Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Eligibility Criteria and Follow-Up
2.3. Exercise Training Sessions Protocol
2.4. Blood Sample Collection and Analysis of Total Antioxidant Capacity
2.5. HS-Omega-3 Index Analysis
2.6. Choline Analysis
2.7. Statistics
3. Results
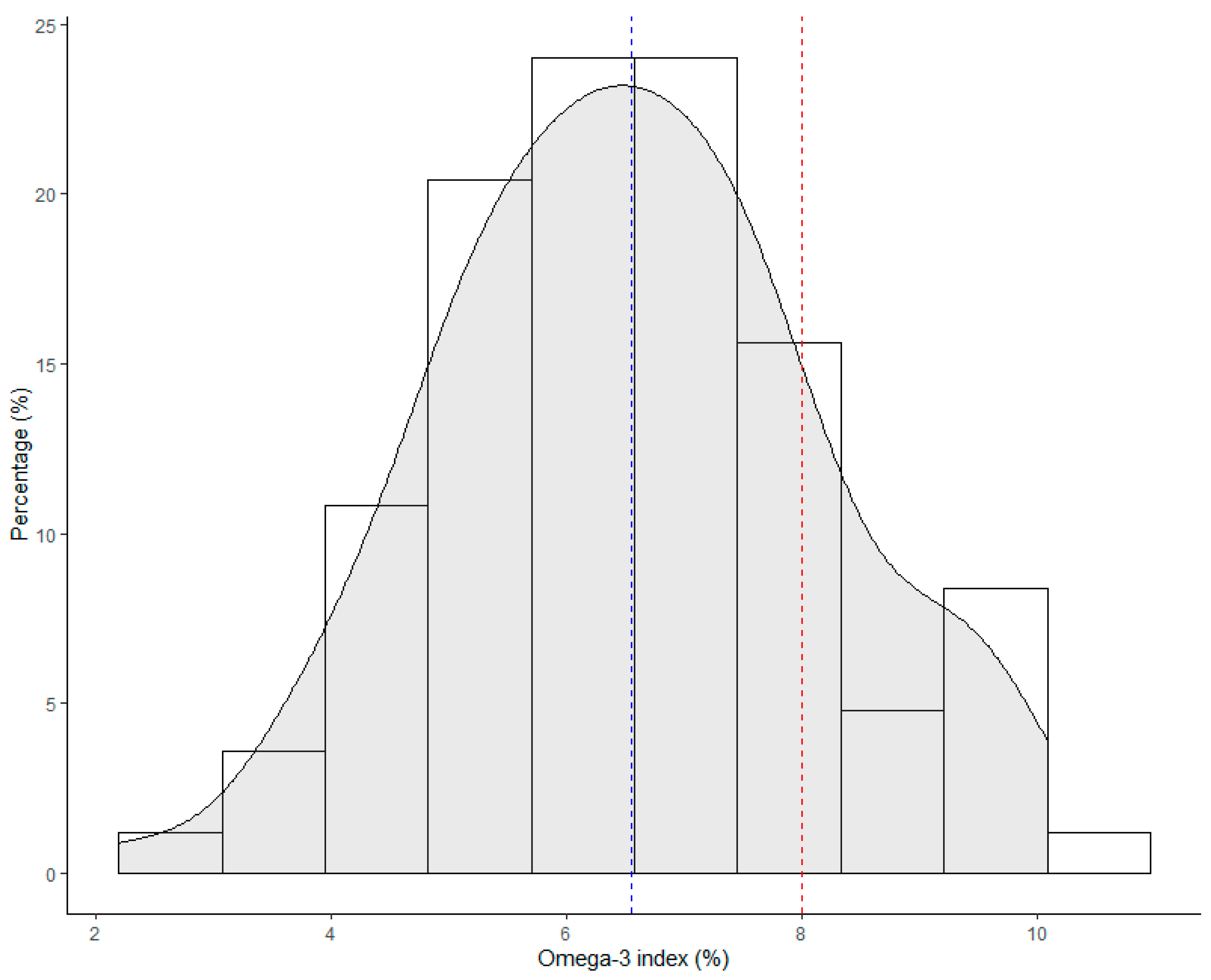
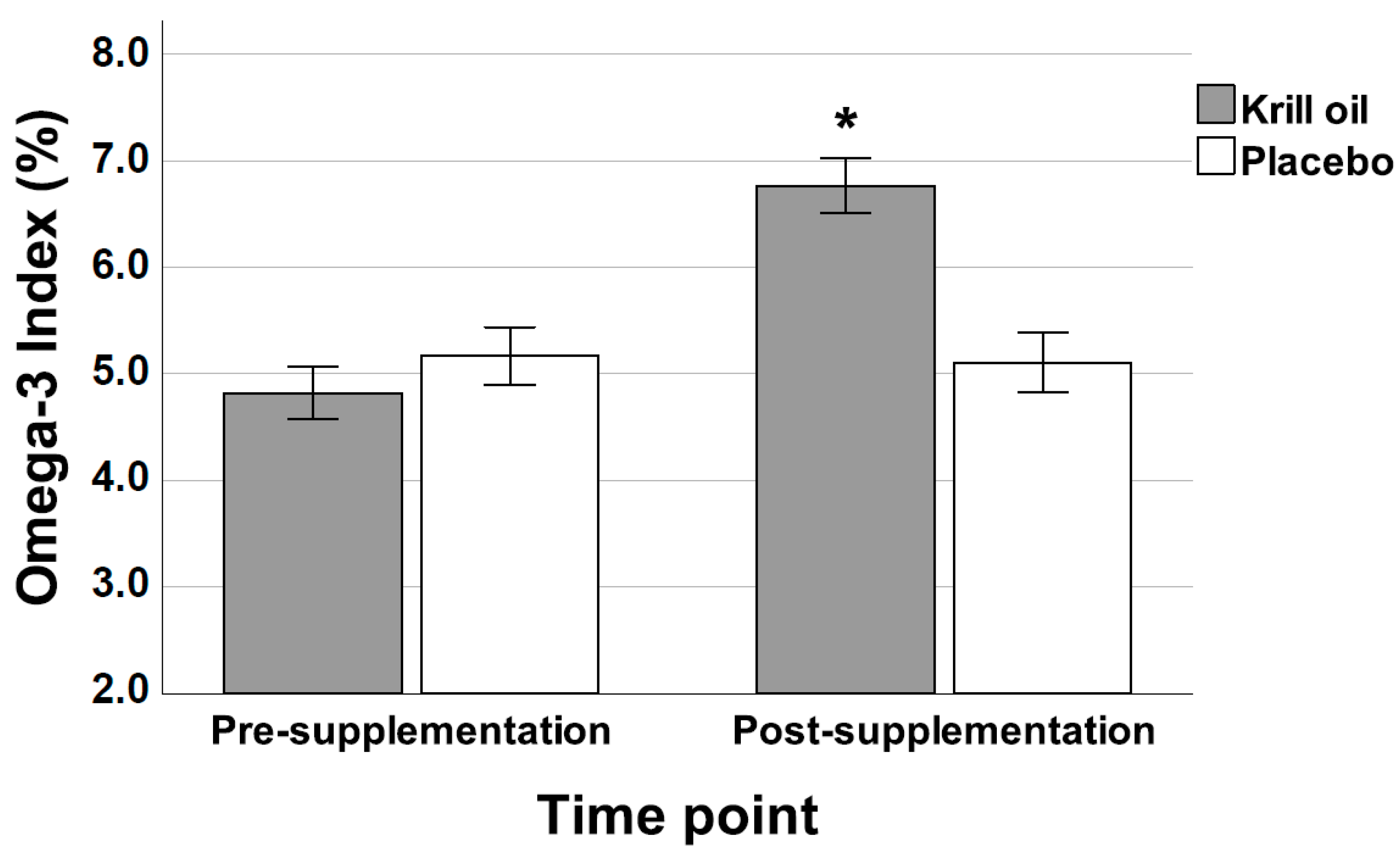
3.1. HS-Omega-3 Index
3.2. Individual Fatty Acids
3.3. Sum of Fatty Acids
3.4. Exercise Training Session Load
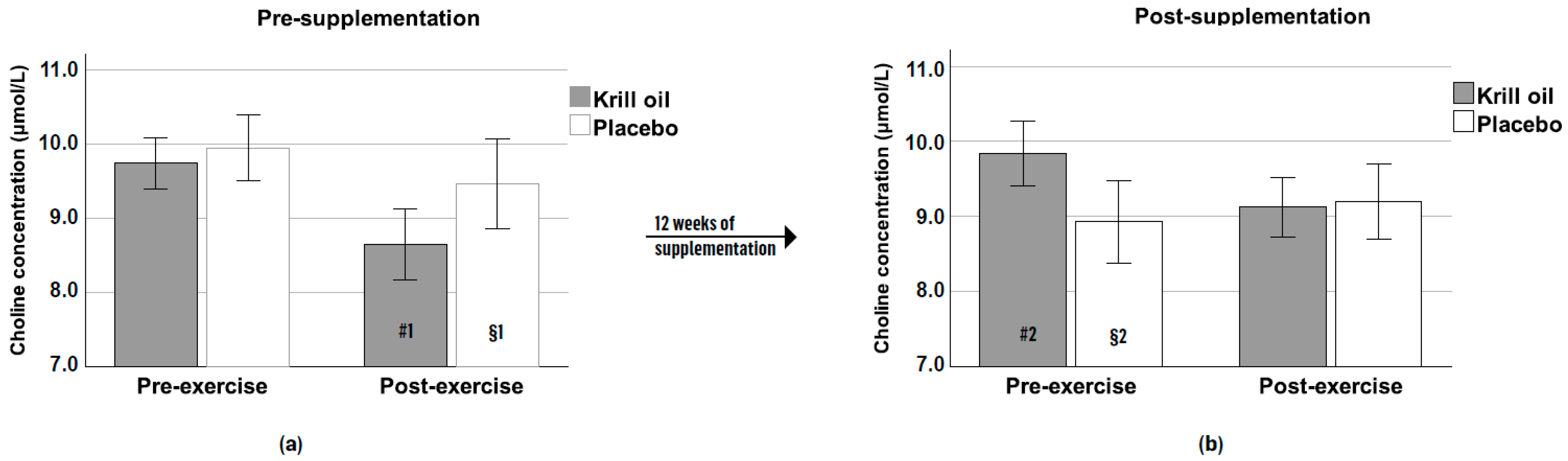
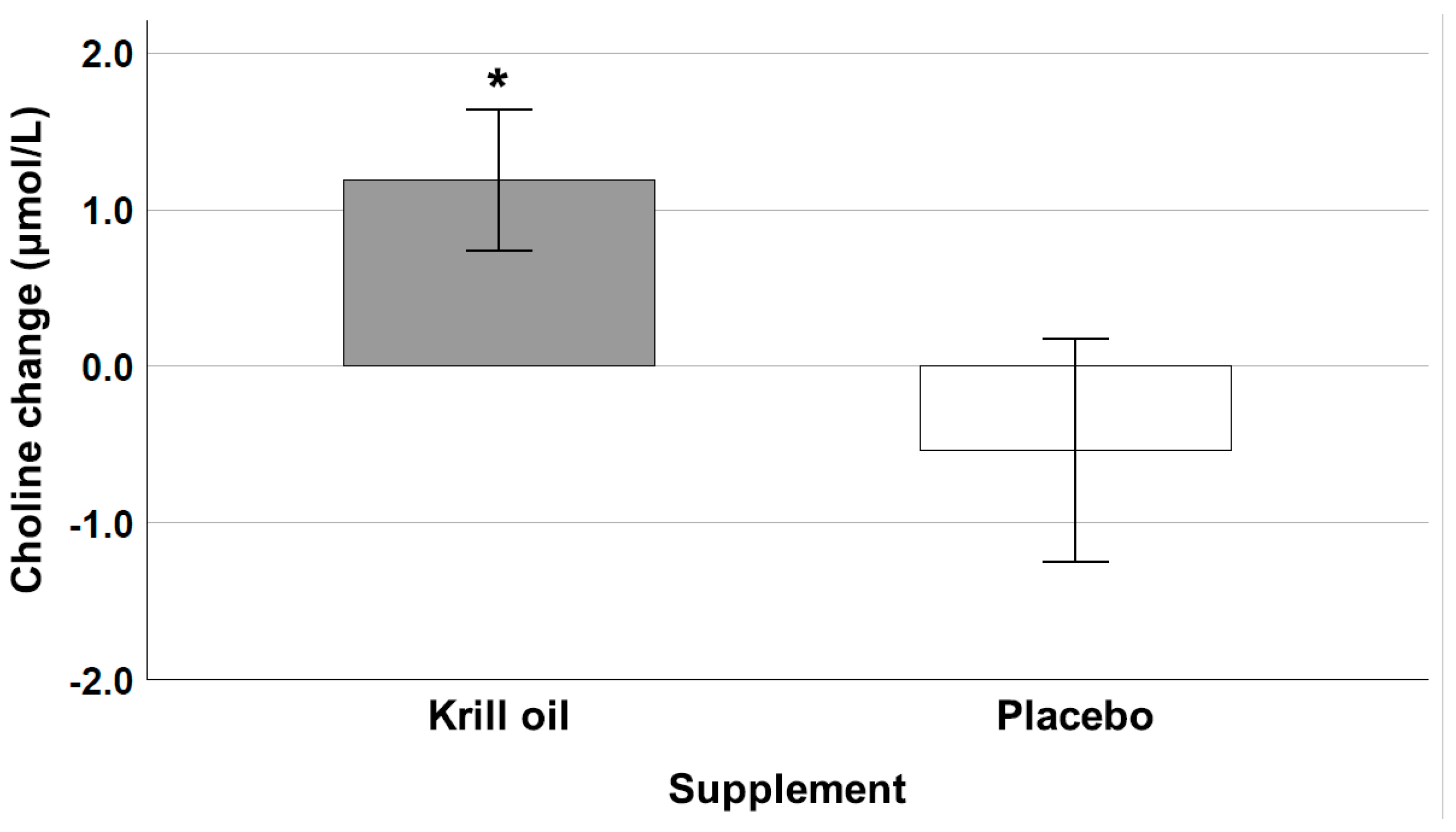
3.5. Choline
3.6. Total Antioxidant Capacity
4. Discussion
- (a)
- Enhanced choline availability
- (b) Optimized HS-Omega-3 Index
- (c) Improved total antioxidant capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butcher, S.J.; Judd, T.B.; Benko, C.R.; Horvey, K.J.; Pshyk, A.D. Relative intensity of two types of CrossFit exercise: Acute circuit and high-intensity interval exercise. J. Fit. Res. 2015, 4, 3–15. [Google Scholar]
- Glassman, G. The CrossFit training guide. Cross Fit J. 2010, 30, 1–115. [Google Scholar]
- Ben-Zeev, T.; Okun, E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. Neuromolecular Med. 2021, 23, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Falk Neto, J.H.; Kennedy, M.D. The multimodal nature of high-intensity functional training: Potential applications to improve sport performance. Sports 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, L.D.; Elliott, B.T.; Yasar, Z.; Bampouras, T.M.; Sculthorpe, N.F.; Sanal-Hayes, N.E.; Hurst, C. High intensity interval training (HIIT) as a potential countermeasure for phenotypic characteristics of sarcopenia: A scoping review. Front. Physiol. 2021, 12, 715044. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; De Sousa, N.M.F. Are extreme conditioning programmes effective and safe? A narrative review of high-intensity functional training methods research paradigms and findings. BMJ Open Sport Exerc. Med. 2018, 4, e000435. [Google Scholar] [CrossRef] [PubMed]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The effects of whey vs. pea protein on physical adaptations following 8-weeks of high-intensity functional training (HIFT): A pilot study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Durkalec-Michalski, K.; Zawieja, E.E.; Podgórski, T.; Łoniewski, I.; Zawieja, B.E.; Warzybok, M.; Jeszka, J. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: A double-blind, randomized cross-over trial. PLoS ONE 2018, 13, e0197480. [Google Scholar] [CrossRef]
