Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey
Abstract
1. Introduction
2. Materials and Methods
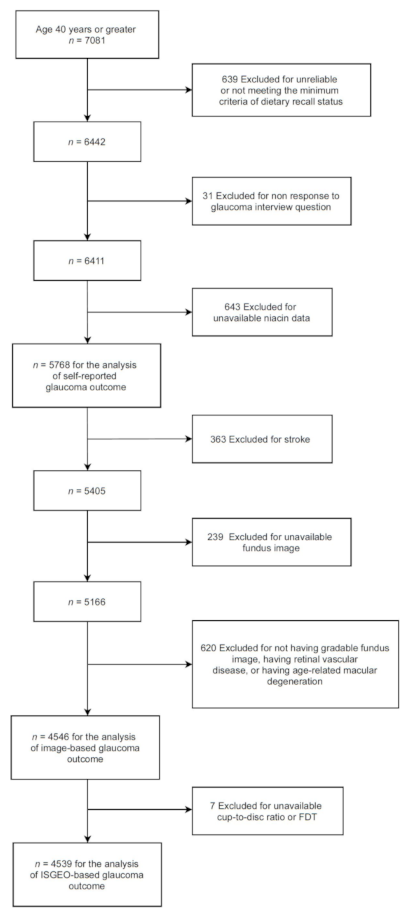
2.1. Sample and Population
2.2. Measures
2.3. Outcome Variables
- (1)
- CDR > 99.5th percentile for the mean NHANES population in either eye, regardless of the FDT results.
- (2)
- CDR asymmetry between eyes > 99.5th percentile for the mean NHANES population, regardless of the FDT results.
- (3)
- CDR > 97.5th percentile for the mean NHANES population in either eye with positive FDT result in the same eye.
- (4)
- CDR asymmetry between eyes > 97.5th percentile for the mean NHANES population with positive FDT result in at least one eye.
2.4. Data Analysis
3. Results
4. Discussion
| Study | Design | Subjects (n) | Niacin Form | Human Doses or Equivalent | Outcomes | Results |
|---|---|---|---|---|---|---|
| Williams et al. [21] | Animal study | DBA/2J mice | NAM doses at 550 and 2000 mg/kg/day | 2.7 g/day and 9.8 g/day in a 60 kg human |
| Dietary supplementation with NAM reduces vulnerability to glaucoma. |
| Tribble et al. [42] | Animal study | C57BL/6J mice | NAM dose at 200, 400 and 800 mg/kg/day | 1.9, 4, and 8 g/day in a 60 kg human |
| NAM buffers against metabolic and bioenergetic insufficiency, and provides neuroprotection against glaucoma-related stresses. |
| Zhang et al. [52] | Animal study | C57BL/6J mice for optic nerve crush (acute model) and DBA/2J mice intracameral microbead injection (chronic model) experiments | Intraperitoneal injection of NR at 1000 mg/kg | N/A |
| Prophylactic systemic treatment with NR is protective in acute and chronic mouse models of RGC damage. |
| Hui et al. [44] | Crossover randomized clinical trial | Early to moderate treated glaucoma (n = 57) | NAM doses at 1.5 g/day for 6 weeks, followed by 3.0 g/day for another 6 weeks |
| Participants received NAM have a better improvement in inner retinal function relative to control. | |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Saxena, R.; Agrawal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- D Voogd, S.; Ikram, M.K.; Wolfs, R.C.; Jansonius, N.M.; Hofman, A.; de Jong, P.T. Incidence of open-angle glaucoma in a general elderly population: The Rotterdam Study. Ophthalmology 2005, 112, 1487–1493. [Google Scholar] [CrossRef]
- Nemesure, B.; Honkanen, R.; Hennis, A.; Wu, S.Y.; Leske, M.C.; Barbados Eye Studies Group. Incident open-angle glaucoma and intraocular pressure. Ophthalmology 2007, 114, 1810–1815. [Google Scholar] [CrossRef]
- Krupin, T.; Liebmann, J.M.; Greenfield, D.S.; Ritch, R.; Gardiner, S.; Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 2011, 151, 671–681. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. For the Early Manifest Glaucoma Trial Group: Reduction of intraocular pressure and glaucoma progression. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y. Central nervous system changes in glaucoma. J. Glaucoma 2013, 22, S24–S25. [Google Scholar] [CrossRef]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog. Retin. Eye Res. 2003, 22, 465–481. [Google Scholar] [CrossRef]
- Weber, A.J.; Chen, H.; Hubbard, W.C.; Kaufman, P.L. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1370–1379. [Google Scholar]
- Gupta, N.; Ang, L.C.; Noel de Tilly, L.; Bidaisee, L.; Yucel, Y.H. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br. J. Ophthalmol. 2006, 90, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Levin, L.A. Glaucoma as a neurodegenerative disease. J. Neuro-Ophthalmol. 2015, 35, S22–S28. [Google Scholar] [CrossRef]
