Lipid Profiles from Dried Blood Spots Reveal Lipidomic Signatures of Newborns Undergoing Mild Therapeutic Hypothermia after Hypoxic-Ischemic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Sampling and Dried Blood Spots
2.3. Lipid Extraction
2.4. Statistical Analysis
3. Results
3.1. Patients and Samples
3.2. Significant Lipid Species Changes between the Single Sample from the Mild HIE Group and the Moderate–Severe HIE Group at S1, S2 and S3
3.3. Significant Lipid Species Changes over the Course of Therapeutic Hypothermia for Newborns with Moderate–Severe HIE
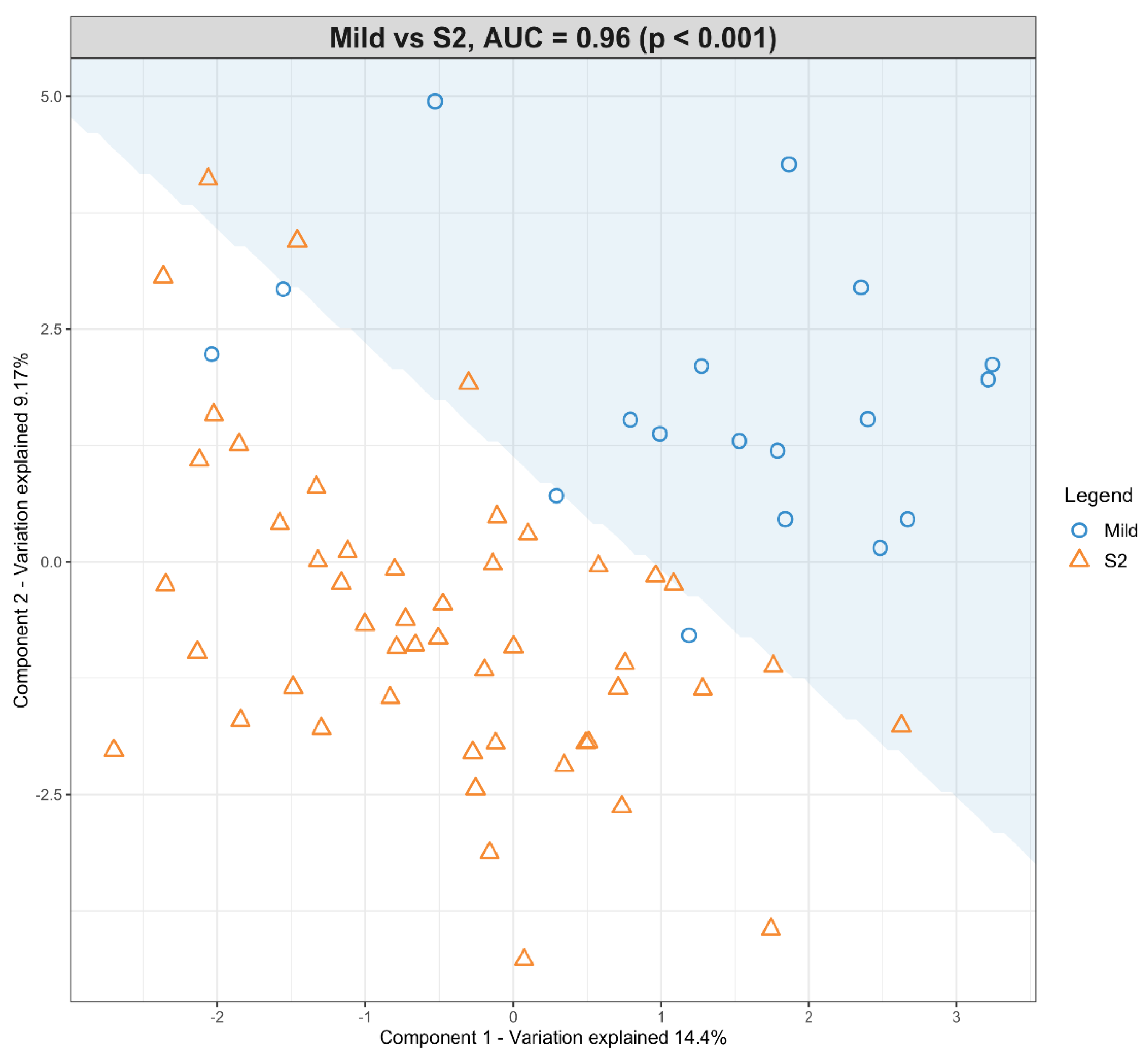
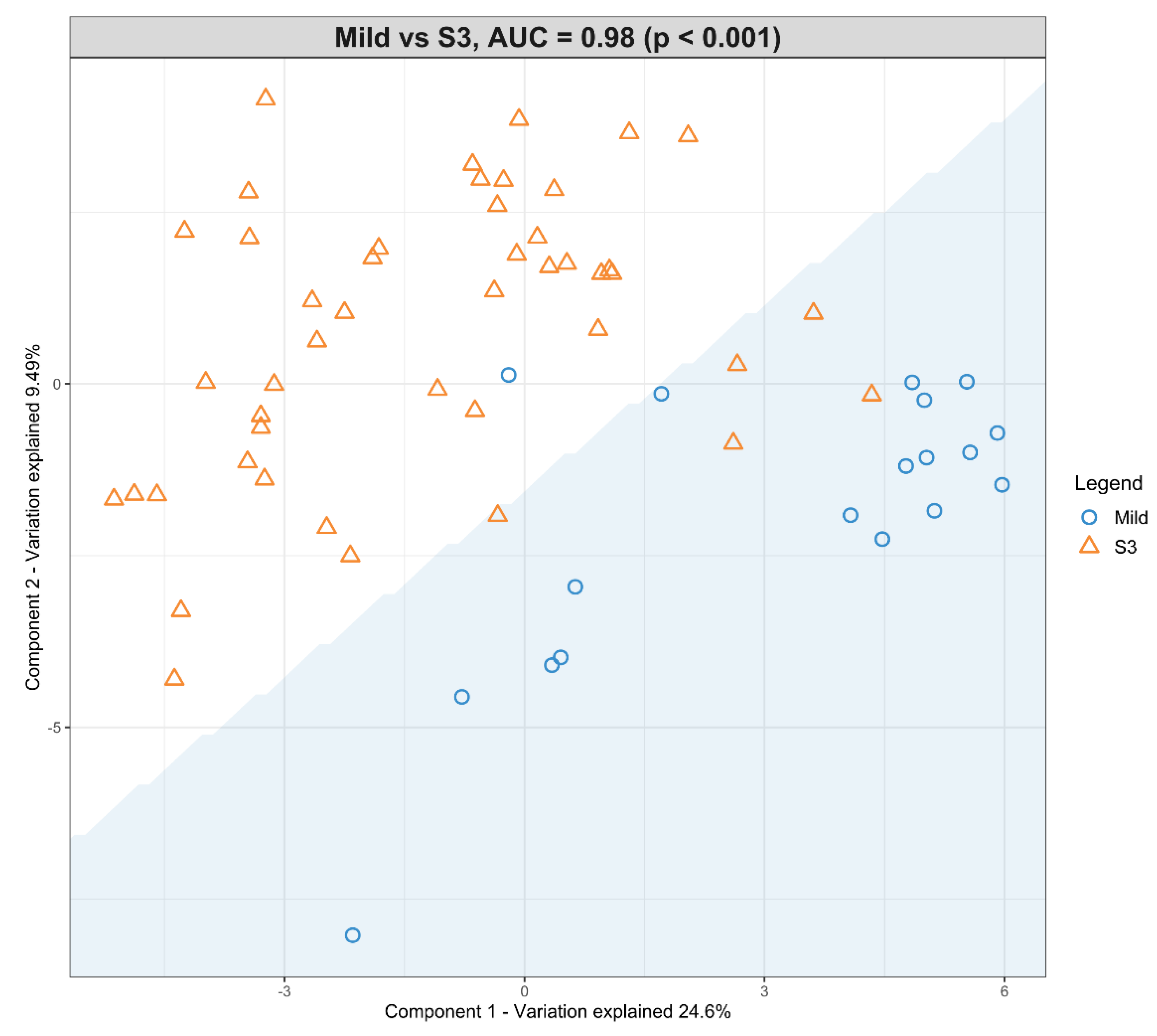
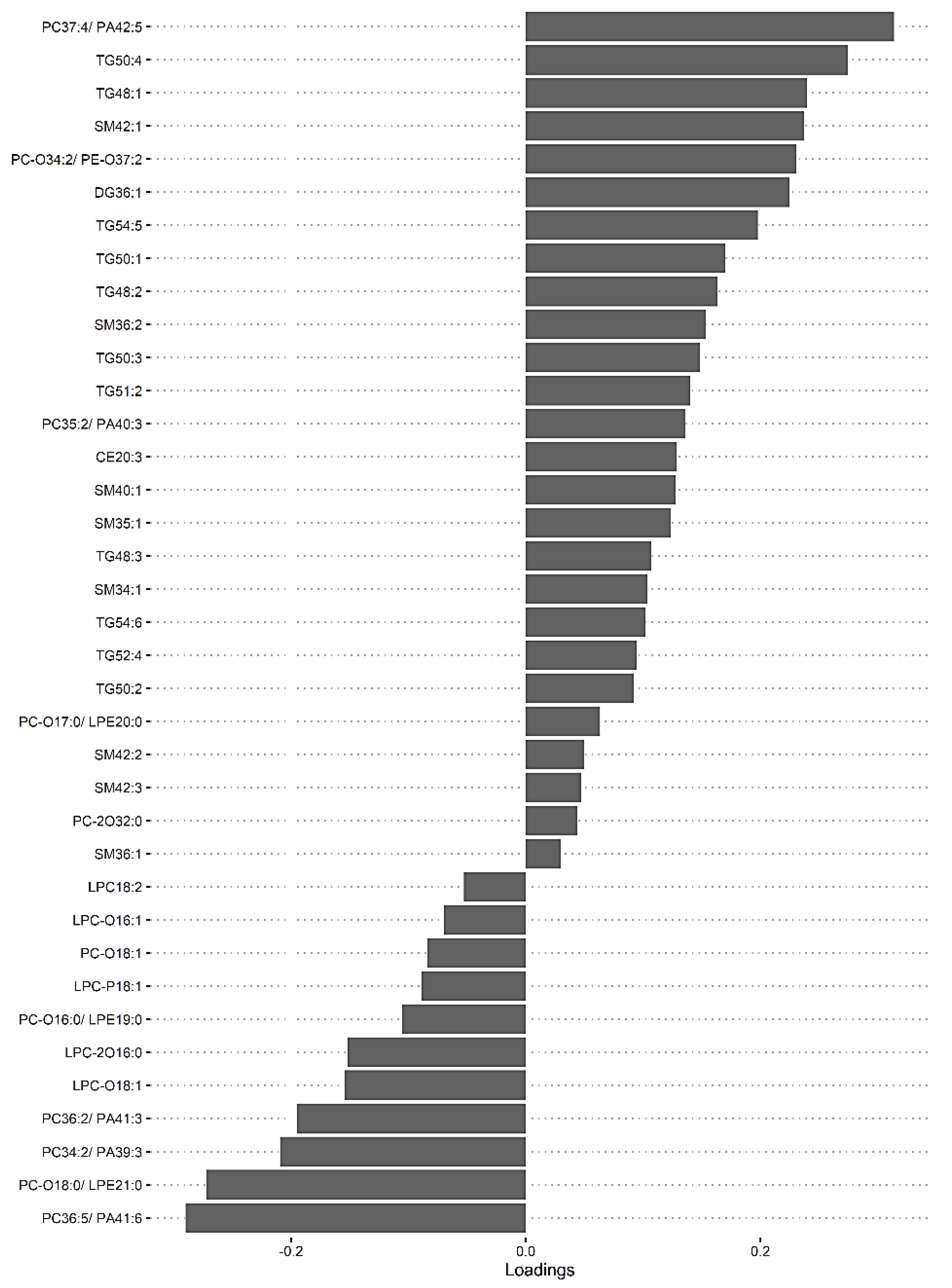
3.4. The Predictive Value of the Significant Changes in Lipid Species in Differentiating Mild HIE Cases from Moderate–Severe HIE Cases Using Sparse Partial Least Squares Discriminant Analysis (sPLS-DA)
3.5. Nutrition
4. Discussion
4.1. The Impact of Nutrition
4.2. The Lipid Classes in the Context of What Is Already Known
4.2.1. Triglyceride
4.2.2. Phosphatidylcholine
4.2.3. Lysophosphatidylcholine
4.2.4. Sphingomyelin
4.3. Strengths
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ASM | acid sphingomyelinase |
| BM | breastmilk |
| Cer | ceramide |
| DBS | dried blood spots |
| DG | diglyceride |
| DHA | docosahexaenoic acid |
| DIHRMS | direct-infusion, high-resolution mass spectrometry |
| FDR | false discovery rate |
| HI | hypoxia-ischemia |
| HIE | hypoxic-ischemic encephalopathy |
| LC-MS | liquid chromatography mass spectrometry |
| LPC | lysophosphatidylcholine |
| MCAO | middle cerebral artery occlusion |
| NBM | nil-by-mouth |
| PA | phosphatidic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PN | parenteral nutrition |
| PUFA | polyunsaturated fatty acids |
| SM | sphingomyelin |
| sPLS-DA | sparse partial least square discriminant analysis |
| TG | triglyceride |
| TH | therapeutic hypothermia |
| W | Wilcoxon signed rank test |
References
- Lawn, J.; Shibuya, K.; Stein, C. No cry at birth: Global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull. World Health Organ. 2005, 83, 409–417. [Google Scholar]
- E Jacobs, S.; Berg, M.; Hunt, R.; O Tarnow-Mordi, W.; E Inder, T.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Excellence NIoCaH. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury, Interventional procedures guidance [IPG347]. In NICE, 2nd ed.; NICE: Machester, UK, 2010. [Google Scholar]
