–9.8) −0.6 ± 22.7 | 0.583 |
| Vitamin | Liposomes | Cyclodextrins | MCT |
|---|---|---|---|
| Vitamin A insufficiency | 23.1% → 15.4% | 22.2% → 33.3% | 26.1% → 34.8% |
| Vitamin D insufficiency | 12.0% → 8.0% | 22.0% → 0% | 13.0% → 13.0% |
| Vitamin E insufficiency | 3.8% → 0% | 5.6% → 22.2% | 8.7% → 13.0% |
| Vitamin K insufficiency | 57.7% → 53.8% | 66.7% →66.7% | 65.2% → 73.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, J.K.; Sobkowiak, P.; Drzymała-Czyż, S.; Krzyżanowska-Jankowska, P.; Sapiejka, E.; Skorupa, W.; Pogorzelski, A.; Nowicka, A.; Wojsyk-Banaszak, I.; Kurek, S.; et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients 2021, 13, 4554. https://doi.org/10.3390/nu13124554
Nowak JK, Sobkowiak P, Drzymała-Czyż S, Krzyżanowska-Jankowska P, Sapiejka E, Skorupa W, Pogorzelski A, Nowicka A, Wojsyk-Banaszak I, Kurek S, et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients. 2021; 13(12):4554. https://doi.org/10.3390/nu13124554
Chicago/Turabian StyleNowak, Jan K., Paulina Sobkowiak, Sławomira Drzymała-Czyż, Patrycja Krzyżanowska-Jankowska, Ewa Sapiejka, Wojciech Skorupa, Andrzej Pogorzelski, Agata Nowicka, Irena Wojsyk-Banaszak, Szymon Kurek, and et al. 2021. "Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial" Nutrients 13, no. 12: 4554. https://doi.org/10.3390/nu13124554
APA StyleNowak, J. K., Sobkowiak, P., Drzymała-Czyż, S., Krzyżanowska-Jankowska, P., Sapiejka, E., Skorupa, W., Pogorzelski, A., Nowicka, A., Wojsyk-Banaszak, I., Kurek, S., Zielińska-Psuja, B., Lisowska, A., & Walkowiak, J. (2021). Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients, 13(12), 4554. https://doi.org/10.3390/nu13124554






