Abstract
Carbohydrate (CHO) supplementation during prolonged exercise postpones fatigue. However, the optimum administration timing, dosage, type of CHO intake, and possible interaction of the ergogenic effect with athletes’ cardiorespiratory fitness (CRF) are not clear. Ninety-six studies (from relevant databases based on predefined eligibility criteria) were selected for meta-analysis to investigate the acute effect of ≤20% CHO solutions on prolonged exercise performance. The between-subject standardized mean difference [SMD = ([mean post-value treatment group–mean post-value control group]/pooled variance)] was assessed. Overall, SMD [95% CI] of 0.43 [0.35, 0.51] was significant (p < 0.001). Subgroup analysis showed that SMD was reduced as the subjects’ CRF level increased, with a 6–8% CHO solution composed of GL:FRU improving performance (exercise: 1–4 h); administration during the event led to a superior performance compared to administration before the exercise, with a 6–8% single-source CHO solution increasing performance in intermittent and ‘stop and start’ sports and an ~6% CHO solution appearing beneficial for 45–60 min exercises, but there were no significant differences between subjects’ gender and age groups, varied CHO concentrations, doses, or types in the effect measurement. The evidence found was sound enough to support the hypothesis that CHO solutions, when ingested during endurance exercise, have ergogenic action and a possible crossover interaction with the subject’s CRF.
1. Introduction
Interest in the role and contribution of carbohydrates (CHOs) as an energy fuel, particularly during endurance exercise, dates back to the beginning of the 20th century [1]. Christensen and Hansen (1939) examined the role of a high-CHO diet and suggested that hypoglycemia causes fatigue during light exercise by affecting the central nervous system (CNS) [2]. In the late 1960s, it was revealed that exercise with glycogen depletion increases the resynthesis of muscle glycogen [3] and also that stored muscle glycogen plays a significant role during exercise [4]. Muscle glycogen stores are primly defined by diet prior to exercise. It has also been shown that the higher the muscle glycogen content, the higher the endurance performance [4,5]. In 1975, a study revealed that CHO feeding during prolonged exercise could increase exercise capacity, which was confirmed by another study in 1983 [5,6]. As scientific interest in the field of dietary supplements gradually grew, during the past 45 years, a great number of researchers have extensively investigated the effects of CHO consumption during endurance exercise, mostly from the perspective of determining the optimal composition and timing of CHO replacement beverages during exercise. Thus, the effect of CHO ingestion during endurance exercise has been reviewed by a great number of authors in the past and also in recent years [7,8,9,10,11], focusing on different areas of the CHO effect on performance.
It is widely accepted that endurance exercise requires a sufficient exogenous amount of CHO to postpone the onset of fatigue; when the CHO quantity is inadequate, performance is impaired [9,10,12]. However, many theories are still the subject of debate, and the conclusions of the relevant reviews have led to conflicting information about the optimum administration timing, dosage, type, and composition of CHO supplements [7,8,9,10,11]. For instance, four modern reviews recommend CHO intake up to 60 g·h−1 for exercise lasting up to 2.5 h and up to 90 g·h−1 when the duration of exercise exceeds 2.5 h [7,8,9]. Nevertheless, Mata et al. (2019) concluded that it is unclear which concentration (6, 8, or 10% etc.) or dose of CHO solution and which CHO substance (maltodextrin (MD), glucose (GL), sucrose (SUC), or a combination) enhance endurance performance better [10]. They also mentioned that “attending to the existing evidence, no universal recommendations regarding CHO intake should suggested to athletes” [10]. In a simplified approach, Brooks (2020) states that as gastrointestinal (GI) emptying and absorption are determinant factors of exogenous glucose availability, beverages containing 4−6% GL could be efficient during exercise for euglycemia maintenance, while GL solutions > 6% are often less effective and blamed for GI discomfort [11]. It is understood, however, that the studies varied in method, exercise duration, performance assessment (e.g., capacity: time to exhaustion vs. performance: fix distance), and total quantity of CHO that was administered, which may explain any inconsistency in the literature.
Unexpectedly, no systematic review or meta-analysis has taken into account the possible effect of the cardio-respiratory fitness (CRF) of the tested subjects on the CHO intake intervention. An extensive comparative and updated review on the effect of CHO supplements on different types of exercise, such as cycling vs. running, also appears to be lacking. Thermal stress-induced energy metabolic changes during prolonged exercise of different modes and intensities are likely to be different [13,14]; yet, the ergogenic role of CHO supplementation in different ambient conditions has not been reviewed in a meta-analysis. Additionally, with only a few exceptions, most past reviews more or less failed to mention how the search for relevant studies was carried out, what the inclusion criteria were for the studies and whether they were appropriate, whether the validity of included studies was assessed, whether the methods and statistics were reliable and appropriate, how conclusions were reached, whether results were explicit, and how the studies were integrated, which can elicit overestimation or underestimation of the intervention effect [15]. No meta-analysis has examined the effect of CHO ingestion with no additives (e.g., caffeine, protein) during endurance exercise on endurance capacity and performance, with the use of an accurate method of combining the results of independent studies, assessing risk-of-bias, and considering the potential limitations of the eligible studies [15].
Therefore, the purpose of this study was to select relevant papers from a specific period (1975–2021) for a systematic/critical review using a meta-analytic technique. It is certain that athletes, coaches, and training instructors will be interested in seeing the actual CHO ergogenic supplementations’ effect on different exercise modalities in association with supplement composition, concentration, administration time, and exercise duration; a systematic review conducted according to predefined methodological criteria will surely be beneficial to this audience. Therefore, the aim of this study was to investigate whether the literature supports the hypothesis that CHO supplementation in a liquid form during exercise enhances performance, taking into account the subjects’ CRF and the relative methodological quality of the papers searched. A further aim was to establish the optimum administration time and the optimal composition and concentration of CHO replacement during endurance exercise, and to contribute to resolving the controversy posed by the previous reviewers’ relevant conflicting findings. On the other hand, this study focused mainly on the scientific evidence for the efficacy of CHO supplementation rather than on understanding the mechanism/s involved.
2. Materials and Methods
2.1. Search Strategy
For the purposes of the present systematic review, a meta-analysis was conducted, based on the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) and Cochrane Handbook for Systematic Reviews of Interventions statements guidelines [16,17]. Figure 1 outlines a summary of the procedures followed in this study.
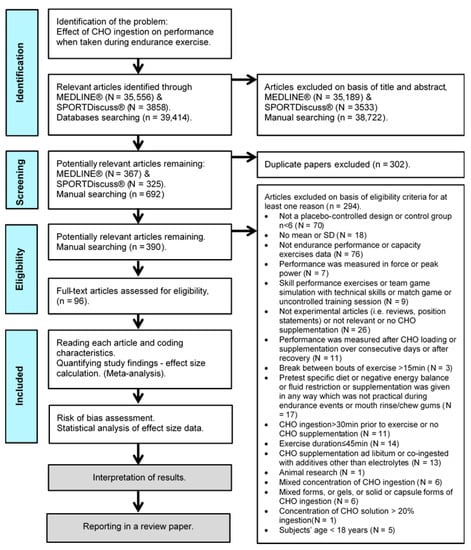
Figure 1.
Prisma flowchart of the study selection process [16].