- Papile, L.-A.; Baley, J.E.; Benitz, W.; Cummings, J.; Carlo, W.A.; Eichenwald, E.; Kumar, P.; Polin, R.A.; Tan, R.C.; Wang, K.S. Hypothermia and neonatal encephalopathy. Pediatrics 2014, 133, 1146–1150. [Google Scholar] [PubMed]
- Sastry, P. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Yahi, N. Brain Lipids in Synaptic Function and Neurological Disease; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Li, Q.; Bozek, K.; Xu, C.; Guo, Y.; Sun, J.; Pääbo, S.; Sherwood, C.C.; Hof, P.R.; Ely, J.J.; Li, Y.; et al. Changes in Lipidome Composition during Brain Development in Humans, Chimpanzees, and Macaque Monkeys. Mol. Biol. Evol. 2017, 34, 1155–1166. [Google Scholar] [CrossRef]
- Yu, Q.; He, Z.; Zubkov, D.; Huang, S.; Kurochkin, I.; Yang, X.; Halene, T.; Willmitzer, L.; Giavalisco, P.; Akbarian, S.; et al. Lipidome alterations in human prefrontal cortex during de-velopment, aging, and cognitive disorders. Mol. Psychiatry 2018, 25, 2952–2969. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Veyrat-Durebex, C.; Bocca, C.; Patin, F.; Vourc’h, P.; Kouassi Nzoughet, J.; Lenaers, G.; Andres, C.R.; Simard, G.; Corcia, P.; et al. Lipidomics Reveals Cerebrospi-nal-Fluid Signatures of ALS. Sci. Rep. 2017, 7, 17652. [Google Scholar] [CrossRef] [PubMed]
- Kloska, A.; Malinowska, M.; Gabig-Cimińska, M.; Jakóbkiewicz-Banecka, J. Lipids and Lipid Mediators Associated with the Risk and Pathology of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 3618. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Mulder, I.; Potočnik, N.O.; Broos, L.A.M.; Prop, A.; Wermer, M.J.H.; Heeren, R.M.A.; Maagdenberg, A.M.J.M.V.D. Distinguishing core from penumbra by lipid profiles using Mass Spectrometry Imaging in a transgenic mouse model of ischemic stroke. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Blaise, B.J.; Schwendimann, L.; Chhor, V.; Hodson, M.P.; Dallmann, G.; Degos, V.; Keller, M.; Gressens, P.; Fleiss, B. Persistently Altered Metabolic Phenotype following Perinatal Excitotoxic Brain Injury. Dev. Neurosci. 2017, 39, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR derived metabolomic profile of neona-tal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef]
- England, P.H. Newborn Blood Spot Screening Programme: Supporting Publications; HM Government: London, UK, 2012. [Google Scholar]