- Gupta, N.; Yucel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Wolfs, R.C.; Kiefte-de Jong, J.C.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Jansonius, N.M. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012, 27, 385–393. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Schouten, J.; Webers, C.A.B. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Ramsden, D. Nicotinamide: A double edged sword. Parkinsonism Relat. Disord. 2005, 11, 413–420. [Google Scholar] [CrossRef]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan. Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cardozo, B.H.; Cochran, K.E.; John, S.W.M. Nicotinamide and WLDS Act Together to Prevent Neurodegeneration in Glaucoma. Front. Neurosci. 2017, 11, 232. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 12 August 2021).
- National Health and Nutrition Examination Survey. 2015–2016 Data Documentation, Codebook, and Frequencies: Dietary Interview Technical Support File—Food Codes (DRXFCD_I). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DRXFCD_I.htm (accessed on 12 August 2021).
- U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA Nutrient Database for Standard Reference, Release 28. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 14 August 2021).
- National Health and Nutrition Examination Survey. 2005–2006 Data Documentation, Codebook, and Frequencies: Ophthalmology—Retinal Imaging (OPXRET_D). Available online: http://wwwn.cdc.gov/nchs/nhanes/2005-2006/OPXRET_D.htm (accessed on 12 August 2021).
- National Health and Nutrition Examination Survey. 2005–2006 Data Documentation, Codebook, and Frequencies: Ophthalmology—Frequency Doubling Technology (OPXFDT_D). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/OPXFDT_D.htm (accessed on 6 August 2021).
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 2002, 86, 238–242. [Google Scholar] [CrossRef]
- Wolfs, R.C.; Borger, P.H.; Ramrattan, R.S.; Klaver, C.C.; Hulsman, C.A.; Hofman, A.; Vingerling, J.R.; Hitchings, R.A.; de Jong, P.T. Changing views on open-angle glaucoma: Definitions and prevalences--The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3309–3321. [Google Scholar]
- Shaikh, Y.; Yu, F.; Coleman, A.L. Burden of undetected and untreated glaucoma in the United States. Am. J. Ophthalmol. 2014, 158, 1121–1129. [Google Scholar] [CrossRef]
- Spry, P.G.; Hussin, H.M.; Sparrow, J.M. Performance of the 24-2-5 frequency doubling technology screening test: A prospective case study. Br. J. Ophthalmol. 2007, 91, 1345–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quigley, H.A. Identification of glaucoma-related visual field abnormality with the screening protocol of frequency doubling technology. Am. J. Ophthalmol. 1998, 125, 819–829. [Google Scholar] [CrossRef]
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Cardozo, B.H.; Foxworth, N.E.; John, S.W.M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 2018, 11, e1356956. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Harun-Or-Rashid, M. Metabolic Vulnerability in the Neurodegenerative Disease Glaucoma. Front. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef]
- Lascaratos, G.; Garway-Heath, D.F.; Willoughby, C.E.; Chau, K.Y.; Schapira, A.H. Mitochondrial dysfunction in glaucoma: Understanding genetic influences. Mitochondrion 2012, 12, 202–212. [Google Scholar] [CrossRef]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Coleman, M.P.; Martin, K.R. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 2008, 28, 1166–1179. [Google Scholar] [CrossRef]