- Escobar, K.A.; Morales, J.; Vandusseldorp, T.A. The effect of a moderately low and high carbohydrate intake on crossfit performance. Int. J. Exerc. Sci. 2016, 9, 460. [Google Scholar]
- Kramer, S.J.; Baur, D.A.; Spicer, M.T.; Vukovich, M.D.; Ormsbee, M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sports Nutr. 2016, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.A.; Ramirez, M.; Heinrich, K.M. Acute caffeine supplementation does not improve performance in trained CrossFit® athletes. Sports 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbina, S.; Hayward, S.; Outlaw, J.; Holt, J.; Burks, B.; Cox, B.; Faillace, E.; Stai, B.; Stone, M.; Wildman, R. Performance and body composition effects of a pre-workout supplement and post-workout protein intake in trained crossfit individuals. J. Int. Soc. Sports Nutr. 2013, 10, S1-P28. [Google Scholar] [CrossRef] [Green Version]
- Gogojewicz, A.; Śliwicka, E.; Durkalec-Michalski, K. Assessment of dietary intake and nutritional status in CrossFit-trained individuals: A descriptive study. Int. J. Environ. Res. Public Health 2020, 17, 4772. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.H.; Batres-Marquez, S.P.; Carriquiry, A.; Schalinske, K.L. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J. 2007, 21, LB46. [Google Scholar] [CrossRef]
- Panel, E.N. Scientific opinion on Dietary Reference Values for choline. EFSA J. 2016, 14, 4484. [Google Scholar]
- Jäger, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr. 2007, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kohlmeier, M.; da Costa, K.-A.; Fischer, L.M.; Zeisel, S.H. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 16025–16030. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.L.; Jenden, D.; Roch, M. Plasma free, phospholipid-bound and urinary free choline all decrease during a marathon run and may be associated with impaired performance. J. Am. Coll. Nutr. 1999, 18, 598–601. [Google Scholar] [CrossRef]
- Conlay, L.; Sabounjian, L.; Wurtman, R. Exercise and neuromodulators. Int. J. Sports Med. 1992, 13, S141–S142. [Google Scholar] [CrossRef]