- Palmer, E.A.; Cooper, H.J.; Dunn, W.B. Investigation of the 12-Month Stability of Dried Blood and Urine Spots Applying Untar-geted UHPLC-MS Metabolomic Assays. Anal. Chem. 2019, 91, 14306–14313. [Google Scholar] [CrossRef]
- National Perinatal Epidemiology Unit. UK TOBY Cooling Register Protocol; National Perinatal Epidemiology Unit: Oxford, UK, 2006. [Google Scholar]
- Azzopardi, D.; Brocklehurst, P.; Edwards, D.; Halliday, H.; Levene, M.; Thoresen, M.; Whitelaw, A.; The TOBY Study Group. The TOBY Study. Whole body hypo-thermia for the treatment of perinatal asphyxial encephalopathy: A randomised controlled trial. BMC Pediatr. 2008, 8, 17. [Google Scholar] [CrossRef]
- Koulman, A.; Prentice, P.; Wong, M.C.; Matthews, L.; Bond, N.J.; Eiden, M.; Griffin, J.L.; Dunger, D.B. The development and validation of a fast and ro-bust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics 2014, 10, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.; Weber, R.J.M.; Broadhurst, D.; Viant, M.R. Direct infusion mass spectrometry metabolomics dataset: A benchmark for data processing and quality control. Sci. Data 2014, 1, 140012. [Google Scholar] [CrossRef] [PubMed]
- Geert van den Berg, R. Chi-Square Independence Test—What and Why? SPSS Tutorials: Amsterdam, The Netherlands, 2016. Available online: https://www.spss-tutorials.com/chi-square-independence-test/ (accessed on 25 November 2021).
- Tutorials, E.S. How to Do Fisher Exact Test in SPSS; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing, Version 3.6.1 ed.; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Laerd Statistics. Wilcoxon Signed-Rank Test Using SPSS Statistics: Lund Research Ltd.; 2018. Available online: https://statistics.laerd.com/spss-tutorials/wilcoxon-signed-rank-test-using-spss-statistics.php (accessed on 15 June 2020).
- Admin. Mann-Whitney U Test: Statology; 2018. Available online: https://www.statology.org/mann-whitney-u-test/ (accessed on 15 June 2020).
- Bradburn, S. The Bonferroni Correction Method Explained: Top Tip Bio. Available online: https://toptipbio.com/bonferroni-correction-method/ (accessed on 15 June 2020).
- mixOmics. PLS-DA 2017. Available online: http://mixomics.org/methods/pls-da/ (accessed on 15 June 2020).
- Lê Cao, K.A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical dis-plays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, S. Understanding AUC-ROC Curve: Towards Data Science; 2018. Available online: https://towardsdatascience.com/understanding-auc-roc-curve-68b2303cc9c5 (accessed on 15 June 2018).
