Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study
Abstract
:1. Introduction
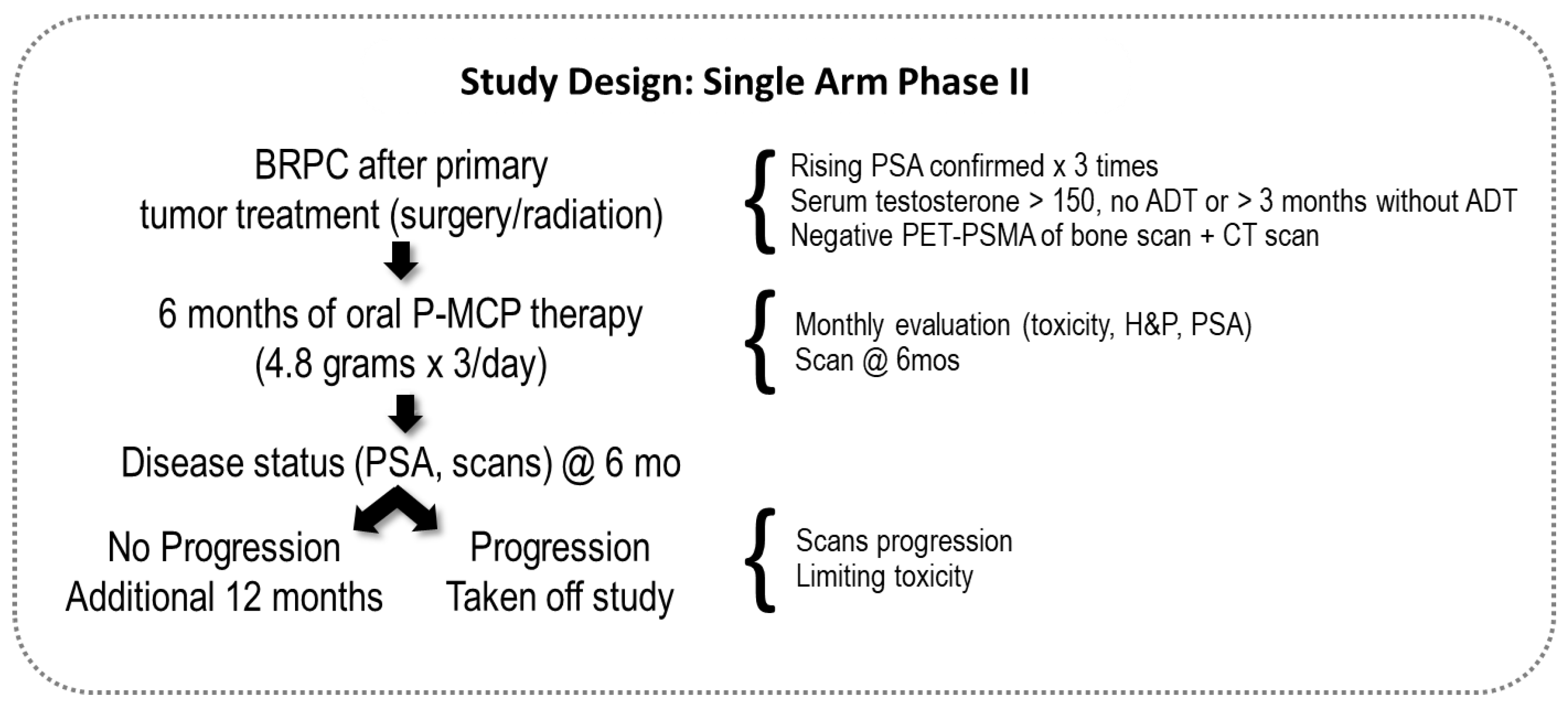
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Toxicity and Disease Status at Follow-Up Monthly Assessments
2.3. Treatment Duration
2.4. Safety Evaluation of Toxicity
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Toxicity and Compliance
3.3. Analysis of the PSADT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Artibani, W.; Porcaro, A.B.; De Marco, V.; Cerruto, M.A.; Siracusano, S. Management of Biochemical Recurrence after Primary Curative Treatment for Prostate Cancer: A Review. Urol. Int. 2017, 100, 251–262. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy for Prostate Cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef]
- Keizman, D.; Zahurak, M.; Sinibaldi, V.; Carducci, M.; Denmeade, S.; Drake, C.; Pili, R.; Antonarakis, E.S.; Hudock, S.; Eisenberger, M. Lenalidomide in Nonmetastatic Biochemically Relapsed Prostate Cancer: Results of a Phase I/II Double-Blinded, Randomized Study. Clin. Cancer Res. 2010, 16, 5269–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pound, C.; Partin, A.; Eisenberger, M.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelefsky, M.J.; Ben-Porat, L.; Scher, H.I.; Chan, H.M.; Fearn, P.A.; Fuks, Z.Y.; Leibel, S.A.; Venkatraman, E. Outcome Predictors for the Increasing PSA State After Definitive External-Beam Radiotherapy for Prostate Cancer. J. Clin. Oncol. 