- Piérard, C.; Béracochéa, D.; Pérès, M.; Jouanin, J.-C.; Liscia, P.; Satabin, P.; Martin, S.; Testylier, G.; Guézennec, C.Y.; Beaumont, M. Declarative memory impairments following a military combat course: Parallel neuropsychological and biochemical investigations. Neuropsychobiology 2004, 49, 210–217. [Google Scholar] [CrossRef]
- da Costa, K.-A.; Badea, M.; Fischer, L.M.; Zeisel, S.H. Elevated serum creatine phosphokinase in choline-deficient humans: Mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004, 80, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Klein, J. Membrane breakdown in acute and chronic neurodegeneration: Focus on choline-containing phospholipids. J. Neural Transm. 2000, 107, 1027–1063. [Google Scholar] [CrossRef] [PubMed]
- Penry, J.T.; Manore, M.M. Choline: An important micronutrient for maximal endurance-exercise performance? Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlay, L.; Wurtman, R.; Blusztajn, K.; Coviella, I.; Maher, T.; Evoniuk, G. Decreased plasma choline concentrations in marathon runners. N. Engl. J. Med. 1986, 315, 892. [Google Scholar]
- von Allwörden, H.N.; Horn, S.; Kahl, J.; Feldheim, W. The influence of lecithin on plasma choline concentrations in triathletes and adolescent runners during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 87–91. [Google Scholar] [CrossRef]
- Beckham, G.; Mizuguchi, S.; Carter, C.; Sato, K.; Ramsey, M.; Lamont, H.; Hornsby, G.; Haff, G.; Stone, M. Relationships of isometric mid-thigh pull variables to weightlifting performance. J. Sports Med. Phys. Fit. 2013, 53, 573–581. [Google Scholar]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [Green Version]
- Mödinger, Y.; Schön, C.; Wilhelm, M.; Hals, P.-A. Plasma kinetics of choline and choline metabolites after a single dose of SuperbaBoostTM krill oil or choline bitartrate in healthy volunteers. Nutrients 2019, 11, 2548. [Google Scholar] [CrossRef] [Green Version]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults-a pilot study. Lipids Health Dis. 2015, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Storsve, A.B.; Johnsen, L.; Nyborg, C.; Melau, J.; Hisdal, J.; Burri, L. Effects of krill oil and race distance on serum choline and choline metabolites in triathletes: A field study. Front. Nutr. 2020, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Da Boit, M.; Hunter, A.M.; Gray, S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 2017, 66, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The effect of krill oil supplementation on exercise performance and markers of immune function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Georges, J.; Sharp, M.H.