- Rutherford, M.; Ramenghi, L.A.; Edwards, A.D.; Brocklehurst, P.; Halliday, H.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: A nested substudy of a randomised con-trolled trial. Lancet Neurol. 2010, 9, 39–45. [Google Scholar] [CrossRef]
- Tharmapoopathy, P.; Chisholm, P.; Barlas, A.; Varsami, M.; Gupta, N.; Ekitzidou, G.; Ponnusamy, V.; Kappelou, O.; Evanson, J.; Rosser, G.; et al. In clinical practice, cerebral MRI in newborns is highly predictive of neurodevelopmental outcome after therapeutic hypothermia. Eur. J. Paediatr. Neurol. 2020, 25, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Richardson, L.; Koulman, A. The validation of biomarkers of metabolic efficacy in infant nutrition. Nutr. Bull. 2018, 43, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Prentice, P.; Acerini, C.; Smith, J.; Hughes, I.A.; Ong, K.; Griffin, J.L.; Dunger, D.; Koulman, A. The translation of lipid profiles to nutritional bi-omarkers in the study of infant metabolism. Metabolomics 2017, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, H.; Ikeda, N.; Ohkawa, N.; Shoji, H.; Shimizu, T.; Naho, I. Influence of i.v. lipid emulsion on lipoprotein subclass in preterm infants. Pediatr. Int. 2018, 60, 839–843. [Google Scholar] [CrossRef]
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; de Alencar, E.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheldon, R.A.; Segal, M.R.; Kelly, M.J.; Pelton, J.G.; Ferriero, D.M.; James, T.L.; Litt, L. 1H nuclear magnetic resonance brain metabolomics in neonatal mice after hypoxia–ischemia distinguished normothermic recovery from mild hypothermia recoveries. Pediatr. Res. 2013, 74, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.H.; Broadhurst, D.I.; Mandal, R.; Wishart, D.S.; Boylan, G.B.; Kenny, L.C.; Murray, D.M. The Metabolomic Profile of Umbilical Cord Blood in Neonatal Hypoxic Ischaemic Encephalopathy. PLoS ONE 2012, 7, e50520. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lv, P.; Ai, W.; Li, L.; Shen, S.; Nie, H.; Shan, Y.; Bai, Y.; Huang, Y.; Liu, H. Lipidomic analysis of plasma in patients with lacunar infarction using nor-mal-phase/reversed-phase two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 3211–3222. [Google Scholar] [CrossRef]
- Koizumi, S.; Yamamoto, S.; Hayasaka, T.; Konishi, Y.; Yamaguchi-Okada, M.; Goto-Inoue, N.; Sugiura, Y.; Setou, M.; Namba, H. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience 2010, 168, 219–225. [Google Scholar] [CrossRef]
- Irie, M.; Fujimura, Y.; Yamato, M.; Miura, D.; Wariishi, H. Integrated MALDI-MS imaging and LC–MS techniques for visualizing spatiotemporal metabolomic dynamics in a rat stroke model. Metabolomics 2013, 10, 473–483. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, C.B.; Wu, H.W.; Kuo, J.S. Direct profiling of phospholipids and lysophospholipids in rat brain sections after is-chemic stroke. Rapid. Commun. Mass Spectrom. 2010, 24, 2057–2064. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Lambertsen, K.L.; Clausen, B.H.; Meyer, M.; Bhandari, D.R.; Larsen, S.T.; Poulsen, S.S.; Spengler, B.; Janfelt, C.; Hansen, H.S. Mass spectrometry imaging of bi-omarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci. Rep. 2016, 6, 39571. [Google Scholar] [CrossRef]
- Sheth, S.A.; Iavarone, A.T.; Liebeskind, D.S.; Won, S.J.; Swanson, R.A. Targeted Lipid Profiling Discovers Plasma Biomarkers of Acute Brain Injury. PLoS ONE 2015, 10, e0129735. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Salihovic, S.; Ganna, A.; Sundström, J.; Broeckling, C.D.; Magnusson, P.K.; Pedersen, N.L.; Siegbahn, A.; Prenni, J.; Fall, T.; et al. A Multi-Cohort Metabolomics Analysis Discloses Sphingomyelin (32:1) Levels to be Inversely Related to Incident Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104476. [Google Scholar] [CrossRef]