2005, 23, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonarakis, E.S.; Feng, Z.; Trock, B.J.; Humphreys, E.B.; Carducci, M.A.; Partin, A.W.; Walsh, P.C.; Eisenberger, M.A. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012, 109, 32–39. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Moul, J.W.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.H. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J. Natl. Cancer Inst. 2003, 95, 1376–1383. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, M.-H.; McLeod, D.; Carroll, P.R.; Moul, J.W.; D’Amico, A.V. Predictors of Prostate Cancer–Specific Mortality After Radical Prostatectomy or Radiation Therapy. J. Clin. Oncol. 2005, 23, 6992–6998. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Zahurak, M.L.; Lin, J.; Keizman, D.; Carducci, M.A.; Eisenberger, M.A. Changes in PSA kinetics predict metastasis-free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents. Cancer 2011, 118, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Arlen, P.M.; Bianco, F.; Dahut, W.L.; D’Amico, A.; Figg, W.D.; Freedland, S.J.; Gulley, J.L.; Kantoff, P.W.; Kattan, M.W.; Lee, A.; et al. Prostate specific antigen working group guidelines on prostate specific antigen doubling time. J. Urol. 2008, 179, 2181–2185. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, E.; Zahurak, M.; Sinibaldi, V.; Carducci, M.A.; Pili, R.; Laufer, M.; Deweese, T.L.; Eisenberger, M.A. Marimastat in the Treatment of Patients with Biochemically Relapsed Prostate Cancer: A Prospective Randomized, Double-Blind, Phase I/II Trial. Clin. Cancer Res. 2005, 11, 4437–4443. [Google Scholar] [CrossRef] [Green Version]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2012, 16, 50–55. [Google Scholar] [CrossRef]