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; Hornberger, T.A.; Harding, F.; Johnson, J.H.; Peele, D.M.; Jäger, R. The effects of krill oil on mTOR signaling and resistance exercise: A pilot study. J. Nutr. Metab. 2018, 2018, 7625981. [Google Scholar] [CrossRef]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef]
- Metcalf, R.G.; Cleland, L.G.; Gibson, R.A.; Roberts-Thomson, K.C.; Edwards, J.R.; Sanders, P.; Stuklis, R.; James, M.J.; Young, G.D. Relation between blood and atrial fatty acids in patients undergoing cardiac bypass surgery. Am. J. Clin. Nutr. 2010, 91, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Schacky, C. The Omega-3 Index as a risk factor for cardiovascular diseases. Prostaglandins Other Lipid Mediat. 2011, 96, 94–98. [Google Scholar] [CrossRef]
- Drobnic, F.; Rueda, F.; Pons, V.; Banquells, M.; Cordobilla, B.; Domingo, J.C. Erythrocyte omega-3 fatty acid content in elite athletes in response to omega-3 supplementation: A dose-response pilot study. J. Lipids 2017, 2017, 1472719. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Burdge, G. α-Linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Alessio, H.M.; Hagerman, A.E.; Fulkerson, B.K.; Ambrose, J.; Rice, R.E.; Wiley, R.L. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med. Sci. Sports Exerc. 2000, 32, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Reid, M.B. Redox interventions to increase exercise performance. J. Physiol. 2016, 594, 5125–5133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzid, M.A.; Filaire, E.; Matran, R.; Robin, S.; Fabre, C. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int. J. Sports Med. 2018, 40, 21–28. [Google Scholar] [CrossRef]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Pilaczyńska-Szcześniak, Ł.; Basta, P.; Foriasz, J.; Arlet, J. Effects of supplementation with Neptune krill oil (euphasia superba) on selected redox parameters and pro-inflammatory markers in athletes during exhaustive exercise. J. Hum. Kinet. 2015, 47, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Jiang, M.; Huang, W.; Liu, S. Antarctic Krill Oil Attenuates Oxidative Stress via the KEAP1-NRF2 Signaling in Patients with Coronary Heart Disease. Evid. Based Complement. Altern. Med. 2020, 2020, 9534137. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52, 382–392. [Google Scholar]