2.2. Databases and Search
The initial electronic literature review was carried out by searching the MEDLINE® (via PubMed®), and SPORTDiscus® (via EBSCOhost) databases until April 2021. We restricted our review to studies published since 1975, which, to our knowledge, was the year of publication of the first study showing that CHO feeding during prolonged exercise improves exercise capacity [5]. We did not review any study dating from August 2021 to the article submission day. We used the Boolean search syntax ((carbohydrate* OR CHO*) AND (endurance OR performance OR capacity OR exercise OR timing)). Full-text studies chosen were limited to those published in English peer-reviewed journals, with human subjects used. Additionally, control clinical trial OR/AND randomized clinical trial filters were activated, and a total of 36,605 studies from MEDLINE® and 3956 studies from SPORTDiscus® were found.
2.3. Eligibility Criteria
From the preliminary articles originally identified through the search of the electronic databases, all irrelevant articles were manually excluded based on their title and abstract. A number of studies were manually picked through predefined eligibility criteria by (two independent: E.Z. and G.M.) reviewers, who screened the potentially relevant papers by checking their titles, abstracts, methods, and results. In order to avoid risk of bias in selecting and rejecting papers, reviewers looked first at methods and then at the results.
The independent variables were: (a) the contents (e.g., GL, FRU) of a liquid form of supplementation only (i.e., CHO ≤ 20%, e.g., solution, drink, beverage); (b) the CHO concentration (%) or the dose (i.e., ingestion rate (CHO g·h−1)); and (c) the timing of CHO supplement ingestion during an endurance exercise (at regular intervals or single bolus dose), which was not less than 30 min before the beginning of the event until its end. The dependent variable was defined as the effect of CHO supplementation on endurance performance, lasting ≥ 45 min (time to exhaustion or time to complete a certain distance in events of variable duration and intensity). The rationale for initially restricting attention to exercise duration effort ≥ 45 min was that endogenous muscle glycogen is not fully depleted in 1-h all-out exercises [18]. During an exercise (>85% O2max) performed continuously for 20–30 min, fatigue in skeletal muscle is caused by an increased accumulation of H⁺ [19]. Thirdly, the ergogenic influence of a CHO solution on an intense exercise of a relatively short duration may be partially explained by the solution’s stimulus on the brain via mouth receptors sensitive to CHO; thus, the ergogenic effect may be not exclusively metabolic in nature but could also be attributed to the CNS [7].
Only papers of controlled interventions, where the authors reported that they used a specific experimental method, were chosen. When an article contained more than one research arm that qualified for inclusion, they were regarded as separate ‘interventions’ denoted as ‘trials’. In particular, studies or trials that did not involve a comparison group in a parallel or crossover design [20], single-subject design studies, studies that used sample sizes (N) less than six subjects per group, and studies that did not provide the numerical means (not depicted) and standard deviation (SD) or standard error (SE = SD divided by the square root of the sample size) for the dependent variable were not included. Editorials, letters to the editor, government’s reports, grey literature, or abstracts or scientific events or other articles indexed by non-scientific databases (not peer-reviewed) that did not contain original results were also excluded from this review. Original studies that reported the use of healthy human subjects (age ≥ 18 years) and were relevant to the topic of interest, i.e., where appropriate, independent and dependent variables could be defined from the article’s title and abstract, were included.
Additionally excluded were studies with substantial rest intervals (>15 min), or CHO supplementation given during recovery from exercise, during team games, during uncontrolled training sessions, during an exercise protocol that included technical sport drills (e.g., ball drills) designed to simulate a fast-paced game, or supplementation administered in many different concentrations or given in solid (e.g., chocolate, energy bar, pudding), gel, capsule, or mixed forms (e.g., solution co-ingested with gel), intravenously, or in any other way, which was not practical during endurance events. Studies that used an intermittent exercise protocol designed to simulate a fast-paced game or ‘stop and start’ sports, using only running, jogging, and walking activities, were included. Studies that used CHO supplementation in combination with electrolytes were chosen when the control group was also provided. Studies that used CHO supplementation: ad libitum, co-ingested with additives (i.e., any neurological stimulant (e.g., caffeine), neurotransmitter or neuromodulator (e.g., taurine), NAD⁺ precursor (e.g., nicotinic acid), substances that may have synergetic effect or are advocated in the medical literature for muscle fatigue reduction after exercise or boosting metabolism (e.g., vitamins, chromium picolinate, carnitine), and potential energetic substrate (e.g., fat, protein) [21,22,23]), in fasted state (except overnight fasting) or in negative energy balance, after pre-experimental CHO preloading or any kind of enriched or specific diet other than normal were excluded. Studies that exclusively used a fluid restriction protocol (pre supplementation or during exercise) or a protocol to investigate during exercise the effects on the hormonal response, immune response, gastric emptying, GI problems, GL oxidation, heart rate, rate of perceived exertion, cognitive performance, reaction time, resistance exercise, peak power, velocity, force, torque, energy cost or technical skills, and CHO mouth rinse or CHO chewing gum response after treatment were also excluded. Finally, after the removal of duplicate articles, 294 articles were excluded and 96 selected for further analysis (Figure 1).
2.4. Data Extraction
The next step was to code the characteristics and outcomes of the selected studies that were likely to influence the true intervention effect sizes [17]. Characteristics provided descriptive information about the study with the following categories: design (e.g., randomization, control/no control, statistical analysis…), protocol test (e.g., continuous, intermittent…), endurance exercise mode (e.g., cycling, running, swimming…), treatment variable (e.g., supplementation that was used, concentration, dose, administration time, composition, form…), subjects (e.g., gender, age, CRF, …), dependent variable (e.g., performance time, exhaustion time …), and environmental conditions (e.g., temperature, altitude …). No contact was made with the studies’ authors. Two reviewers (A.K. and K.P.) independently processed data extraction from the initially selected studies. Disagreements between reviewers with regard to including or excluding data of a given study were resolved by consensus.
2.5. Risk-of-Bias Assessment and Deficiencies in Scientific Design or Reporting
A modified version of the Cochrane risk-of-bias assessment tool for systematic reviews was employed to assess potential risk-of-bias in the eligible studies [24]. The modification was based on empirical evidence showing that they have a biasing effect on the estimates of a treatment’s effectiveness. This evidence derived from previous systematic reviews [25,26,27,28] and their importance to the reviewer in determining whether confidence should be placed on the author’s conclusions based on the CONsolidated Standards of Reporting Trials 2010 guidelines [29,30]. Nine risk-of-bias items were used for all eligible studies (i.e., eligibility criteria; statistical power calculation; subject’s familiarization; time series control of treatment allocation over the study period; subjects blinded to treatment; researchers blinded to treatment; reliability of measures; validity of measures; complication or dropout ≥ 15%), which were graded as low (+), some concerns (?), and high (−) risk-of-bias. Two external researchers (K.P. and A.K.), unaware of this study’s purpose and of any data that could help identify the studies’ authorship (e.g., authors’ names and affiliations, year, and type of publication), assessed the studies’ risk-of-bias based on answers to the signaling questions independently [24]. Any disagreement between the two researchers was resolved by discussion; if no consensus could be reached, a third researcher (T.T.) made the final decision.