- Conti, S.; Vexler, A.; Hagoel, L.; Kalich-Philosoph, L.; Corn, B.W.; Honig, N.; Shtraus, N.; Meir, Y.; Ron, I.; Eliaz, I.; et al. Modified Citrus Pectin as a Potential Sensitizer for Radiotherapy in Prostate Cancer. Integr. Cancer Ther. 2018, 17, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pienta, K.J.; Naik, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J. Natl. Cancer Inst. 1995, 87, 348–353. [Google Scholar] [CrossRef]
- Guess, B.W.; Scholz, M.C.; Strum, S.B.; Lam, R.Y.; Johnson, H.J.; Jennrich, R.I. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: A phase II pilot study. Prostate Cancer Prostatic Dis. 2003, 6, 301–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonarakis, E.S.; Chen, Y.; Elsamanoudi, S.I.; Brassell, S.A.; Da Rocha, M.V.; Eisenberger, M.A.; McLeod, D.G. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: Analysis of the Center for Prostate Disease Research National Database. BJU Int. 2010, 108, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Hogan, V.; Raz, A. Extracellular galectin-3 programs multidrug resistance through Na+/K+-ATPase and P-glycoprotein signaling. Oncotarget 2015, 6, 19592–19604. [Google Scholar] [CrossRef] [Green Version]
- Balan, V.; Nangia-Makker, P.; Kho, D.H.; Wang, Y.; Raz, A. Tyrosine-phosphorylated Galectin-3 Protein Is Resistant to Prostate-specific Antigen (PSA) Cleavage. J. Biol. Chem. 2012, 287, 5192–5198. [Google Scholar] [CrossRef] [Green Version]