- Kaneko, S.; Wang, J.; Kaneko, M.; Yiu, G.; Hurrell, J.M.; Chitnis, T.; Khoury, S.J.; He, Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 2006, 26, 9794–9804. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Sasaki, Y.; Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004, 305, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Otmani, A.; Sun, S.; Ellis, S.A.; Cimaglia, G.; Vohra, R.; Joe, M.; Lardner, E.; Venkataraman, A.P.; Dominguez-Vicent, A.; et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 2021, 43, 101988. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; John, S.W.M. Glaucoma as a Metabolic Optic Neuropathy: Making the Case for Nicotinamide Treatment in Glaucoma. J. Glaucoma 2017, 26, 1161–1168. [Google Scholar] [CrossRef]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- Stratford, M.R.; Rojas, A.; Hall, D.W.; Dennis, M.F.; Dische, S.; Joiner, M.C.; Hodgkiss, R.J. Pharmacokinetics of nicotinamide and its effect on blood pressure, pulse and body temperature in normal human volunteers. Radiother. Oncol. 1992, 25, 37–42. [Google Scholar] [CrossRef]
- Petley, A.; Macklin, B.; Renwick, A.G.; Wilkin, T.J. The pharmacokinetics of nicotinamide in humans and rodents. Diabetes 1995, 44, 152–155. [Google Scholar] [CrossRef]
- Fahmideh, F.; Marchesi, N.; Barbieri, A.; Govoni, S.; Pascale, A. Non-drug interventions in glaucoma: Putative roles for lifestyle, diet and nutritional supplements. Surv. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, R.M.; Allen, L.H.; Bailey, L.B.; Bernfield, M. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998; pp. 374–389. [Google Scholar] [CrossRef]
- Mills, E.; Prousky, J.; Raskin, G.; Gagnier, J.; Rachlis, B.; Montori, V.M.; Juurlink, D. The safety of over-the-counter niacin. A randomized placebo-controlled trial [ISRCTN18054903]. BMC Clin. Pharmacol. 2003, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Song, S.B. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules 2020, 10, 687. [Google Scholar] [CrossRef]
- Conze, D.; Brenner, C.; Kruger, C.L. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep. 2019, 9, 9772. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Chrenek, M.A.; Girardot, P.E.; Wang, J.; Sellers, J.T.; Geisert, E.E.; Brenner, C.; Nickerson, J.M.; Boatright, J.H.; et al. Systemic Treatment with Nicotinamide Riboside Is Protective in Two Mouse Models of Retinal Ganglion Cell Damage. Pharmaceutics 2021, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stodkilde-Jorgensen, H.; Moller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018, 108, 343–353. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef]
- Jung, K.I.; Kim, Y.C.; Park, C.K. Dietary Niacin and Open-Angle Glaucoma: The Korean National Health and Nutrition Examination Survey. Nutrients 2018, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Patty, L.; Wu, C.; Torres, M.; Azen, S.; Varma, R.; Los Angeles Latino Eye Study Group. Validity of self-reported eye disease and treatment in a population-based study: The Los Angeles Latino Eye Study. Ophthalmology 2012, 119, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Lucy, K.A.; Wollstein, G. Structural and Functional Evaluations for the Early Detection of Glaucoma. Expert Rev. Ophthalmol. 2016, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Self-Reported Glaucoma | Self-Reported No Glaucoma | p Value a |