2.6. Statistical Analyses
Data were analyzed using the Review Manager software, Version 5.3.4. (Cochrane Collaboration Copenhagen, The Nordic Cochrane Centre, Copenhagen, Denmark). The effect size (ES) of CHO supplementation on exercise performance was calculated as the between-subject standardized mean difference (SMD = ([mean post-value treatment group–mean post-value control group]/pooled variance)). Due to the nature of the test performance assessment, performance mean data were inserted into Review Manager software for analysis in a negative way (i.e., multiplied by −1) so that both studies (time-to-exhaustion and self-paced time-trial) corresponded to the same direction by means of the effect size in performance enhancement [32,33,34]. Studies with small sample sizes have a biased ES [35], and thus, each SMD was multiplied by a correction factor (g) to allow an unbiased estimate of ES [36,37,38]. The correction factor was calculated from the formula: g = [1 − 3/(4Ni-9)], where, Ni = pooled sample size. According to Higgins et al., (2011), SMD values of 0.2–0.4 indicate a small effect, 0.5–0.7 indicate a medium effect, and >0.8 indicate a large effect [15].
Furthermore, subgroup analysis was carried out to see if they had an advantage effect on their own and also to identify aspects of any possible study heterogeneity. Depending on the related published studies’ data, study trials were classified as follows: (a) with regard to the subjects’ characteristics, into three gender classes (male (M), female (F), combined (MF)), four age classes (young (18–29 years), adults I (30–39 years), adults II (40–49 years) [39]), and four CRF classes based on maximal oxygen uptake data (fair, good, excellent, superior) [40]; (b) with regard to exercise task, into three exercise mode classes (cycling, running, other (triathlon, duathlon, swimming, walking, loaded marching, roller-skiing)), two exercise protocol test classes (capacity: time to exhaustion, performance: time trial), three exercise type classes (intermittent: sessions interspersed with short rest or recovery periods involve activity of lower intensity; continuous: no break between sessions, regardless of the intensity of the sessions; intermittent shuttle: sessions that simulate the activity pattern of ‘stop and start’ sports), and four exercise time classes ((T), (45 min ≤ T ≤ 60 min, 60 min < T ≤ 120 min, 120 min < T ≤ 240 min, T > 240 min)); (c) with regard to supplementation, into seven CHO concentration classes (0% < CHO ≤ 2%, 2% < CHO ≤ 4%, 4% < CHO ≤ 6%, 6% < CHO ≤ 8%, 8% < CHO ≤ 10%, 10% < CHO ≤ 15%, 15% < CHO ≤ 20%), five CHO dose classes (CHO dose ≤ 40 g·h−1, 40 g·h−1 < CHO dose ≤ 60 g·h−1, 60 g·h−1 < CHO dose ≤ 80 g·h−1, 80 g·h−1 < CHO dose ≤ 100 g·h−1, CHO dose > 100 g·h−1), six CHO type classes (GL, MD, SUC, maltose (MAL), FRU, galactose (GAL)), 12 multiple transportable CHO classes ((MTC), (GL:FRU, GL:SUC, GL:MD, MD:FRU, MD:Dextrose (DEX), MD:SUC, GL:MD:FRU, GL:MD:DEX, GL:SUC:FRU, GL:MD:MAL:Saccharides, SUC:MD:IsoMAL, unclear CHO substances mixture), three CHO solution formulation classes (single-source CHO solution, double-source CHO solution, triple-or-more-source CHO solution), four administration time classes (prior to or at the beginning, during, prior to or at the beginning + during, late in exercise), and two supplement temperature administration classes (cool (<18 °C), neutral (18–26 °C)); and d) with regard to ambient conditions, into three thermal condition classes (cool (<18 °C), neutral (18–26 °C), heat (>26 °C)).
An assessment of the consistency of effects across eligible studies in the subgroups was also carried out. The between-study heterogeneity was assessed using the I² statistic and the Chi-square test. According to Higgins et al. (2003) and Sedgwick (2015), values of the I2 statistic of 0–50% represent low heterogeneity, 50–74% moderate heterogeneity, and ≥75% high heterogeneity [41,42]. It was assumed that subgrouped studies were characterized by a high degree of homogeneity due to similar clinical and methodological aspects. Consequently, SMD could be compared to find possible differences between treatments and controlled with a fixed-effect meta-analysis model (estimating the same underlying intervention effect) by means of the inverse-variance method when I2 < 50% [43]. However, the fixed-effects analysis may not be the proper option to account for the accessible random-effects within the analysis. On the other hand, irrespective of the I2 statistics, the random-effect meta-analysis model (which is usually used in case of I2 ≥ 50%) may provide a more conservative estimate that may be viewed as an ‘average intervention effect’ [44] and thus it was programmed to compute the pooled effect size by calculating the SMD in the current study. Nevertheless, the decision between fixed- and random-effects meta-analyses has been the subject of much debate and since many authors have argued that a fixed-effect analysis can be interpreted in the presence of heterogeneity, and that it makes fewer assumptions than a random-effects meta-analysis [45,46], Cochrane organization does not provide a universal recommendation [17]. For this reason, based on fixed-effects analysis assumptions [45,46] and Cochrane organization recommendations (that it may be reasonable to present both analyses) [17], we also ran fixed-effects analysis and report these results, only in two cases, where the presence of homogeneity (I2 < 50%) supported our actions and better interpreted our well-founded assumptions. So, the outcomes of this meta-analysis are derived from the random-effect meta-analysis model throughout the paper except otherwise stated. The presence or not of publication bias was investigated by funnel plots using the Review Manager software, Version 5.3.4. (Cochrane Collaboration Copenhagen, The Nordic Cochrane Centre, Copenhagen, Denmark) and Egger’s regression analysis using the Meta-Essentials tools (Rotterdam School of Management, Erasmus University, The Netherlands).
The descriptive data of the eligible studies is presented as means. Pooled estimates of the ES derived by either subgroup or comprehensive meta-analyses are presented as SMD and 95% confidence intervals (CIs) in square brackets (SMD [95% CI]). The alpha level for statistical significance was set at p ≤ 0.05 a priori.
3. Results
3.1. Study Characteristics
An overview, along with the subjects’ characteristics, descriptive characteristics of the protocols used, and the effect (in SMD [95% CI]) of experimental CHO supplementation as compared to the control on an exercise task of the reviewed articles included in this meta-analysis, is reported in alphabetical order, by first author surname, in Table 1. The comparison of experimental CHO supplementation vs. control on exercise outcome, including raw data (in mean and SD [95% CI]) and risk-of-bias judgments of all trials is presented in a forest plot (Figure S1). The overview authors’ judgments for each risk-of-bias item are depicted as a percentage in Figure 2.

Table 1.
Overview of the reviewed articles and the effect of experimental carbohydrate supplementation as compared to a control on an exercise task in SMD with 95% CI. Data presented as mean or range or otherwise stated. Where not reported in the articles, sufficient averaged data per sex subgroups and averaged pooled values for mixed sample groups are referred to, respectively.
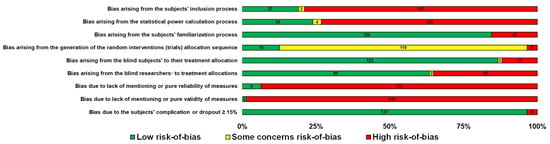
Figure 2.