- Bayés García, R.; Quiles Guardia, P.; Neira Antonio, B.; Molina Font, J. Effect of perinatal hypoxia on blood triglycerides and total cholesterol including high density lipoproteins. An. Esp. Pediatr. 1988, 29, 15–22. [Google Scholar]
- Gibson, G.E.; Blass, J.P. Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J. Neurochem. 1976, 27, 37–42. [Google Scholar] [CrossRef]
- Hamilton, P.; Cady, E.; Wyatt, J.; Hope, P.; Delpy, D.; Reynolds, E. Impaired energy metabolism in brains of newborn infants with increased cerebral echodensities. Lancet 1986, 327, 1242–1246. [Google Scholar] [CrossRef]
- Hope, P.L.; Costello, A.M.; Cady, E.B.; Delpy, D.T.; Tofts, P.; Chu, A.; A Hamilton, P.; O Reynolds, E.; Wilkie, D.R. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 1984, 2, 2. [Google Scholar] [CrossRef]
- Pathak, D.; Berthet, A.; Nakamura, K. Energy failure: Does it contribute to neurodegeneration? Ann. Neurol. 2013, 74, 506–516. [Google Scholar] [CrossRef]
- Muniraman, H.; Gardner, D.; Skinner, J.; Paweletz, A.; Vayalakkad, A.; Chee, Y.H.; Clifford, C.; Sanka, S.; Venkatesh, V.; Curley, A.; et al. Biomarkers of hepatic injury and function in neonatal hypoxic ischemic encephalopathy and with therapeutic hypothermia. Eur. J. Pediatr. 2017, 176, 12951303. [Google Scholar] [CrossRef] [PubMed]
- Fattuoni, C.; Palmas, F.; Noto, A.; Fanos, V.; Barberini, L. Perinatal Asphyxia: A Review from a Metabolomics Perspective. Molecules 2015, 20, 7000–7016. [Google Scholar] [CrossRef] [PubMed]
- Lucke, A.M.; Shetty, A.N.; Hagan, J.L.; Walton, A.; Stafford, T.D.; Chu, Z.D.; Rhee, C.J.; Kaiser, J.R.; Cortes, M.S. Early proton magnetic resonance spectroscopy dur-ing and after therapeutic hypothermia in perinatal hypoxic-ischemic encephalopathy. Pediatr. Radiol. 2019, 49, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Drgová, A.; Likavcanová, K.; Dobrota, D. Changes of phospholipid composition and superoxide dismutase activity during global brain ischemia and reperfusion in rats. Gen. Physiol. Biophys. 2004, 23, 337–346. [Google Scholar]
- Luptakova, D.; Baciak, L.; Pluhacek, T.; Skriba, A.; Sediva, B.; Havlicek, V.; Juranek, I. Membrane depolarization and aberrant lipid dis-tributions in the neonatal rat brain following hypoxic-ischaemic insult. Sci. Rep. 2018, 8, 6952. [Google Scholar] [CrossRef]
- Nishida, A.; Emoto, K.; Shimizu, M.; Uozumi, T.; Yamawaki, S. Brain ischemia decreases phosphatidylcholine-phospholipase D but not phosphatidylinositol-phospholipase C in rats. Stroke 1994, 25, 1247–1251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassell, K.J.; Ezzati, M.; Alonso-Alconada, D.; Hausenloy, D.J.; Robertson, N.J. New horizons for newborn brain protection: En-hancing endogenous neuroprotection. Arch. Dis. Child Fetal Neonatal Ed. 2015, 100, F541–F552. [Google Scholar] [CrossRef]
- Adam, B.W.; Orsini, J.J.; Martin, M.; Hall, E.M.; Zobel, S.D.; Caggana, M.; Hannon, W.H. The preparation and storage of dried-blood spot qual-ity control materials for lysosomal storage disease screening tests. Clin. Biochem. 2011, 44, 704–710. [Google Scholar] [CrossRef]
- Jain, M.; Ngoy, S.; Sheth, S.A.; Swanson, R.; Rhee, E.P.; Liao, R.; Clish, C.; Mootha, V.K.; Nilsson, R. A systematic survey of lipids across mouse tissues. Am. J. Physiol. Metab. 2014, 306, E854–E868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood-Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Herr, D.R.; Farooqui, T.; Ling, E.-A.; A Farooqui, A. Role of sphingomyelinases in neurological disorders. Expert Opin. Ther. Targets 2015, 19, 1725–1742. [Google Scholar] [CrossRef]
- Adibhatla, R.H.; Dempsey, R.J. Lipid metabolism in ischemic neuronal death. Recent Res. Dev. Neuro-Chem. 1999, 2, 533–549. [Google Scholar]
- Jones, Z.B.; Ren, Y. Sphingolipids in spinal cord injury. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 52–69. [Google Scholar] [PubMed]