|---|---|---|---|
| Estimated Mean/% Proportion (SE) | Estimated Mean/% Proportion (SE) | ||
| Age, years | 65.8 (1.17) | 56.9 (0.38) | <0.001 b |
| Gender | |||
| Male | 49.2 (0.04) | 45.4 (0.01) | 0.32 |
| Female | 50.8 (0.04) | 54.6 (0.01) | |
| Race/ethnicity | |||
| Non-Hispanic White | 72.6 (0.04) | 78.1 (0.02) | 0.02 |
| Non-Hispanic Black | 16.4 (0.03) | 9.8 (0.01) | |
| Mexican and Hispanic | 6.2 (0.01) | 8.0 (0.01) | |
| Other races | 4.8 (0.02) | 4.1 (0.01) | |
| Educational level | |||
| Less than high school | 24.4 (0.03) | 17.3 (0.01) | 0.01 |
| High school graduation or equivalent | 30.8 (0.04) | 26.6 (0.01) | |
| More than high school | 44.8 (0.04) | 56.1 (0.02) | |
| Annual household income, USD ($) | |||
| <20,000 | 18.0 (0.05) | 14.0 (0.01) | 0.32 |
| ≥20,000 | 82.0 (0.05) | 86.0 (0.01) | |
| Diabetes | |||
| No | 76.2 (0.02) | 89.2 (0.01) | <0.001 |
| Yes | 23.8 (0.02) | 10.8 (0.01) | |
| Daily total energy, kcal | 1690.7 (46.3) | 2015.1 (19.6) | <0.001 b |
| Daily Niacin Intake | Glaucoma | Participants | Crude OR (95% CI) | p Value | Model 1 OR (95% CI) | p Value | Model 2 OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Self-reported | Quartile 1 | 122 | 1442 | Ref | Ref | Ref | |||
| Quartile 2 | 111 | 1442 | 1.08 (0.71 to 1.65) | 0.704 | 1.15 (0.75 to 1.76) | 0.518 | 1.42 (0.89 to 2.27) | 0.133 | |
| Quartile 3 | 83 | 1442 | 0.57 (0.43 to 0.76) | <0.001 | 0.63 (0.46 to 0.87) | 0.006 | 0.95 (0.61 to 1.50) | 0.833 | |
| Quartile 4 | 77 | 1442 | 0.57 (0.37 to 0.90) | 0.018 | 0.73 (0.44 to 1.20) | 0.207 | 1.39 (0.74 to 2.58) | 0.292 | |
| p trend | 0.001 | 0.054 | 0.557 | ||||||
| Fundus Image | Quartile 1 | 35 | 1036 | Ref | Ref | Ref | |||
| Quartile 2 | 34 | 1091 | 0.78 (0.41 to 1.47) | 0.424 | 0.80 (0.43 to 1.49) | 0.470 | 0.77 (0.40 to 1.48) | 0.415 | |
| Quartile 3 | 29 | 1188 | 0.42 (0.25 to 0.72) | 0.002 | 0.43 (0.25 to 0.75) | 0.004 | 0.76 (0.21 to 0.99) | 0.050 | |
| Quartile 4 | 27 | 1231 | 0.36 (0.20 to 0.67) | 0.002 | 0.42 (0.23 to 0.75) | 0.005 | 0.50 (0.23 to 1.05) | 0.067 | |
| p trend | <0.001 | <0.001 | 0.040 | ||||||
| ISGEO criteria | Quartile 1 | 29 | 1034 | Ref | Ref | Ref | |||
| Quartile 2 | 33 | 1089 | 1.18 (0.50 to 2.80) | 0.691 | 1.20 (0.51 to 2.79) | 0.669 | 1.20 (0.56 to 2.58) | 0.624 | |
| Quartile 3 | 26 | 1187 | 0.60 (0.27 to 1.32) | 0.197 | 0.59 (0.26 to 1.33) | 0.196 | 0.63 (0.34 to 1.17) | 0.140 | |
| Quartile 4 | 23 | 1229 | 0.60 (0.24 to 1.49) | 0.258 | 0.62 (0.24 to 1.63) | 0.321 | 0.72 (0.34 to 1.54) | 0.387 | |
| p trend | 0.105 | 0.159 | 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taechameekietichai, T.; Chansangpetch, S.; Peerawaranun, P.; Lin, S.C. Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey. Nutrients 2021, 13, 4263. https://doi.org/10.3390/nu13124263
Taechameekietichai T, Chansangpetch S, Peerawaranun P, Lin SC. Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey. Nutrients. 2021; 13(12):4263. https://doi.org/10.3390/nu13124263
Chicago/Turabian StyleTaechameekietichai, Teerajet, Sunee Chansangpetch, Pimnara Peerawaranun, and Shan C. Lin. 2021. "Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey" Nutrients 13, no. 12: 4263. https://doi.org/10.3390/nu13124263
APA StyleTaechameekietichai, T., Chansangpetch, S., Peerawaranun, P., & Lin, S. C. (2021). Association between Daily Niacin Intake and Glaucoma: National Health and Nutrition Examination Survey. Nutrients, 13(12), 4263. https://doi.org/10.3390/nu13124263