Overview of the authors’ judgments for each risk-of-bias item as a percentage in 142 trials.
In total, 96 studies (142 trials) involving 1560 subjects in experimental groups (1534 in control groups) satisfied the inclusion criteria and were approved for further analysis. So, 142 SMDs were assessed and the total SMD [95% CI] estimate of 0.43 [0.35, 0.51] was significant, (p < 0.001). All studies used similar methods (pre-post design). The sample population of the control groups used was usually identical to that of the experimental groups in a crossover design, with the exception of three studies, which used a different sample population as control groups in a parallel design (Table 1). The participating subjects were mainly male, and their mean age was 19.3–44.0 (Table 1). Eighty-nine studies were lab-based and only seven field-based. The sport types of exercise used in the trials were cycling, running, triathlon, duathlon, roller-skiing, walking, loaded marching, swimming, and arm cranking (100, 35, 2, 1, 1, 1, 1, and 1, respectively; Table 1).
3.2. Effect of Carbohydrate Supplementation
Subgroup analysis showed that there were no significant differences between the age classes and subjects’ gender in the effect measurement (Figures S2 and S3). However, it is worth mentioning that although there is a tendency of the effect size to be reduced as the subjects’ CRF level increases according to the random-effects analysis (Figure 3), the fixed-effects analysis shows a significant effect size reduction as the subjects’ CRF level increases (p = 0.03, Figure S4).
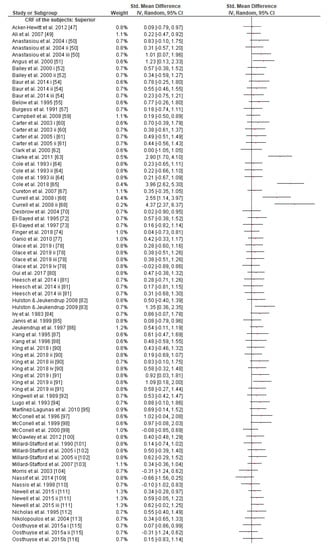
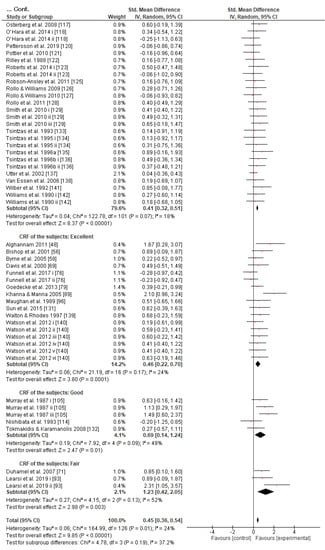
Figure 3.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 127 interventions [47,48,49,50,51,52,54,55,56,57,58,59,60,61,62,63,64,65,67,68,69,70,71,72,73,74,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,109,110,111,112,113,114,115,116,117,118,120,121,122,123,125,126,127,128,129,131,133,134,135,136,137,138,139,140,141,142]. Subgroup analyses show the results with regards to the exercise task in four exercise duration time groups (45 min ≤ T ≤ 60 min, 60 min < T ≤ 120 min, 120 min < T ≤ 240 min, T > 240 min). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; T, time; i–vi denote different intervention arms (trials) within the same study.
Regarding the exercise task, subgroup analysis showed that there were no significant differences between exercise modes, protocol tests, or type classes in the effect measurement (Figures S5–S7). What is remarkable, however, is the greater SMD in the cycling (0.47 [0.38, 0.57]) compared to running mode (0.35 [0.17, 0.52]) subgroup classes, though the effect is not significantly different. The effect of CHO interventions compared to control trials in the four exercise task duration time classes was significantly different between classes, (p ≤ 0.05, Figure 4). This revealed the advantageous role of CHO supplementation when CHO supplements are ingested during endurance exercise lasting 1–2 h (0.41 [0.27, 0.55]) or 2–4 h (0.51 [0.40, 0.62]) in comparison to exercise sessions lasting less than 1 h (0.15 [−0.13, 0.43]) or more than 4 h (0.19 [−0.16, 0.55]).
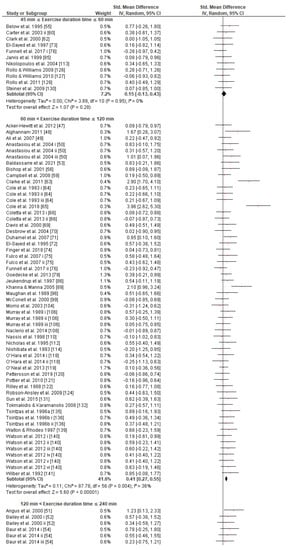
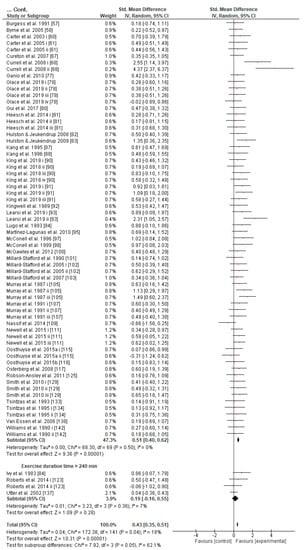
Figure 4.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 142 interventions [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142]. Subgroup analyses show the results with regards to the exercise task in four exercise duration time groups (45 min ≤ T ≤ 60 min, 60 min T ≤ 120 min, 120 min T ≤ 240 min, T 240 min). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; T, time; i–vi denote different intervention arms (trials) within the same study.
Concerning the CHO supplementation, subgroup analysis showed that there were no significant differences in the SMD between different CHO supplementation concentrations, doses, types, composites, formulations, administration times, and temperatures of supplement administration classes (Figures S8–S13). It was, however, found by the random-effects analysis that CHO supplementation composed of GL:FRU has a slight tendency for a greater effect on performance in comparison to other MTC compositions (marginally insignificant, Figure 5). This tendency becomes a significantly greater effect of GL:FRU formation on performance in comparison to other MTC compositions using the fixed-effects analysis model, (p = 0.04, Figure S14). Further subgroup analysis showed that the effect measurement of the CHO supplement ingested was significantly higher when a double-source CHO solution formulation (0.57 [0.37, 0.76]) was used, in comparison to a triple-or-more-source CHO solution formulation (0.30 [0.11, 0.49]), (p ≤ 0.05; Figure 6). Regarding the administration of high CHO doses, the effect of CHO dose at rates > 100 g·h−1 had a significantly lower effect measurement (0.17 [−0.23, 0.57]) in comparison to dose rates of 81–100 g·h−1 (0.82 [0.31, 1.34]), (p ≤ 0.05, Figure 7). Moreover, CHO supplementation during exercise had a significantly higher effect (0.47 [0.37, 0.58]) in comparison to CHO supplementation when administered prior to or at the beginning of the exercise (0.12 [−0.21, 0.44]) (p ≤ 0.05, Figure 8). Lastly, not enough evidence was found to confirm or reject the hypothesis that the absence of differences in the effect measurement between different ambient thermal conditions after CHO supplementation was due to the limited studies available (Figure S15).