- Lipid MAPS. Recommendations and Tools: Classification, Nomenclature and Structure Drawing 2018. Available online: http://www.lipidmaps.org/data/classification/lipid_cns.html (accessed on 1 October 2018).
- Jia, Z.; Tie, C.; Wang, C.; Wu, C.; Zhang, J. Perturbed Lipidomic Profiles in Rats with Chronic Cerebral Ischemia Are Regulated by Xiao-Xu-Ming Decoction. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
| Perinatal Characteristics | Mild HIE | Moderate–Severe HIE | p Value |
|---|---|---|---|
| No TH | TH | ||
| n | 18 | 46 | |
| Male | 5 (28%) | 25 (54%) | 0.06 |
| Birth weight (g) | 3520 (3080, 3900) | 3450 (3105, 3860) | 0.81 |
| 10 min Apgar score | 9 (8, 10) | 6 (5, 7) | <0.001 * |
| Chest compressions | 2 (11%) | 9 (20%) | 0.48 |
| Inotropes used | 0 (0%) | 16 (35%) | 0.003 * |
| Worst pH in first hour | 6.95 (6.93, 7.00) | 6.92 (6.84, 7.00) | 0.24 |
| Worst base deficit in first hour | −13.2 (−14.0, −10.9) | −16.2 (−18.0, −14.9) | <0.001 * |
| Sentinel event | 6 (33%) | 11 (24%) | 0.54 |
| Meconium aspiration | 0 (0%) | 9 (20%) | 0.05 * |
| Seizures | 0 (0%) | 25 (54%) | <0.001 * |
| Unfavourable MRI | - | 8 (17%) | - |
| Moderate–Severe HIE at S1 vs. Mild | |||||
|---|---|---|---|---|---|
| Lipid Species | Moderate–Severe HIE Lipid Median | Mild HIE Lipid Median | Change in Relative Intensity as a Multiple (95% Confidence Interval) | p Value | Adjusted p Value (FDR) |
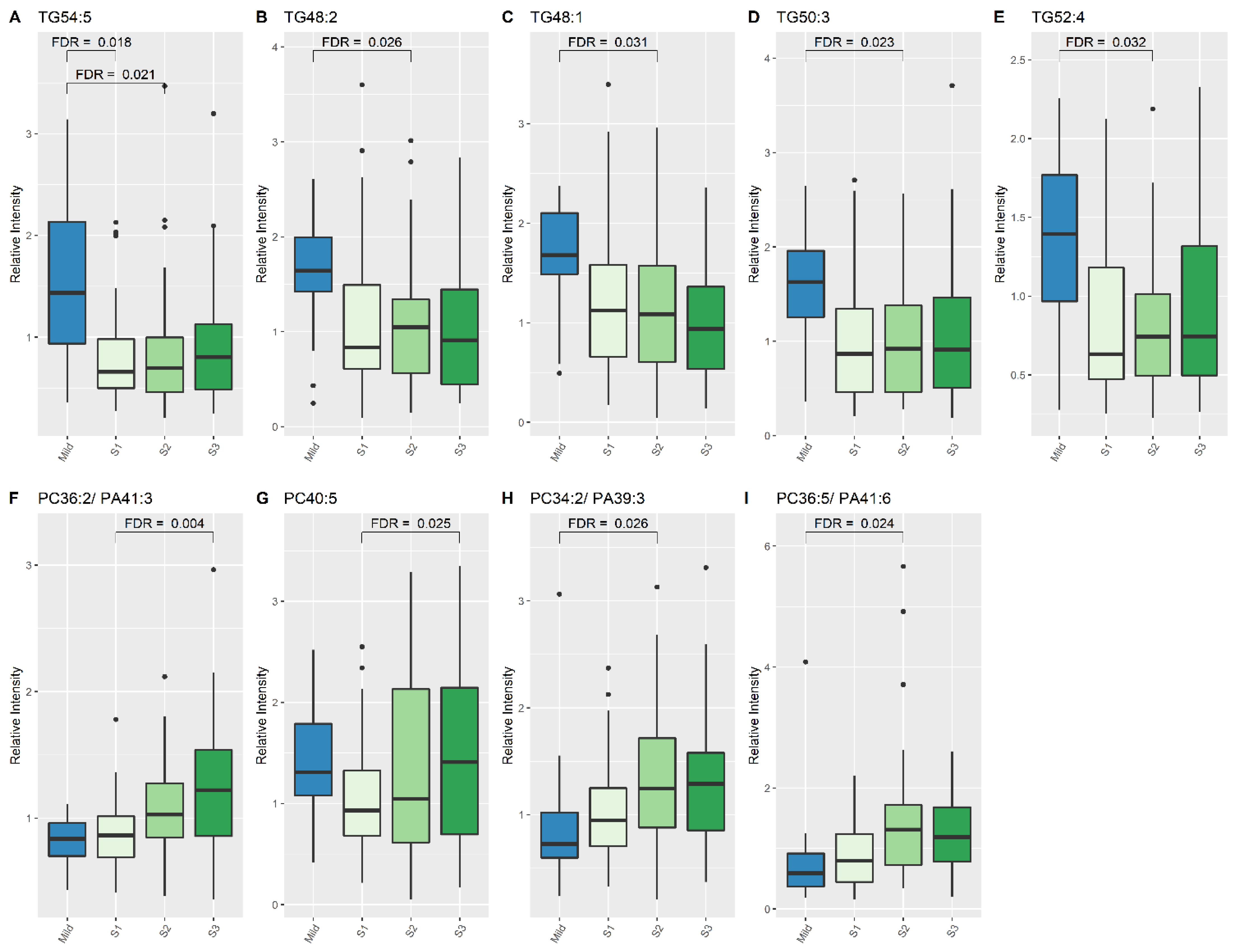
| TG (54:5) | 0.66 | 1.432 | 2.031 (1.4345, 2.768) | <0.001 | 0.018 |
| Moderate–Severe HIE at S2 vs. Mild | |||||
| Lipid Species | Moderate–Severe HIE Lipid Median | Mild HIE Lipid Median | Change in Relative Intensity as a Multiple (95% Confidence Interval) | pValue | AdjustedpValue (FDR) |
| PC (34:2)/PE (37:2)/PA (39:3) | 1.247 | 0.726 | 0.616 (0.487, 0.829) | 0.002 | 0.026 |
| PC (36:5)/PE (39:5)/PA (41:6) | 1.31 | 0.589 | 0.494 (0.327, 0.736) | 0.001 | 0.024 |
| TG (48:1) | 1.087 | 1.678 | 1.542 (1.183, 2.061) | 0.002 | 0.031 |
| TG (48:2) | 1.049 | 1.642 | 1.605 (1.236, 2.214) | 0.001 | 0.026 |
| TG (50:3) | 0.92 | 1.628 | 1.708 (1.267, 2.493) | 0.001 | 0.023 |
| TG (52:4) | 0.741 | 1.394 | 1.691 (1.219, 2.253) | 0.003 | 0.032 |
| TG (54:5) | 0.695 | 1.432 | 2.018 (1.447, 2.836) | <0.001 | 0.021 |
| Lipid Species | Moderate–Severe HIE Lipid Median | Mild HIE Lipid Median | Change in Relative Intensity as a Multiple (95% Confidence Interval) | p Value | Adjusted p Value (FDR) |
|---|---|---|---|---|---|
| PC (34:2)/PE (37:2)/PA (39:3) | 1.291 | 0.726 | 0.631 (0.479, 0.825) | 0.001 | 0.012 |
| PC (35:2)/PE (38:2)/PA (40:3) | 0.761 | 1.119 | 1.509 (1.171, 1.947) | 0.004 | 0.017 |
| PC (36:2)/PE (39:2)/PA (41:3) | 1.125 | 0.838 | 0.725 (0.5910, 0.872) | 0.003 | 0.015 |
| PC (36:5)/PE (39:5)/PA (41:6) | 1.186 | 0.589 | 0.495 (0.351, 0.692) | 0 | 0.006 |
| PC-O (16:0)/LPE (19:0) | 1.209 | 0.595 | 0.552 (0.3784, 0.862) | 0.014 | 0.045 |
| PC-O (18:0)/LPE (21:0) | 1.238 | 0.533 | 0.505 (0.361, 0.717) | 0.001 | 0.011 |
| PC-2O (32:0) | 1.275 | 1.518 | 1.182 (1.094, 1.303) | 0.001 | 0.011 |
| PC-O (34:2)/PE-O (37:2) | 0.837 | 1.498 | 1.770 (1.294, 2.445) | 0.002 | 0.015 |
| PC-O (34:1)/PE-O (37:1) | 1.283 | 1.52 | 1.175 (1.053, 1.318) | 0.008 | 0.032 |
| PC (37:4)/PE (40:4)/PA (42:5) | 0.692 | 1.525 | 2.073 (1.430, 2.990) | 0 | 0.006 |
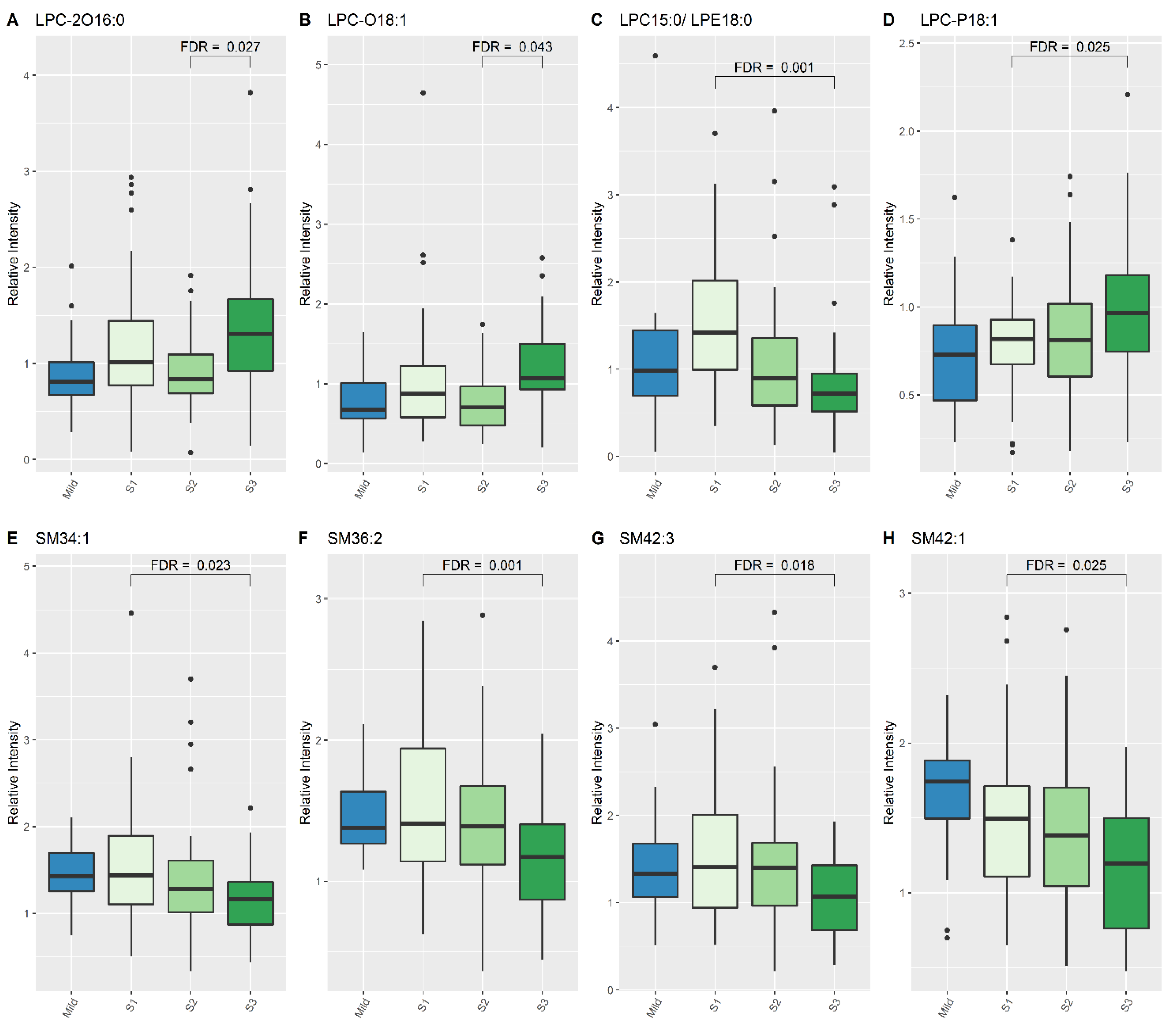
| LPC-O (18:1) | 1.094 | 0.677 | 0.619 (0.493, 0.855) | 0.002 | 0.015 |
| LPC-P (18:1) | 0.976 | 0.726 | 0.7195 (0.553, 0.932) | 0.017 | 0.05 |
| SM (34:1) | 1.266 | 1.429 | 1.235 (1.029, 1.471) | 0.017 | 0.05 |
| SM (35:1) | 1.118 | 1.765 | 1.403 (1.122, 2.015) | 0.004 | 0.019 |
| SM (36:2) | 1.185 | 1.38 | 1.219 (1.061, 1.433) | 0.01 | 0.035 |
| SM (40:1) | 1.225 | 1.624 | 1.361 (1.127, 1.778) | 0.003 | 0.015 |
| SM (42:1) | 1.221 | 1.741 | 1.405 (1.188, 1.724) | 0 | 0.006 |