- Earnest, C.P.; Lupo, M.; White, K.; Church, T. Effect of astaxanthin on cycling time trial performance. Int. J. Sports Med. 2011, 32, 882–888. [Google Scholar] [CrossRef]
- Malmsten, C.; Lignell, A. Dietary Supplementation with Astaxanthin-Rich Algal Meal Improves Strength Endurance–A Double Blind Placebo Controlled Study on Male Students. Carotenoid Sci. 2008, 13, 20–22. [Google Scholar]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar]
- Faulkner, J.A. Physiology of Swimming and Diving; Exercise Physiology; Academic Press: Baltimore, MD, USA, 1968. [Google Scholar]
- Yuhasz, M.S. The Effects of Sports Training on Body Fat in Man with Predictions of Optimal Body Weight; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 1962. [Google Scholar]
- Midttun, Ø.; Kvalheim, G.; Ueland, P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Yan, J.; Caudill, M.A. Choline and one-carbon metabolite response to egg, beef and fish among healthy young men: A short-term randomized clinical study. Clin. Nutr. Exp. 2016, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Kanter, M.M.; Williams, M.H. Antioxidants, carnitine, and choline as putative ergogenic aids. Int. J. Sport Nutr. Exerc. Metab. 1995, 5, S120–S131. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), maintenance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360),“maternal health; pregnancy and nursing”(ID 514),“to fulfil increased omega-3 fatty acids need during pregnancy”(ID 539),“skin and digestive tract epithelial cells maintenance”(ID 525), enhancement of mood (ID 536),“membranes cell structure”(ID 4295),“anti-inflammatory action”(ID 4688) and maintenance of normal blood LDL-cholesterol concentrations (ID 4719) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2078. [Google Scholar]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n–3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siscovick, D.S.; Raghunathan, T.; King, I.; Weinmann, S.; Wicklund, K.G.; Albright, J.; Bovbjerg, V.; Arbogast, P.; Smith, H.; Kushi, L.H. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995, 274, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: A decade in review. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmied, C.; Borjesson, M. Sudden cardiac death in athletes. J. Intern. Med. 2014, 275, 93–103. [Google Scholar] [CrossRef]
