Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design and Procedures
2.3. Quantitative PCR (qPCR) Preparation and Analysis
2.4. Statistical Analysis
3. Results
3.1. Dietary Intake, Body Composition and Training Data
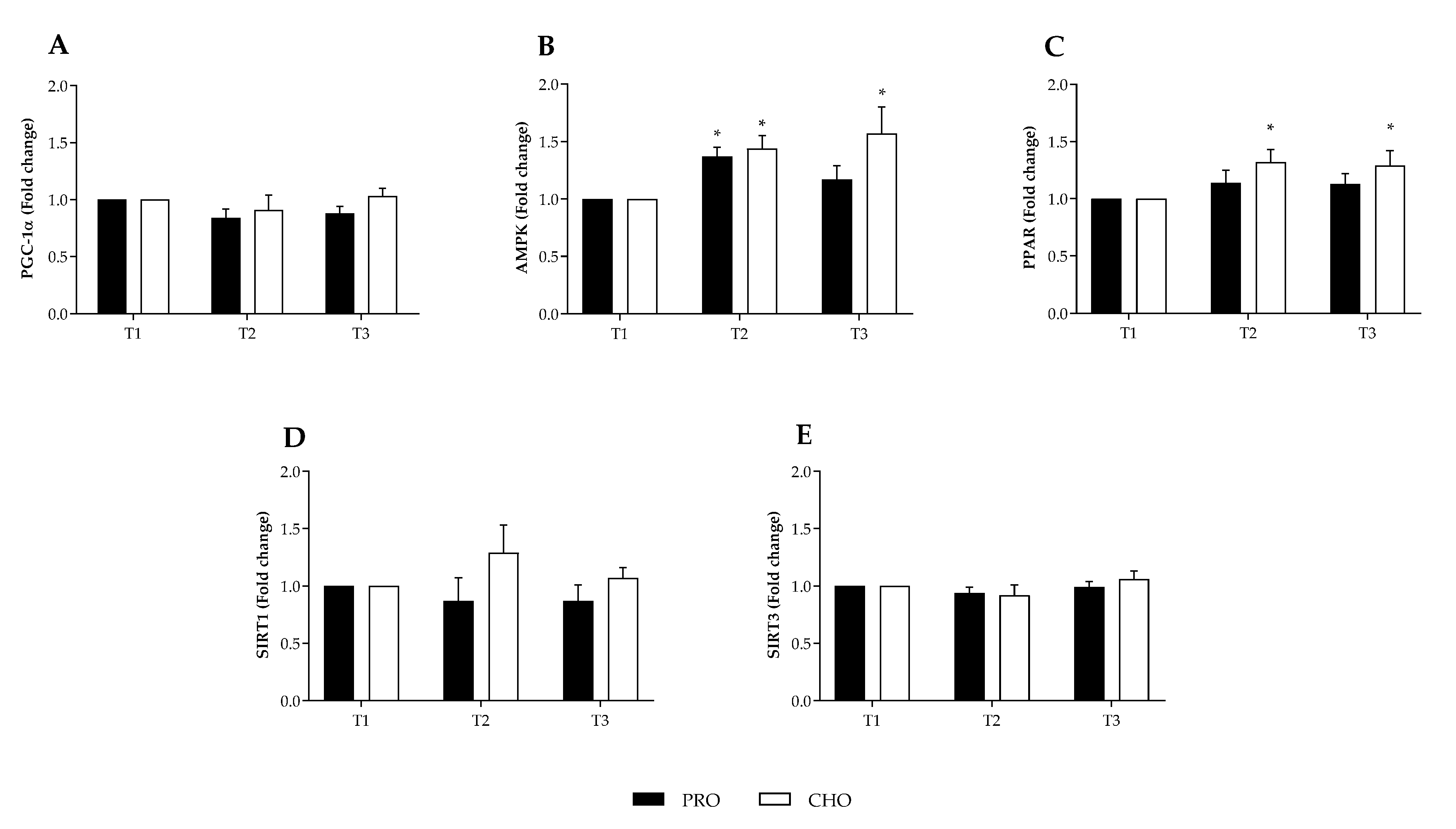
3.2. Transcription Biomarkers
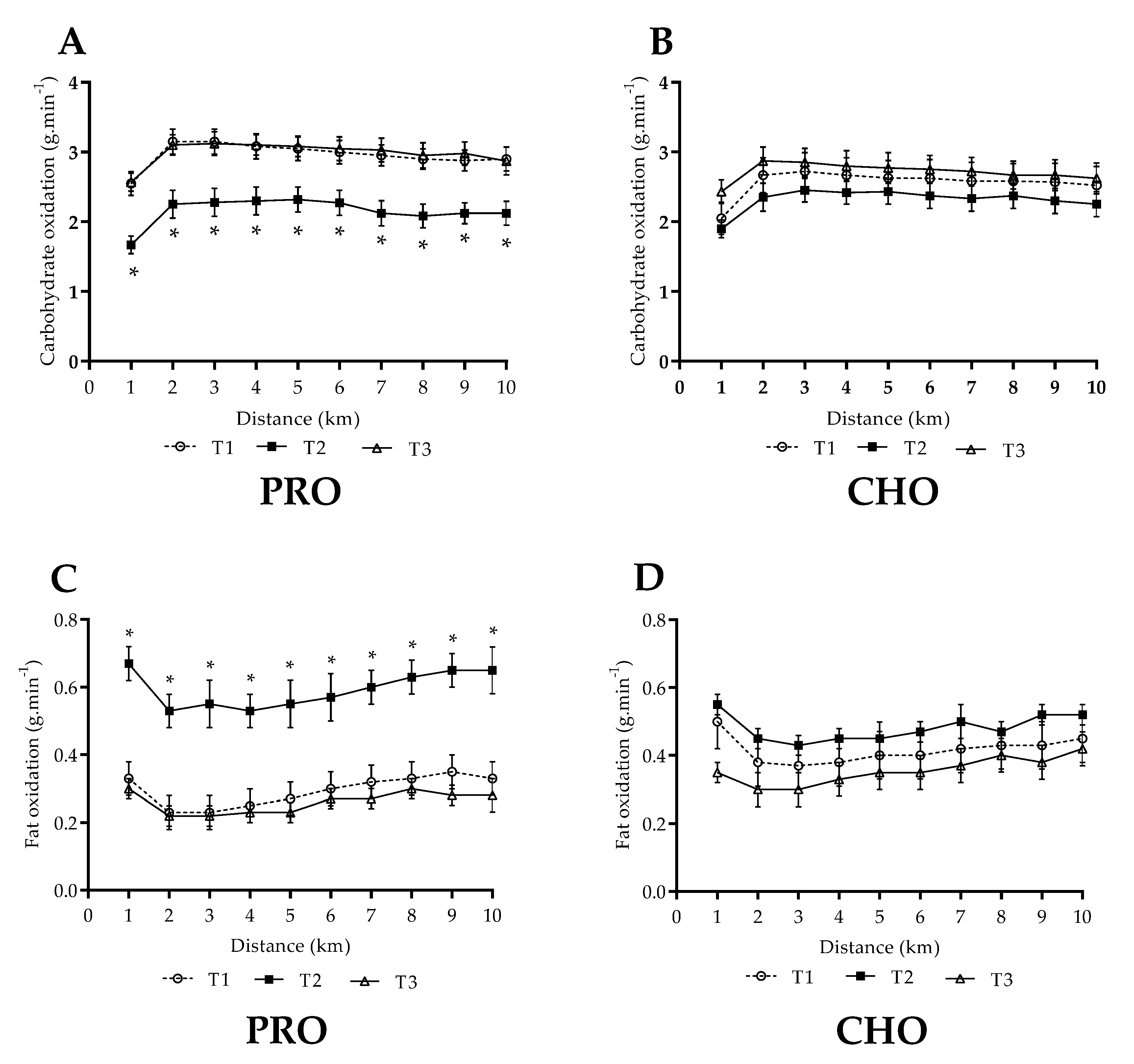
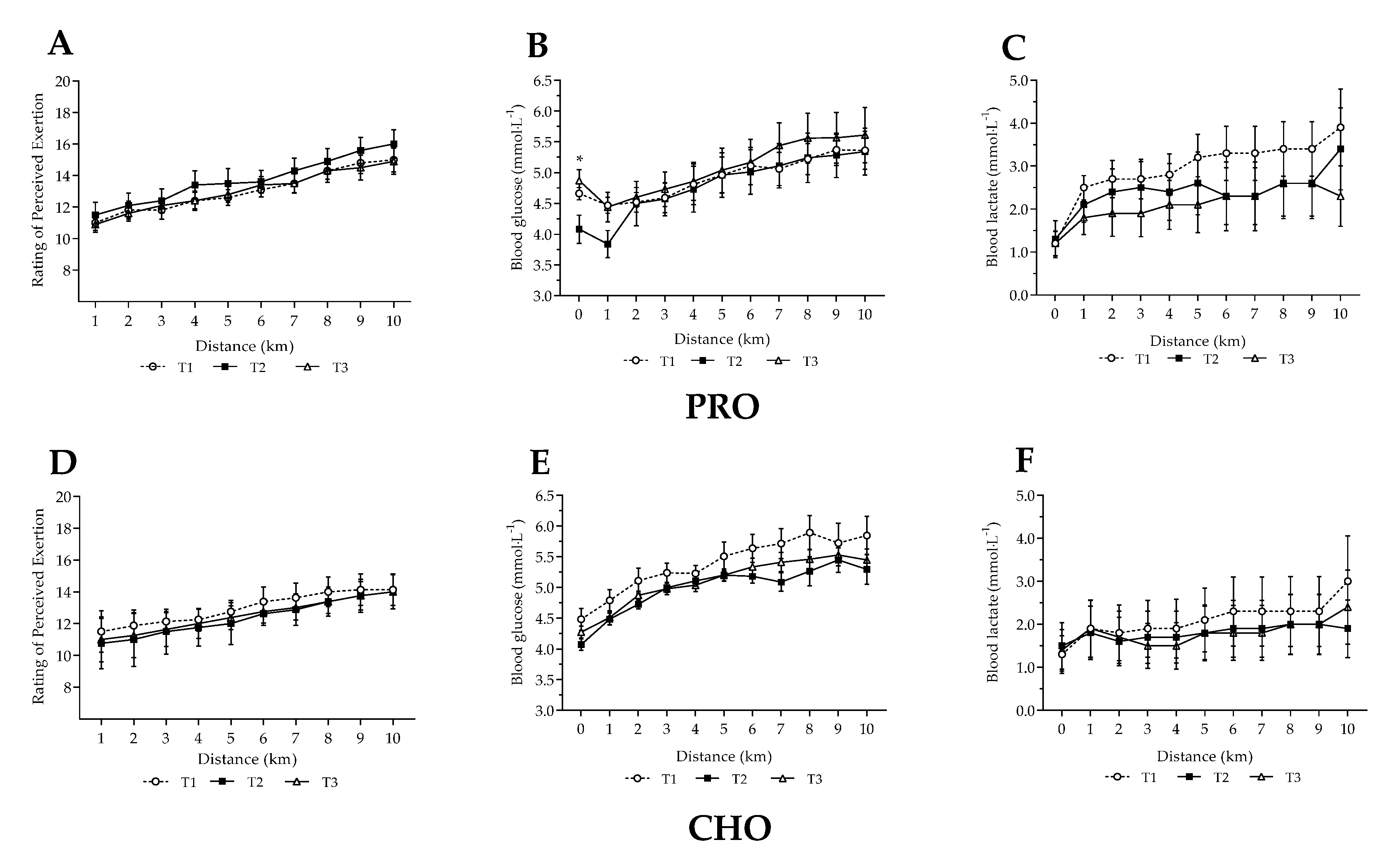
3.3. Steady State Fuel Utilisation and Related Measures
3.4. MaxSE Time Trial Results
4. Discussion
4.1. Applications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myburgh, K.H. What makes an endurance athlete world-class? Not simply a physiological conundrum. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 171–190. [Google Scholar] [CrossRef]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, J.X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Vega, R.B.; Huss, J.M.; Kelly, D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000, 20, 1868–1876. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Suwa, M.; Nakano, H.; Radak, Z.; Kumagai, S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coactivator-1α protein expressions in rat skeletal muscle. Metabolism 2008, 57, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draznin, B.; Wang, C.; Adochio, R.; Leitner, J.W.; Cornier, M.A. Effect of dietary macronutrient composition on AMPK and SIRT1 expression and activity in human skeletal muscle. Horm. Metab. Res. 2012, 44, 650–655. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M.; Kelly, D.P.; Holloszy, J.O. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef]