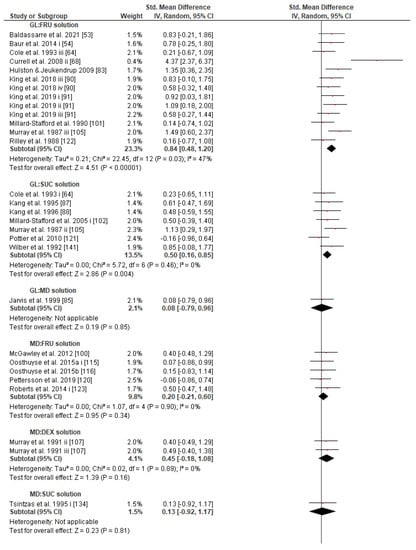
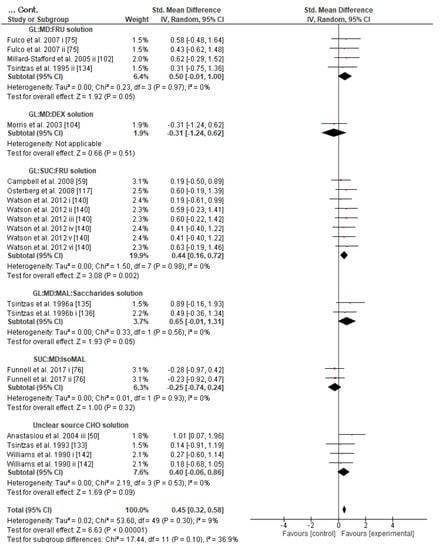
Figure 5.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 50 interventions [50,53,54,59,64,68,75,76,83,85,87,88,90,91,100,101,102,104,105,107,117,121,122,133,134,135,136,140,141,142]. Subgroup analyses show the results with regards to supplementation in 12 MTC groups (GL:FRU, GL:SUC, GL:MD, MD:FRU, MD:DEX, MD:SUC, GL:MD:FRU, GL:MD:DEX, GL:SUC:FRU, GL:MD:MAL:Saccharides, SUC:MD:IsoMAL, unclear CHO substances mixture). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate, CI, confidence interval; DEX, dextrose; FRU, fructose; GAL, galactose; GL, glucose; IV, inverse variance; MD, maltodextrin; MAL, maltose; MTC, multiple transportable carbohydrate; SD, standard deviation; Std, standardized; SUC, sucrose; i–vi denote different intervention arms (trials) within the same study.
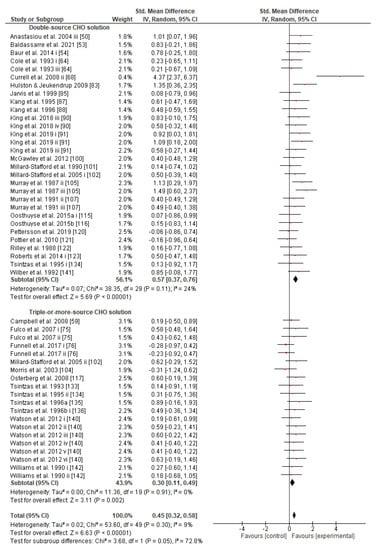
Figure 6.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 50 interventions [50,53,54,59,64,68,75,76,83,85,87,88,90,91,100,101,102,103,104,105,107,115,116,117,120,121,122,123,133,134,135,136,140,141,142]. Subgroup analyses show the results with regards to supplementation in two carbohydrate formulation groups (double-source CHO solution, triple-or-more-source CHO solution). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study.
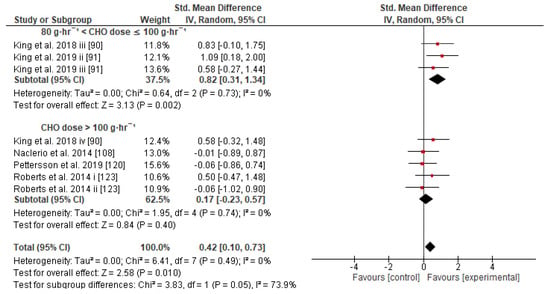
Figure 7.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 8 interventions [90,91,108,120,123]. Subgroup analyses show the results with regards to supplementation in two carbohydrate dose groups (80 g·h−1 < CHO dose ≤ 100 g·h−1, CHO dose > 100 g·h−1). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study.
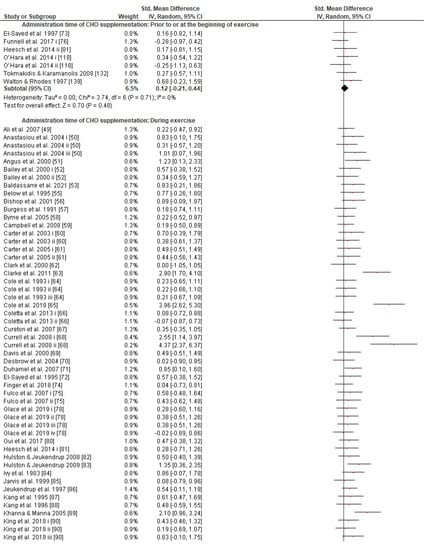
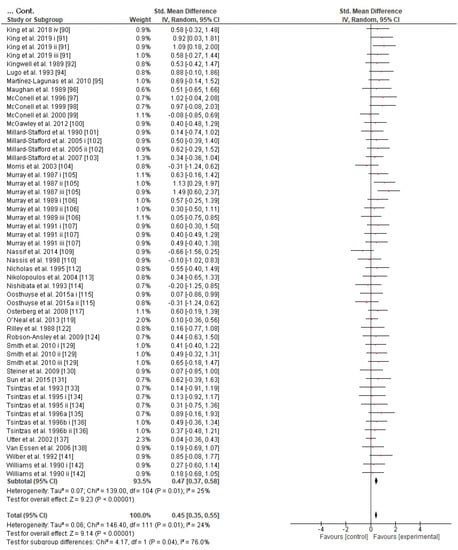
Figure 8.
Forest plot shows the effects (red square symbol) of experimental carbohydrate supplementation as compared to a control on exercise outcome for 112 interventions [49,50,51,52,53,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,78,80,81,82,83,84,85,86,87,88,89,90,91,92,94,95,96,97,98,99,100,101,102,103,104,105,106,107,109,110,112,113,114,115,117,118,119,122,124,129,130,131,132,133,134,135,136,137,138,139,141,142]. Subgroup analyses show the results with regards to supplementation in two carbohydrate administration time groups (prior to or at the beginning of exercise, during exercise). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random effects meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study.
3.3. Risk of Bias
Evidence of some risk of bias was found in all included studies (Figure 2). The predominant risk of bias derived from the lack of reliability and validity of measurements in the studies (Figure 2). Risk of bias also derived from the subjects’ inclusion and statistical power process in the vast majority of the included studies (Figure 2). Furthermore, the Egger’s test of the intercept and inspection of the funnel plot suggested no potential publication bias (Figure S16).