| DG (36:1) | 0.794 | 1.652 | 1.968 (1.368, 2.866) | 0.002 | 0.013 |
| TG (48:1) | 0.939 | 1.678 | 1.727 (1.306, 2.512) | 0 | 0.006 |
| TG (48:2) | 0.909 | 1.642 | 1.646 (1.180, 2.667) | 0.002 | 0.015 |
| TG (48:3) | 0.81 | 1.662 | 1.735 (1.152, 2.476) | 0.011 | 0.038 |
| TG (50:1) | 0.841 | 1.775 | 1.929 (1.341, 2.821) | 0.003 | 0.015 |
| TG (50:2) | 0.847 | 1.459 | 1.693 (1.100, 2.365) | 0.011 | 0.038 |
| TG (50:3) | 0.91 | 1.628 | 1.631 (1.163, 2.450) | 0.004 | 0.017 |
| TG (50:4) | 0.92 | 1.828 | 1.722 (1.319, 2.294) | 0.001 | 0.009 |
| TG (51:2) | 0.74 | 1.149 | 1.613 (1.116, 2.110) | 0.009 | 0.034 |
| TG (52:4) | 0.744 | 1.394 | 1.535 (1.068, 2.224) | 0.016 | 0.049 |
| TG (54:5) | 0.804 | 1.432 | 1.739 (1.232, 2.462) | 0.003 | 0.015 |
| S1 vs. S3 for Moderate–Severe HIE | |||||
|---|---|---|---|---|---|
| Lipid Species | Median Level at Birth | Median Level at Re-Warming | Change in Relative Intensity as a Multiple (95% Confidence Interval) | p Value | Adjusted p Value (FDR) |
| PC (36:2)/PE (39:2)/PA (41:3) | 0.865 | 1.222 | 0.711 (0.599, 0.832) | <0.001 | 0.004 |
| PC (40:5) | 0.932 | 1.409 | 0.576 (0.459, 0.832) | 0.002 | 0.025 |
| LPC (15:0)/LPE (18:0) | 1.42 | 0.717 | 1.935 (1.456, 2.442) | <0.001 | 0.001 |
| LPC-P (18:1) | 0.818 | 0.964 | 0.811 (0.713, 0.932) | 0.002 | 0.025 |
| SM (34:1) | 1.437 | 1.163 | 1.443 (1.174, 1.729) | 0.001 | 0.023 |
| SM (36:2) | 1.407 | 1.171 | 1.382 (1.22, 1.580) | <0.001 | 0.001 |
| SM (42:1) | 1.496 | 1.197 | 1.402 (1.126, 1.667) | 0.002 | 0.025 |
| SM (42:3) | 1.405 | 1.067 | 1.535 (1.193, 1.956) | 0.001 | 0.018 |
| S2 vs. S3 for Moderate–Severe outcome HIE | |||||
| Lipid Species | Median Level at Cooling | Median Level at Re-Warming | Change in Relative Intensity as a Multiple (95% Confidence Interval) | p Value | Adjusted p Value (FDR) |
| LPC-2O (16:0) | 0.837 | 1.308 | 0.693 (0.554, 0.847) | <0.001 | 0.027 |
| LPC-O (18:1) | 0.704 | 1.073 | 0.647 (0.519, 0.826) | 0.001 | 0.043 |
| Lipid Species | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| PC (34:2)/PE (37:2)/PA (39:3) | −0.13725 | 0 | 0 |
| PC (36:5)/PE (39:5)/PA (41:6) | −0.46582 | −5.03 × 104 | 0 |
| TG (48:1) | 0 | 0 | 0 |
| TG (48:2) | 0 | 0 | −0.12742 |
| TG (50:3) | 0.400286 | 0 | 0 |
| TG (52:4) | 0.221072 | 0 | 0 |
| TG (54:5) | 0.745032 | 0 | 0 |
| Lipid Species | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| PC (34:2)/PE (37:2)/PA (39:3) | −0.20961 | 0.127569 | 0 |
| PC (35:2)/PE (38:2)/PA (40:3) | 0.135575 | 0 | 0 |
| PC (36:5)/PE (39:5)/PA (41:6) | −0.29015 | 0.141899 | 0 |
| PC (36:2)/PE (39:2)/PA (41:3) | −0.19513 | 0.168625 | 0 |
| PC (37:4)/PE (40:4)/PA (42:5) | 0.313908 | 0 | 0 |
| PC-O (16:0)/LPE (19:0) | −0.10542 | −0.11515 | 0 |
| PC-O (18:0)/LPE (21:0) | −0.27236 | 0 | 0 |
| PC-O (34:2)/PE-O (37:2) | 0.230711 | 0 | 0 |
| PC-O (34:1)/PE-O (37:1) | 0 | 0.069747 | 0 |
| PC-2O (32:0) | 0.043895 | 0 | 0 |
| LPC-O (18:1) | −0.15428 | 0 | 0 |
| LPC-2O (16:0) | −0.15171 | 0 | 0 |
| LPC-P (18:1) | −0.08909 | 0.067468 | 0 |
| SM (34:1) | 0.103771 | 0 | 0.484361 |
| SM (35:1) | 0.123691 | 0.015681 | 0 |
| SM (36:2) | 0.153531 | −0.06324 | 0 |
| SM (40:1) | 0.127328 | 0 | 0 |
| SM (42:1) | 0.237286 | 0 | 0 |
| DG (36:1) | 0.224861 | 0 | 0 |
| TG (48:1) | 0.239505 | 0 | 0 |
| TG (48:2) | 0.16305 | 0 | 0 |
| TG (48:3) | 0.106817 | 0.044888 | 0 |
| TG (50:1) | 0.169699 | 0 | 0 |
| TG (50:2) | 0.091848 | 0 | 0 |
| TG (50:3) | 0.14831 | 0 | 0 |
| TG (50:4) | 0.274731 | 0 | 0 |
| TG (51:2) | 0.14014 | 0 | 0 |
| TG (52:4) | 0.094162 | 0.01366 | 0 |
| TG (54:5) | 0.197721 | 0 | 0 |
| Reference | Biofluid/Tissue | Species and Population | Discriminant Lipid Species and Observed Changes in Disease Group |
|---|---|---|---|
| HIE | |||
| Liu et al. 2013 [37] | Whole brain extract | Mice—6 no reoxygenation, 6 controls, 6 hypothermia, 6 normothermia, 6 rewarming, 6 long normothermia | Increase: choline and PC |