- Burke, L.M. Re-examining high-fat diets for sports performance: Did we call the ‘nail in the coffin’ too soon? Sports Med. 2015, 45, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.C.; Bryce, G.R.; Conlee, R.K. Adaptations to a high-fat diet that increase exercise endurance in male rats. J. Appl. Physiol. 1984, 56, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Christie, C.; Wilson, G.; Dennis, S.C.; Noakes, T.D.; Hopkins, W.G.; Lambert, E.V. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999, 48, 1509–1517. [Google Scholar] [CrossRef]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Elmer-English, R.; Dennis, S.C.; Schloss, I.; Noakes, T.D.; Lambert, E.V. Effects of medium-chain triacylglycerol ingested with carbohydrate on metabolism and exercise performance. Int. J. Sport Nutr. 1999, 9, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise. Med. Sci. Sports Exerc. 2002, 34, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Furber, M.; Anton-Solanas, A.; Koppe, E.; Ashby, C.; Roberts, M.; Roberts, J. A 7-day high protein hypocaloric diet promotes cellular metabolic adaptations and attenuates lean mass loss in healthy males. Clin. Nutr. Exp. 2017, 14, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Winter, E.M.; Jones, A.M.; Davison, R.C.R.; Bromley, P.D.; Mercer, T.H. (Eds.) The British Association of Sport and Exercise Sciences: Sport and Exercise Physiology Testing Guidelines (Volume I: Sport Testing); Taylor and Francis: Abingdon, Oxfordshire, UK, 2006. [Google Scholar]
- Magkos, F.; Yannakoulia, M. Methodology of dietary assessment in athletes: Concepts and pitfalls. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Borg, G. Ratings of Perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2004, 26, S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wu, J.; Chang, J.S.; Zhang, P.; Wang, J.; Zhang, Y.; Gettys, T.W.; Zhang, Y. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1αmRNA in mouse skeletal muscle. BioMed Res. Int. 2014, 2014, 402175. [Google Scholar] [CrossRef] [Green Version]
- Mata, F.; Valenzuela, P.L.; Gimenez, J.; Tur, C.; Ferreria, D.; Domínguez, R.; Sanchez-Oliver, A.J.; Sanz, J.M.M. Carbohydrate availability and physical performance: Physiological overview and practical recommendations. Nutrients 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of muscle glycogen metabolism during exercise: Implications for endurance performance and training adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentjens, R.L.; Moseley, L.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and fructose during exercise. J. Appl. Physiol. 2004, 96, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Tarpey, M.D.; Kass, L.S.; Tarpey, R.J.; Roberts, M.G. Assessing a commercially available sports drink on exogenous carbohydrate oxidation, fluid delivery and sustained exercise performance. J. Int. Soc. Sports Nutr. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edinburgh, R.M.; Koumanov, F.; Gonzalez, J.T. Impact of pre-exercise feeding status on metabolic adaptations to endurance-type exercise training. J. Physiol. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Angus, D.J.; Cox, G.R.; Clark, S.A.; Cummings, N.K.; Desbrow, B.; Hargreaves, M. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med. Sci. Sports Exerc. 2002, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Suzuki, K. Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention: A narrative review. Sports 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.K.; Mukai, K.; Lally, J.S.; Maher, A.C.; Gurd, B.J.; Heigenhauser, G.J.F.; Spriet, L.L.; Holloway, G.P. AMP-activated protein kinase is required for exercise-induced peroxisome proliferator-activated receptor γ co-activator 1α translocation to subsarcolemmal mitochondria in skeletal muscle. J. Physiol. 2013, 591, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Norrbom, J.; Sundberg, C.J.; Ameln, H.; Kraus, W.E.; Jansson, E.; Gustafsson, T. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J. Appl. Physiol. 2004, 96, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitsiladis, Y.P.; Maughan, R.J. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. J. Physiol. 1999, 517, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Psilander, N.; Frank, P.; Flockhart, M.; Sahlin, K. Exercise with low glycogen increases PGC-1α gene expression in human skeletal muscle. Eur. J. Appl. Physiol. 2013, 113, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]

| Group | Age (years) | Height (cm) | Body Mass (kg) | O2max (L·Min−1) | Relative O2max (mL·kg−1·Min−1) |
|---|---|---|---|---|---|
| PRO (n = 8) | 25 ± 4 | 179.4 ± 6.4 | 69.5 ± 3.3 | 4.38 ± 0.35 | 63.1 ± 4.8 |
| CHO (n = 8) | 27 ± 5 | 181.6 ± 3.5 | 67.6 ± 6.1 | 4.39 ± 0.28 | 65.3 ± 6.4 |
| Group | Energy Intake | Carbohydrate | Protein | Fat | ||||
|---|---|---|---|---|---|---|---|---|
| kcal·d−1 | kcal·kg−1·d−1 | g·d−1 | g·kg−1·d−1 | g·d−1 | g·kg−1·d−1 | g·d−1 | g·kg−1·d−1 | |
| PRO | 3185 ± 84 | 48 ± 1.2 | 239 ± 6.3 | 3.4 ± 0.9 | 319 ± 8.4 | 4.6 ± 0.1 | 106 ± 2.8 | 1.5 ± 0.04 |
| CHO | 3281 ± 69 | 49 ± 1.0 | 492 ± 10.4 * | 7.3 ± 0.1 * | 82 ± 1.7 * | 1.2 ± 0.03 * | 109 ± 2.3 | 1.5 ± 0.03 |