- Glinskii, O.V.; Sud, S.; Mossine, V.V.; Mawhinney, T.P.; Anthony, D.C.; Glinsky, G.V.; Pienta, K.J.; Glinsky, V.V. Inhibition of Prostate Cancer Bone Metastasis by Synthetic TF Antigen Mimic/Galectin-3 Inhibitor Lactulose-l-Leucine. Neoplasia 2012, 14, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Harazono, Y.; Gao, X.; Hogan, V.; Raz, A. Galectin-3 Inhibits Osteoblast Differentiation through Notch Signaling. Neoplasia 2014, 16, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Harazono, Y.; Hogan, V.; Chen, W.; Ali-Fehmi, R.; Mehra, R.; Raz, A. Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis. Cancer Res. 2016, 76, 1391–1402. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Zimel, M.; Heath, E.; Hogan, V.; Raz, A. Galectin-3 in bone tumor microenvironment: A beacon for individual skeletal metastasis management. Cancer Metastasis Rev. 2016, 35, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Heilbrun, L.K.; Hogan, V.; Smith, D.; Heath, E.; Raz, A. Positive associations between galectin-3 and PSA levels in prostate cancer patients: A prospective clinical study-I. Oncotarget 2016, 7, 82266–82272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, C.J.; Olatoye, D.; Xie, S.; Zhou, X.; Denmeade, S.R.; Eisenberger, M.A.; Antonarakis, E.S.; Carducci, M.A.; Rosner, G.L. The effect of the frequency and duration of PSA measurement on PSA doubling time calculations in men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2013, 17, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Pre-Treatment (n = 60) |