- de Groot, R.H.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans–A systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Corbi, G.; Righetti, S.; Casiraghi, E.; Chiappero, F.; Martegani, S.; Pina, R.; De Vivo, I.; Simopoulos, A.P.; Scapagnini, G. Relationship between distance run per week, omega-3 index, and arachidonic acid (AA)/Eicosapentaenoic acid (EPA) ratio: An observational retrospective study in non-elite runners. Front. Physiol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Tepsic, J.; Vucic, V.; Arsic, A.; Blazencic-Mladenovic, V.; Mazic, S.; Glibetic, M. Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur. J. Appl. Physiol. 2009, 107, 359–365. [Google Scholar] [CrossRef]
- Anzalone, A.; Carbuhn, A.; Jones, L.; Gallop, A.; Smith, A.; Johnson, P.; Swearingen, L.; Moore, C.; Rimer, E.; McBeth, J. The omega-3 index in National Collegiate Athletic Association division I collegiate football athletes. J. Athl. Train. 2019, 54, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritz, P.P.; Rogers, M.B.; Zabinsky, J.S.; Hedrick, V.E.; Rockwell, J.A.; Rimer, E.G.; Kostelnik, S.B.; Hulver, M.W.; Rockwell, M.S. Dietary and biological assessment of the Omega-3 status of collegiate athletes: A cross-sectional analysis. PLoS ONE 2020, 15, e0228834. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C.; Kemper, M.; Haslbauer, R.; Halle, M. Low omega-3 index in 106 German elite winter endurance athletes: A pilot study. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.B.; Madrigal, L.A. Associations between whole blood and dietary omega-3 polyunsaturated fatty acid levels in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 497–505. [Google Scholar] [CrossRef]
- Tiryaki-Sönmez, G.; Schoenfeld, B.; Vatansever-Ozen, S. Omega-3 fatty acids and exercise: A review of their combined effects on body composition and physical performance. Biomed. Hum. Kinet. 2011, 3, 23. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C. Exercise effects on systemic immunity. Immunol. Cell Biol. 2000, 78, 496–501. [Google Scholar] [CrossRef]
- Ninomiya, T.; Nagata, M.; Hata, J.; Hirakawa, Y.; Ozawa, M.; Yoshida, D.; Ohara, T.; Kishimoto, H.; Mukai, N.; Fukuhara, M. Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: The Hisayama Study. Atherosclerosis 2013, 231, 261–267. [Google Scholar] [CrossRef]
| Product | Krill Oil | Placebo |
|---|---|---|