4. Discussion
More than 45 years of research into the effects of CHO supplementation on performance during endurance exercise has provided a wealth of evidence to suggest that CHO contributes to fatigue postponement; yet, there have been no clear conclusions about the optimum administration timing, dosage, and type of CHO. Meta-analyzing the efficacy of CHO (≤20%) solution compared with control (placebo) on prolonged exercise performance in subjects over 18 years old found that regardless of the CHO supplementation time administration, form, and concentration, and exercise type, mode, or protocol, consumption of CHO solution has positive effects on performance. However, a crossover interaction between SMD and the subjects’ CRF level could exist. Carbohydrate solution seems to have a tendency to favor cyclists’ performance more than that of runners. As the exercise duration increases from 1 up to 4 h, the effect size of CHO intake on performance is also increased. A 6–8% CHO solution composed of two CHOs (GL:FRU) than more components appeared sufficient to increase the chances of a better performance. Interval frequency administration during the event proved superior to administration before the exercise. Moreover, though the effect size on performance seems to be unaffected by the use of different CHO doses schedules, it was found that a CHO dose of 80–100 g·h−1 has a significantly greater effect size on endurance performance in comparison to a CHO dose of more than 100 g·h−1.
4.1. Effects of CHO Supplementation on Endurance Exercise and Gastrointestinal Symptoms
In 2011, two meta-analyses reported a significant acute effect of CHO supplementation on endurance exercise performance (time trials or time to exhaustion tests) [143,144]. In the first one, the mean effect size of CHO solution intake on performance, derived from 50 studies, ranged from 0.30 to 0.53 [144]; in the second one, it derived from 73 studies (122 trials) and ranged from ~2 to ~6% [143]. Three years later, another meta-analysis demonstrated a positive influence on endurance performance in 82% of the 61 studies included (n = 679 subjects) and no effect in the remaining 18% [145]. Another systematic review, also comparing CHO solution(s) with placebo or water conditions, showed an improvement of exercise performance in 13 of the 17 studies included [146]. Similarly, we found strong evidence supporting that CHO solutions favor performance in prolonged exercise in 88.9% of the trials included, with an overall SMD = 0.43, (n = 1560).
On the other hand, high concentrated CHO sport solutions (>8% CHO) may in some cases impede the process of fluid absorption, hinder performance, [147,148,149,150], and additionally cause unpleasant GI distress [151,152]. It has been observed that GI symptoms are associated with endurance exercise after CHO feeding mainly in long-distance running and triathlon [153,154]. Mesenteric blood flow is reduced when exercise intensity is high, and notably when participants are hypohydrated. This is thought to be one of the main causes of the development of GI symptoms among other physiological, mechanical, psychological, or nutritional factors [155,156]. These GI symptoms might impede performance, making it difficult for athletes to win or even to follow the race [155,156]. In this review, we found three trials in which GI problems were reported during running only; one of them did not use FRU [134] and the other two used FRU in combination [134,135]. Fructose seems to be absorbed slowly from the intestinal tract and may be responsible for a significant osmotic effect in the intestines, which may cause GI problems [157]. This may have been the case in the above studies; however, there is insignificant evidence to further investigate this hypothesis in this study, as the causal mechanisms for most GI problems are still unclear. Until this is resolved, it may be wise for the athlete to avoid single FRU as a CHO supplement.
4.2. Effects of Mode, Protocol, and Type of Exercise
CHO supplementation supposedly has different effects on performance in different modes of exercise. This may be due to different muscle groups at work (for instance, during cycling, the upper body muscles do not contribute as much as in a triathlon or cross-country skiing) or to possible differences in the CHO absorption rate, which may be aided by small stomach movements during different exercise modes (for instance, running vs. cycling). Undoubtedly, the athletes’ weight is one of the determinant factors of energy expenditure, running economy, and, consequently, endurance exercise performance [158]. As running is less mechanically efficient than cycling, runners during given mechanical work exercise would be expected to have greater energy requirements than cyclists; therefore, a runner’s performance would be more CHO dependent [159]. This simply means that when both athletes ingest the same amount of CHO supplements, the cyclist may have a greater advantage in exercise performance compared to the runner, under similar work exercise conditions. It has been reported, however, that the ergogenic action of exogenous CHO during exercise, cycling or running, although depending on different physiological and metabolic mechanisms, is similar and of a comparably relative intensity; exogenous CHO oxidation rates between long running and cycling are identical as well [160,161]. Our findings show that CHO supplementation in cyclists tends to have a larger effect size on performance in comparison to runners (Figure S5). These results may be explained by what was mentioned above; however, this topic requires further investigation. The lack of an adequate number of comparative studies on other exercise modes prevents us from drawing conclusions.
Previous studies have demonstrated that fatigue during prolonged exercise in subjects fed CHO supplement occurs at approximately the same time as muscle glycogen depletion [162,163,164,165,166]. On the contrary, there seems to be no dose–response association between CHO uptake and improved exercise capacity [160]. However, our results indicate that the effect size of CHO supplementation on performance has no significant difference between capacity and performance tests (Figure S6). It must be noted, by the way, that performance tests better simulate real-world competitive endurance sports and are more reliable [167]. Therefore, the effects of CHO supplementation on performance between time trial and time to exhaustion tests seem identical and are in agreement with other relevant systematic reviews [144,145].
Originally, most of the studies investigated the role of CHO supplements during continuous (rather than intermittent) endurance exercise and showed an increase in endurance efficiency. During the prolonged intermittent and intermittent shuttle exercise (periods of intermittent bursts of high or lower intensity exercise (punctuated by rest or lower intensity activities)), energy is derived conjointly from anaerobic and aerobic metabolism [8,168]. So, in conditions of prolonged multi-sprint activities, the intra-muscular phosphocreatine and muscle glycogen stores are gradually depleted, and deterioration of performance is unavoidable [8,168,169]. Consequently, the research community has relatively recently extended its interest to investigating CHO supplements on performance during various intermittent exercise disciplines, especially in popular team games [8,168,169]. Nevertheless, the multi-complex nature of ‘stop and start’ sports (which makes it hard not only to simulate them but also to measure the subjects’ physical performance (i.e., for non-technical skills) in a reliable and accurate manner) and the various methodological approaches applied among studies create conditions that complicate an interpretation of the results. The CHO concentration of sport solution that was used so far in these studies was ~6–8% and the quantity of CHO (GL, SUC, and/or MD) ingested was ~30–60 g·h−1 [169]. Based on the relevant studies, for ‘stop and start’ sports (lasting from 1 to 2.5 h) that have shown an improved performance (or no effect), consumption of CHO at rates of 30–60 g·h−1 as an acute fueling strategy is generally recommended [8,9,169]. Similarly, we originally found strong evidence that CHO intake solutions have a significant and similar impact on performance during prolonged intermittent, intermittent shuttle, and continuous endurance exercise (Figure S7). However, the positive effect of CHO intake solutions on intermittent shuttle exercise (60 min < T ≤ 120 min) in the included studies derived from trials that had used mainly single-source CHO solutions (MD or SUC at a concentration range of 6.4–7%, [48,49,63,79]), with only one of them having used a CHO dose intervention (SUC 32.6 g·h−1 using a 7% solution, [79]). It is also worth mentioning here that in the three trials that had used >8% concentrated solutions in a range of CHO doses ~40–120 g·h−1, no positive result was found [76,108].