| Gene Name | Gene Abbreviation | Assay on Demand Number |
|---|---|---|
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) | PPARGC1 | Hs01016719_m1 |
| AMP-activated protein kinase 1 (AMPK) | PRKAA1 | Hs01562315_m1 |
| Peroxisome proliferator-activated receptor delta (PPARδ) | PPARδ | Hs00987011_m1 |
| Silent information regulator-T1 (SIRT1) | SIRT1 | Hs01009005_m1 |
| Silent information regulator-T3 (SIRT3) | SIRT3 | Hs00953477_m1 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | GAPDH | Hs99999905_m1 |
| T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|
| Distance (km) | Average Pace (km·h−1) | Distance (km) | Average Pace (km·h−1) | Distance (km) | Average Pace (km·h−1) | |
| PRO | 51 ± 26 | 14.7 ± 1.7 | 51 ± 28 | 13.8 ± 1.5 * | 50 ± 28 | 14.2 ± 1.3 |
| CHO | 62 ± 20 | 13.9 ± 0.8 | 64 ± 24 | 14.1 ± 1.4 | 65 ± 22 | 13.9 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furber, M.; Pyle, S.; Roberts, M.; Roberts, J. Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners. Nutrients 2021, 13, 4391. https://doi.org/10.3390/nu13124391
Furber M, Pyle S, Roberts M, Roberts J. Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners. Nutrients. 2021; 13(12):4391. https://doi.org/10.3390/nu13124391
Chicago/Turabian StyleFurber, Matthew, Simone Pyle, Michael Roberts, and Justin Roberts. 2021. "Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners" Nutrients 13, no. 12: 4391. https://doi.org/10.3390/nu13124391
APA StyleFurber, M., Pyle, S., Roberts, M., & Roberts, J. (2021). Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners. Nutrients, 13(12), 4391. https://doi.org/10.3390/nu13124391