| Product Name | NKOTM krill oil | Virgin olive oil |
| Manufacturer | Aker BioMarine, Norway | Lonza Group, Switzerland |
| Amount per capsule (mg) | 500 | 500 |
| Capsules per day | 5 | 5 |
| Weeks of administration | 12 | 12 |
| EPA/DHA (g/100 g) | 16/6 | <1/<1 |
| Total phospholipids (g/100 g) | 46 | <1 |
| Choline (g/100 g) | 6 | <1 |
| Esterified astaxanthin (mg/kg) | 691 | <1 |
| Time | Phase | Activity | |
|---|---|---|---|
 | 3 months | Subject selection and characterization | 1. Subject selection according to eligibility criteria |
| 2. Subject characterization (medical examination, ECG, max exercise test, anthropometry, standard blood analysis) | |||
| 15 days | Group determination | ||
| 1 day | First training day | 3. Blood analysis:Omega-3 Index, TAC, inflammatory parameters | |
| 4. Power training session (Heart rate, lactate, RPE) | |||
| 5. Blood analysis: TAC, inflammatory parameters | |||
| 12 weeks | Supplementation period: Placebo (n = 16) / Krill oil (n = 19) | ||
| 1 day | Second training day | 3. Blood analysis:Omega-3 Index, TAC, inflammatory parameters | |
| 4. Power training session (Heart rate, lactate, RPE) | |||
| 5. Blood analysis: TAC, inflammatory parameters | |||
| Product | Krill Oil | Placebo |
|---|---|---|
| n (male/female) | 19 (16/3) | 16 (11/5) |
| Age (years) | 32.5 ± 9.6 | 33.7 ± 8.0 |
| BMI (m/kg2) | 23.9 ± 2.4 | 24.3 ± 2.7 |
| Body fat (%) | 11.0 ± 2.6 | 11.2 ± 2.6 |
| Resting heart rate (bpm) | 65.9 ± 9.55 | 70.8 ± 13.7 |
| Maximum heart rate (bpm) | 183.6 ± 11.9 | 182.9 ± 8.9 |
| Maximal oxygen consumption (VO2max ml/min/kg) | 47.9 ± 5.6 | 44.9 ± 4.9 |
| Heart rate after 1 min of recovery | 149 ± 15 | 149 ± 18 |
| Fatty Acid (%) | Suppl. | Pre | Post | Fatty Acid (%) | Suppl. | Pre | Post | |
|---|---|---|---|---|---|---|---|---|
| Polyunsaturated | 18:3 n-3 (ALA) | Krill oil | 0.08 | 0.08 | ∑Trans | Krill oil | 0.61 | 0.58 |
| Placebo | 0.08 | 0.08 | Placebo | 0.61 | 0.59 | |||
| 20:5 n-3 (EPA) | Krill oil | 0.41 | 1.34 **b | ∑Saturated | Krill oil | 38.84 | 38.69 | |
| Placebo | 0.44 | 0.40 | Placebo | 39.24 | 38.96 | |||
| 22:5 n-3 (DPA) | Krill oil | 1.60 | 2.46 **b | ∑Monounsaturated | Krill oil | 18.29 | 18.13 | |
| Placebo | 1.65 | 1.65 | Placebo | 17.90 | 17.96 | |||
| 22:6 n-3 (DHA) | Krill oil | 4.41 | 5.31 **a | |||||
| Placebo | 4.72 | 4.71 | ||||||
| 18:2 n-6 (linoleic) | Krill oil | 13.41 | 13.05 | |||||