|---|---|
| Age (years): Median (range) | 73 (53–89) |
| Gleason: % (n) | |
| 6 | 30% (n = 18) |
| 7 | 47% (n = 28) |
| 8–10 | 23% (n = 14) |
| Local therapy: % (n) | |
| Radical prostatectomy | 13% (n = 8) |
| Radiation therapy (RT) | 57% (n = 34) |
| Surgery + RT | 30% (n = 18) |
| Prior ADT | 52% (n = 31) |
| PSA (ng/mL): Median (range) | 4.13 (0.25–30) |
| PSA progression (increase of ≥25% within the six months prior to treatment initiation) | 88% (n = 53) |
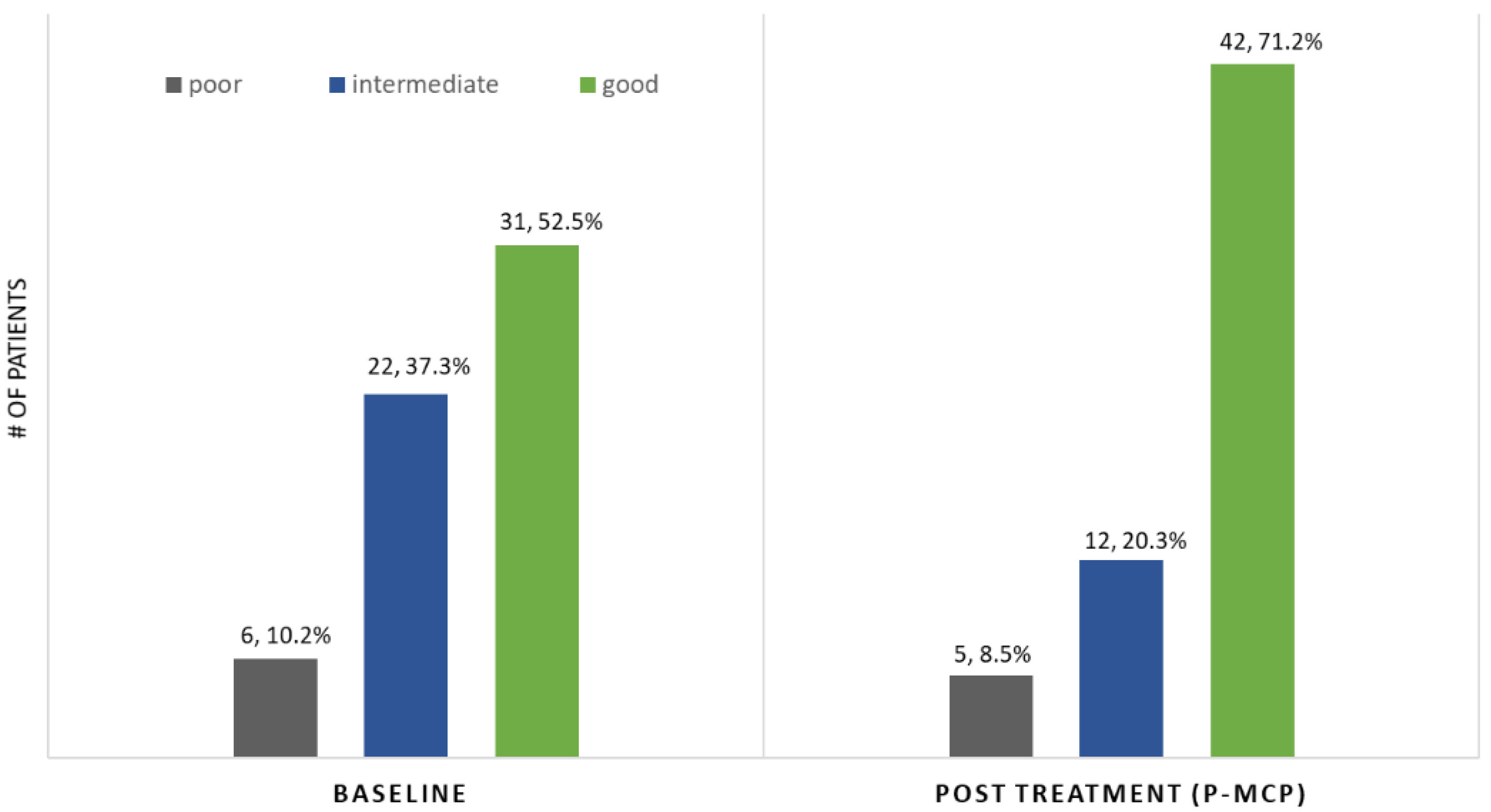
| PSADT (months) risk grouping: % (n) | |
| Poor < 3 | 10% (n = 6) |
| Intermediate 3–8.99 | 38% (n = 23) |
| Good ≥ 9 | 52% (n = 31) |
| PSADT (months): Median (range) | |
| Whole cohort | 9.12 (1.4–55) |
| Poor PSADT risk | 2.3 (1.6–2.82) |
| Intermediate risk | 5.21 (3.23–8.94) |
| Good risk | 14.74 (9.10–54.6) |
| Parameter | Whole Cohort (n = 59) | According to Pre-Study PSADT (months) Risk Grouping | ||
|---|---|---|---|---|
| Poor <3.00 (n = 6) | Intermediate 3.00–8.99 (n = 22) | Good ≥9.00 (n = 31) | ||
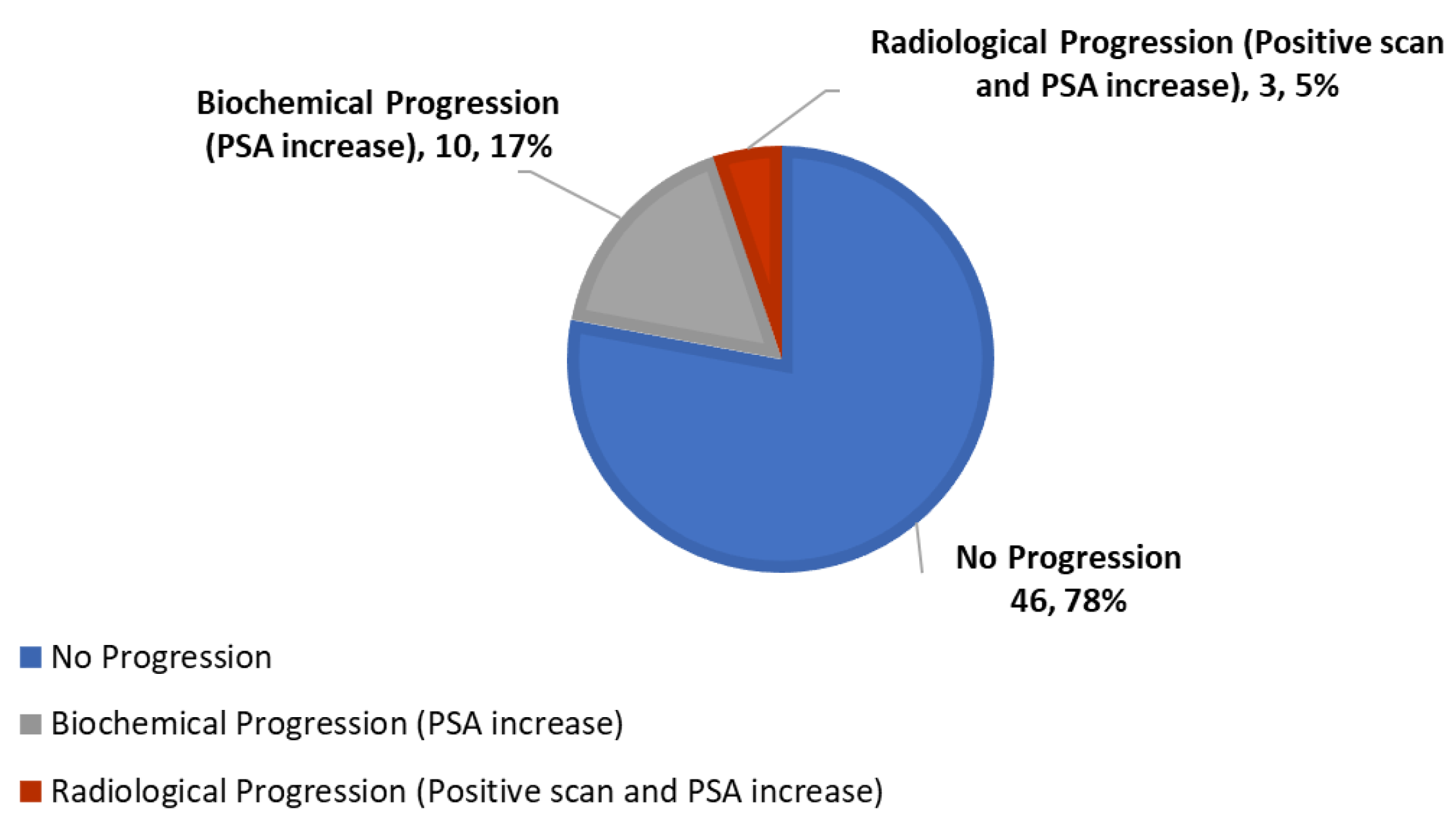
| Overall response to therapy (Decrease or stabilization of PSA, and/or lengthening of PSADT, with negative scans) | 78% (n = 46) | 66% (n = 4) | 77% (n = 17) | 81% (n = 25) |
| PSA response | ||||
| Stable–decreased | 58% (n = 34) | 0% (n = 0) | 45% (n = 10) | 77% (n = 24) |
| Progression | 42% (n = 25) | 100% (n = 6) | 55% (n = 12) | 23% (n = 7) |