4.3. Effects of CHO Supplementation and Exercise Duration
Theoretically, CHO availability via the blood is not limited during exercise in individuals taking CHO supplements. Our findings show that: (a) as exercise duration increases up to 4 h, so does the effect size of CHO intake on performance (Figure 4); and (b) the positive SMD remains significant in exercise lasting more than 4 h; however, the effect is tempered. As muscle glycogen is gradually reduced during a 1–4-h all-out endurance exercise, the positive impact of CHO intake on exercise performance increases. As the duration of the exercise is prolonged (>4 h) and the intensity inevitably decreases, the performance should depend less on the availability of CHOs [19], since the percentage of energy contribution of CHO will be tempered in comparison to fats [170,171,172,173]. Thus, it is not surprising that most athletes (subjects) consume less CHO (~20–40 g·h−1) than the guidelines suggest [9] in endurance races lasting from 4 to 24 h [174,175,176,177,178]. The positive effect of CHO intake on performance in exercise lasting 45 min to 1 h appears to also be tempered in the included studies (Figure 4) (although a 6.4% CHO solution administration showed better results, [60,113,126,127,128]). Possible explanations are that (a) muscle glycogen is not fully depleted in this duration [18], (b) muscle fatigue is possibly due to the accumulation of H⁺ [19], and (c) the ergogenic effect of CHO solution may not be exclusively metabolic but could also be attributed to the CNS [7]. Similar evidence was also reported in one meta-analysis and one systematic review [144,145].
4.4. Effects of CRF and Gender on CHO Supplementation
Given that (a) GL expenditure is closely correlated with exercise intensity [179], (b) training reduces the flux of GL (so athletes may rely more on fat catabolism) for a specific power output [179], (c) endurance preparation reduces endogenous oxidation of blood glucose during prolonged exercise [180], (d) trained athletes could resynthesize better GL from lactate in comparison to untrained ones [11,181], and (e) muscle glycogen has a direct impact on CHO availability and CRF [182], it could be assumed that the more the athletes trained, the less they depend on exogenous CHO during prolonged exercise [11,179,183]. Indeed, we observed an interaction between SMD and the subjects’ CRF level (Figure 3 and Figure S4). Two previous reviews had partially discussed the potentially limited effect of exogenous CHO on the performance of trained endurance athletes as opposed to untrained ones [160,182] without reaching a permanent conclusion. The present study is probably the first one to report clear evidence as the fixed effect analysis indicated; nonetheless, more research is needed on this topic, since the conservative random effect analysis failed to prove any CRF effect on the efficacy of CHO intake during exercise (Figure 3).
In terms of sex, the already existing guidelines often assumed a similarly acute exercise effect of CHO supplementation in males and females. A study with a mixed-gender group [184] revealed that males and females responded in a similar fashion to CHO supplementation enriched with protein. On the contrary, it has been well documented that females oxidize more fat during endurance exercise than males and seem to metabolize endogenous CHO in different degrees, a process influenced by estrogen levels [12,52,185,186,187,188]. Our findings indicate no different effects of CHO supplementation on endurance performance between male (111 trials) and female subjects (8 trials) (Figure S2). However, it should be noted that in two of the trials, the menstrual status of the female participants was not controlled [78,139]. The well-established sexual dimorphism in muscle mass, hemoglobin concentration, level of reproductive hormones, and CHO oxidation [185,189,190,191], as well as the limited number of trials with female-only populations do not allow us to draw safe comparative conclusions.
4.5. Effect of Different CHO Supplement Concentrations and CHO Solution Formulations
The energy-dependent sodium-glucose link transporter (SGLT1) actively transports GL (which may also derive from other hydrolyzed CHO sources, such as starch, MAL, MD, or GL-polymer) and GAL through the intestinal mucosa [7,192,193,194]. Other CHOs, such as FRU and SUC, use GLUT5 (facilitated diffusion) and SCRT (if SUC is not hydrolyzed into GL and FRU) protein transporters, respectively [7,192,193,194]. Between GL-only and FRU-only (i.e., single-source CHO solution formulation), FRU has been theorized to be a better source of CHO than GL because it is absorbed more slowly than GL and therefore does not stimulate an insulin response [7,195,196]. Accordingly, the oxidation rate of ingested FRU during endurance exercise is slower than GL [197,198,199] or similar [200]. With the exception of FRU, GAL, IsoMAL, and starches (oxidation rate up to 40 g·h−1) [201], exogenous CHO is oxidized at a maximum rate of approximately 1 g·min−1 (60 g·h−1) [7,202,203,204,205]. Glucose-polymer has also been shown to be more digestible, with faster gastric emptying than GL, and could hence provide GL and fluids more effectively [206,207,208,209,210]. Moreover, a combination of different types of CHO like GL:FRU in a ratio of 2:1 (referred to as MTC, because they use multiple protein transporters (SGLT1 and GLUT5) [211]) speeds up oxidation rates up to 25% more than was previously thought (due to the GL barrier of 60 g·h−1). A series of studies also demonstrated even higher oxidation rates, up to 75%, using MTC (GL:FRU in ratio of 2:1) [201,212]. Moreover, it seems that ingestion of a solution composed of MTC during prolonged exercise (≥2–2.5 h) at high rates (>60 g·h−1) benefits performance more than the consumption of sport solutions containing GL-only (or MD-only) and minimizes the chances of GI discomfort [7,68,213,214,215,216].
The main goal of the intake of CHO sport solution during exercise is euhydration and euglycemia maintenance, and the minimum GL concentration required to boost water absorption is 0.9% [217]. So, in order to sustain an adequate glycogen supply and prevent hypoglycemia in exercise events lasting >1 h, a 6–8% CHO solution was generally recommended in many reviews [12,146,218,219]. Briefly, most modern reviews give more appreciation to solutions of 6–8% CHO concentration than to less or more concentrated drinks, and more credit to solutions composed of MTC than to single-source CHO solutions (e.g., GL or MD-only), in order to achieve high oxidation rates (>90 g·h−1), and consequently ensure better performance during prolonged exercise [7,9,12,143,145,146,216,218,219,220,221]. Similarly, the present meta-analysis provides evidence that CHO supplementation composed of GL:FRU is superior to other MTC compositions (fixed effect analysis; Figure S14). According to our results, the 6–8% CHO concentrated solutions show the highest SMD (Figure S8), and the double-source CHO solutions increase performance significantly more than triple-or-more-source CHO solutions (Figure 6). These findings combined show that a 6–8% CHO concentrated solution composed of two types of CHO (GL:FRU in a ratio of 2:1) is sufficient to increase the chances of a better performance. This statement has to be considered with caution since the effect of GL:FR on performance, in the present study, becomes weak as far as the random effect analysis is performed (Figure 5). It appears that further investigation is needed. With regard to interventions of various single-source CHO-only solutions (53 trials), although no significant differences in efficacy were found (Figure S10), the vast majority of trials (49) appear to have used MD-only, GL-only, or SUC-only solutions, which also showed greater SMD values in comparison to MAL-only, FRU-only, or GAL-only solutions (4 trials) [145]. This is obviously the reason why in some reviews, GL, MD, and SUC are recommended in cases where a single-source CHO solution is preferred [145,169].