| Placebo | 13.68 | 13.30 | ||||||
| 20:4 n-6 (ARA) | Krill oil | 15.76 | 15.10 * | |||||
| Placebo | 15.76 | 16.24 a | ||||||
| ∑n-3 | Krill oil | 6.51 | 9.18 **b | |||||
| Placebo | 6.90 | 6.83 | ||||||
| ∑n-6 | Krill oil | 35.76 | 33.42 ** | |||||
| Placebo | 35.36 | 35.66 b | ||||||
| n-6/n-3 | Krill oil | 5.84 | 3.77 ** | |||||
| Placebo | 5.27 | 5.37 b | ||||||
| ARA/EPA | Krill oil | 50.72 | 13.61 ** | |||||
| Placebo | 41.33 | 48.41 b | ||||||
| First Training Session | Second Training Session | |||
|---|---|---|---|---|
| Krill Oil | Placebo | Krill Oil | Placebo | |
| Subject number | 14 | 8 | 14 | 8 |
| HR 20 (beats/min) | 173 (9) | 171 (12) | 168 (11) | 166 (13) |
| Lactate (mM/mL) | 8.2 (2.2) | 8.5 (3.1) | 8.8 (2.1) | 7.3 (2.3) |
| HR 40 (beats/min) | 184 (7) | 179 (9) | 179 (8) | 178 (10) |
| Lactate (mM/mL) | 11.6 (1.8) | 12.3 (3.1) | 12.8 (2.0) | 11.6 (3.3) |
| RPE | 8.3 (0.7) | 8.4 (0.6) | 8.3 (0.9) | 8.5 (0.7) |
| Pre-Supplementation | Post-Supplementation | |||||
|---|---|---|---|---|---|---|
| Before 1st Session | After 1st Session | Δ%1 | Before 2nd Session | After 2nd Session | Δ%2 | |
| Placebo (n = 8) | 186.0 (±24.9) | 140.7 (±28.0) | −24.4 (±9.8) | 179.0 (±58.5) | 141.5 (±44.4) | −15.8 (±31.0) |
| Krill oil (n = 14) | 158.8 (±34.6) | 115.4 (±28.2) | −25.2 (±20.3) | 149.2 (±45.8) | 137.2 (±43.7) | −0.2 (±38.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobnic, F.; Storsve, A.B.; Burri, L.; Ding, Y.; Banquells, M.; Riera, J.; Björk, P.; Ferrer-Roca, V.; Domingo, J.C. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients 2021, 13, 4237. https://doi.org/10.3390/nu13124237
Drobnic F, Storsve AB, Burri L, Ding Y, Banquells M, Riera J, Björk P, Ferrer-Roca V, Domingo JC. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients. 2021; 13(12):4237. https://doi.org/10.3390/nu13124237
Chicago/Turabian StyleDrobnic, Franchek, Andreas B. Storsve, Lena Burri, Yunpeng Ding, Montserrat Banquells, Joan Riera, Per Björk, Ventura Ferrer-Roca, and Joan Carles Domingo. 2021. "Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes" Nutrients 13, no. 12: 4237. https://doi.org/10.3390/nu13124237
APA StyleDrobnic, F., Storsve, A. B., Burri, L., Ding, Y., Banquells, M., Riera, J., Björk, P., Ferrer-Roca, V., & Domingo, J. C. (2021). Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients, 13(12), 4237. https://doi.org/10.3390/nu13124237