| PSADT months (median range) | 15.3 (1.4–677) | 2.35 (1.4–2.97) | 6.5 (3.2–8.1) | 20.4 (9.2–677) |
| PSADT risk grouping | 9% (n = 5) | 20% (n = 12) | 71% (n = 42) | |
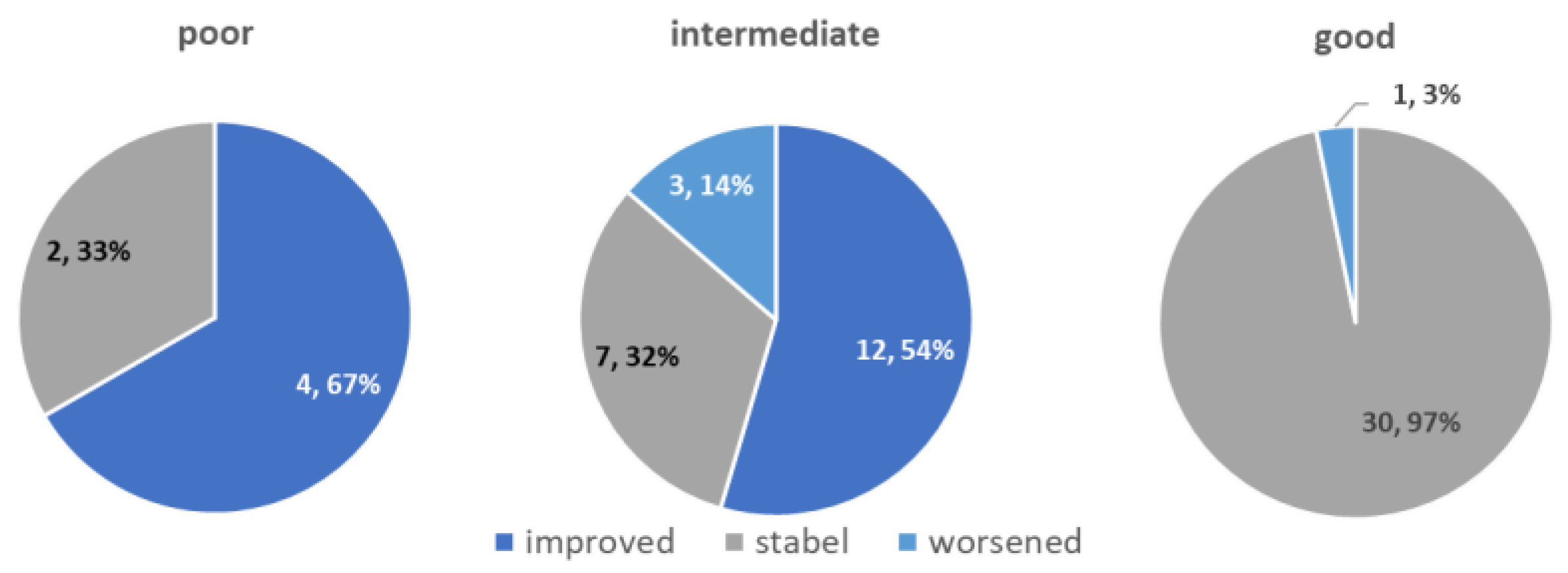
| PSADT lengthening | 75% (n = 44) | 66% (n = 4) | 82% (n = 18) | 71% (n = 22) |
| Better PSADT risk grouping | 27% (n = 16) | 66% (n = 4) | 55% (n = 12) | not applicable |
| Radiologic response | ||||
| Negative scans | 95% (n = 56) | 83% (n = 5) | 91% (n = 20) | 100% (n = 31) |
| Disease progression | 5% (n = 3) | 17% (n = 1) | 9% (n = 2) | 0% (n = 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients 2021, 13, 4295. https://doi.org/10.3390/nu13124295
Keizman D, Frenkel M, Peer A, Kushnir I, Rosenbaum E, Sarid D, Leibovitch I, Mano R, Yossepowitch O, Margel D, et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients. 2021; 13(12):4295. https://doi.org/10.3390/nu13124295
Chicago/Turabian StyleKeizman, Daniel, Moshe Frenkel, Avivit Peer, Igal Kushnir, Eli Rosenbaum, David Sarid, Ilan Leibovitch, Roy Mano, Ofer Yossepowitch, David Margel, and et al. 2021. "Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study" Nutrients 13, no. 12: 4295. https://doi.org/10.3390/nu13124295
APA StyleKeizman, D., Frenkel, M., Peer, A., Kushnir, I., Rosenbaum, E., Sarid, D., Leibovitch, I., Mano, R., Yossepowitch, O., Margel, D., Wolf, I., Geva, R., Dresler, H., Rouvinov, K., Rapoport, N., & Eliaz, I. (2021). Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients, 13(12), 4295. https://doi.org/10.3390/nu13124295