| Walsh et al. 2012 [38] | Umbilical cord blood | Human newborns—40 asphyxia vs. matched controls Human newborns—31 HIE vs. matched controls | Increase: LPC (16:0), PC (34:1), PC (36:4), PC (38:4) and PC (38:5) Increase: PC (38:4) |
| Reinke et al. 2013 [15] | Umbilical cord blood | Human newborns—34 Asphyxia vs. matched control Human newborns—25 HIE vs. matched controls | Increase: choline and O-phosphocholine Increase: choline |
| Adult Stroke | |||
| Yang et al. 2017 [39] | Plasma | Human adults—29 lacunar infarct patients vs. 15 healthy controls | Decrease: PE species, especially PE (35:2) Increase: LPC species—LPC (20:4), LPC (20:5), LPC (22:6) and LPC (24:0). TG species, especially TG (56:5) |
| Koizumi et al. 2010 [40] | Brain tissue sections | Sprague–Dawley Rats—MCAO model of acute stroke vs. normal brain sections | Decrease: PC (16:0/18:1) due to overactivation of PLA2 Increase: LPC (16:0) |
| Irie et al. 2014 [41] | Whole brain sections | Male Wistar Rats—MCAO model of stroke vs. healthy controls | Decrease: CDP-choline and PE species at reperfusion |
| Wang et al. 2010 [42] | Cerebral cortex | Rats—MCAO model of acute stroke | Decrease: PC species and SM species Increase: LPC species due to lipid breakdown and neuroinflammation. |
| Nielsen et al. 2016 [43] | Whole brain coronal slices | Male C57BL/6 male Mice—MCAO model of stroke vs. healthy controls | Increase during ischemia: LPC species, especially LPC (16:0) in acute ischemia, PS species and NAPE 1 species Decrease during ischemia: SM species decreased and disappeared by 24 h Increase during reperfusion: spingosine-1-phosphate and DHA |
| Sheth et al. 2015 [44] | Rodent whole brain slices and plasma from humans | MCAO stroke model in mice vs. TBI model in rats Plasma from 9 human patients with acute ischemic stroke vs. 5 patients with stroke mimics and no stroke | Increase: within 24 h of stroke sharp increase in the sphingolipid (SL) score, using SM (36:0) and Cer (42:1) species with BBB disruption. The SL score correlates with the volume of infarct. |
| Lind et al. 2020 [45] | Plasma | Human adults—3 independent populations of ischemic stroke | Decrease: SM (32:1) in ischemic stroke. The lower the level of SM (32:1), the higher the rate of incident ischemic stroke. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nixon, R.; Ip, T.H.R.; Jenkins, B.; Yip, P.K.; Clarke, P.; Ponnusamy, V.; Michael-Titus, A.T.; Koulman, A.; Shah, D.K. Lipid Profiles from Dried Blood Spots Reveal Lipidomic Signatures of Newborns Undergoing Mild Therapeutic Hypothermia after Hypoxic-Ischemic Encephalopathy. Nutrients 2021, 13, 4301. https://doi.org/10.3390/nu13124301
Nixon R, Ip THR, Jenkins B, Yip PK, Clarke P, Ponnusamy V, Michael-Titus AT, Koulman A, Shah DK. Lipid Profiles from Dried Blood Spots Reveal Lipidomic Signatures of Newborns Undergoing Mild Therapeutic Hypothermia after Hypoxic-Ischemic Encephalopathy. Nutrients. 2021; 13(12):4301. https://doi.org/10.3390/nu13124301
Chicago/Turabian StyleNixon, Rebekah, Ting Hin Richard Ip, Benjamin Jenkins, Ping K. Yip, Paul Clarke, Vennila Ponnusamy, Adina T. Michael-Titus, Albert Koulman, and Divyen K. Shah. 2021. "Lipid Profiles from Dried Blood Spots Reveal Lipidomic Signatures of Newborns Undergoing Mild Therapeutic Hypothermia after Hypoxic-Ischemic Encephalopathy" Nutrients 13, no. 12: 4301. https://doi.org/10.3390/nu13124301
APA StyleNixon, R., Ip, T. H. R., Jenkins, B., Yip, P. K., Clarke, P., Ponnusamy, V., Michael-Titus, A. T., Koulman, A., & Shah, D. K. (2021). Lipid Profiles from Dried Blood Spots Reveal Lipidomic Signatures of Newborns Undergoing Mild Therapeutic Hypothermia after Hypoxic-Ischemic Encephalopathy. Nutrients, 13(12), 4301. https://doi.org/10.3390/nu13124301