4.6. Effect of CHO Administration Time and Dose
Regarding CHO administration time (i.e., prior to vs. during vs. late in exercise), there seems to be a lack of relevant comparative studies and only a few reviews have addressed this topic, albeit not extensively [12,160]. Briefly, it is reported that interval CHO administration during exercise is prioritized over feeding prior to exercise on its own [12]. Given that we did not study the CHO administration time pattern (e.g., feeding every 10 min vs. 20 min vs. 30 min vs. 40 min), our results reveal the advantage of interval CHO ingestion during exercise for performance as opposed to administration prior to exercise on its own (Figure 8). However, a prior-to-exercise CHO feeding protocol also has a significant effect on performance that cannot be neglected, and it could be used as an added strategy or on its own as long as it does not cause gastrointestinal discomfort or provoke insulin disturbance and therefore possible rebound hypoglycemia (Figure S12).
Concerning the CHO dose–response, our results show that the effect size on performance is unaffected by the use of different CHO dose schedules (g·h−1), probably because of the large variance of SMD among different trials that used different experimental methods (Figure S9). It also appears that performance is benefited significantly more from a CHO dose of 80–100 g·h−1 in comparison to a CHO dose >100 g·h−1 (Figure 7), and also more in comparison to 60–80 g·h−1, though not significantly (p = 0.15). On the other hand, some reviews consider that CHO intake of up to 60 g·h−1 for exercise lasting up to 2.5 h and up to 90 g·h−1 when the exercise duration exceeds 2.5 h should be recommended [7,9,220]. For the most part, these recommendations seem based on a previous review [221], which in turn seems to have premised its arguments on four previous studies about trained male endurance cyclists only [68,129,215,222]. The first study (N = 8) did not actually test the dose–response in terms of CHO g·h−1 intake but tested the CHO ingestion at a rate of 1.8 g·min−1 during 120 min of exercise where the significant effect was confirmed [68]. The second study (N = 9) compared the effect of two different iso-caloric beverages, containing GL or GL:FRU (no placebo/control group), at an ingestion rate of 2.4 g·min−1 ~144 g·h−1 on exercise lasting more than 3 h [215]. The third study (N = 12) compared ingestion of a placebo or CHO at doses of 15, 30, and 60 g·h−1 (but not a higher dose, such as 80 or 90 g·h−1) on exercise lasting more than 2 h [129]. The last study (in an abstract form at that time, N = 51) suggested an CHO ingestion dose between 60 and 80 g·h−1 for an optimum performance enhancement when exercise lasts 2 h [222]. Based on this evidence, it is more reasonable to suggest a CHO ingestion dose of 60–80 g·h−1 for exercise lasting up to 2.5 h in the trained male cyclists population only, rather than recommend a CHO intake up to 60 g·h−1 for exercise lasting up to 2.5 h and up to 90 g·h−1 when the duration of exercise exceeds 2.5 h for the general population in various exercise modes.
In 2013, Jekendrup (one of the leading researchers in the field) argued that, with a few exceptions, decent dose–response research was notably absent and many of the published reports had substantial methodological flaws, which made it difficult to establish a valid relationship between the quantity of CHO consumption and the efficiency of the dose–response [223]. In the absolute sense of the dose–response term (i.e., g·h−1), relevant research studies are still limited at the present time [169]. We only found a total of 11 relevant studies that satisfied our inclusion criteria (Figure S9). Regardless of this, most modern reviews recommend a CHO intake up to 60 g·h−1 during an endurance event lasting up to 2.5 h, and up to 90 g·h−1 when the duration of exercise exceeds 2.5 h [7,9,143,220]. Nevertheless, as the duration of an all-out endurance exercise decreases and the intensity increases accordingly, performance should depend more on the availability of CHOs, since CHOs will contribute to a higher energy percentage than fats [19,170,171,172,173]. Therefore, much more energy would need to be derived from CHO sources as a percentage in an exercise lasting up to 2.5 h than when the exercise exceeds 2.5 h [170]. A CHO ingestion dose of ~80 g·h−1 has also been suggested for an optimum performance benefit when exercise lasts ~2 h [224]. So, in endurance events lasting up to 2.5 h, if we supply 90 g·h−1 of MTC (e.g., GL: 60 g·h−1 and FRU: 30 g·h−1, taking advantage of both different CHO transporters, SGLT1 and GLUT5, respectively), theoretically, the chance of an optimum CHO oxidation rate [225] will be increased [226], thus increasing the chances of a higher performance in comparison to 60 g·h−1 GL-only (or MD-only) ingestion [227]. In this case, if the extra CHOs are not fully oxidized in the first 2.5 h of the endurance task, the residual CHOs will probably help reduce the overall amount of CHOs needed for replenishment in the later stages of the exercise or post exercise and may favor the athlete for upcoming exercise events series. Consequently, with our findings in mind, since GI comfort is not compromised [216,228], the recommended CHO dose could also be raised up to 90 g·h−1 (instead of 60 g·h−1) composed of MTC for endurance events lasting 1–2.5 h [10,90,145,146].
The main reason for recommending a CHO dose up to 90 g·h−1 (instead of 60 g·h−1) when exercise duration exceeds 2.5 h is to compensate for the metabolic demands due to the increased glycogen depletion rates [7,9,143,220]. However, this guideline is very general and may not consider all the multifactorial metabolic demands of various ultra-exercise events, as (a) most guidelines do not take into account body mass (BM) and sex differences. (b) Controversially, the above recommendation equates in theory the energy requirements in exogenous CHOs for endurance races that just last >2.5 to those for races that last, for instance, 4, 8, 12, and 24 h, which obviously have different intensities and metabolic demands. As the duration of an ultra-endurance event increases and the intensity decreases accordingly, the performance should depend less on the availability of exogenous CHOs [19,170,171,172,173]. We found that the effect size of CHO intake on performance is smaller for an exercise duration >4 h (4 trials) (Figure 3). It was also revealed that (a) runners’ (subjects’) consumption was surprisingly 14.93 g·h−1 in race durations ~4.28 h [175]; (b) most athletes’ consumption rate ranged from 0.27–0.64 g·kg−1·h−1 in race durations ~24 h [174] or 20–40 g·h−1 in single-stage and multistage ultramarathon events [176,177,178]; and (c) mean consumption was 62.2 g·h−1 in race durations of 24 h [229], which is less than the prevailing recommendation. Therefore, as new evidence keeps coming up in the literature, we believe that the issue of exercise duration deserves further investigation, perhaps even a reconsideration of the hitherto proposed recommendations.
However, another question arises regarding dose: Why are the doses recommended so far expressed mainly in g·h−1 units and not, correspondingly, in individual BM, as proposed [9], given that heavier athletes consume more energy than lighter ones? On the one hand, a meta-analysis has reported that the ergogenic action of a CHO dose up to 80 g·h−1 depends on the athlete’s size among other factors [144]. On the other hand, it has been concluded that that there is no evidence for expressing CHO dose guidelines in relation to BM because they are not correlated [201], a conclusion that was later adopted by several other reviewers [7,9,143,220]. This viewpoint appears to be based on the results of a series of CHO oxidation rate studies [161,230,231,232,233]. However, research in these studies was conducted in the same laboratory, on trained male cyclist subjects only (BM ranged from ~58–84 kg), whereas exercise performance was not tested, which limited the results’ generalizability. Moreover, the subjects in these studies followed their own diet (except the last 1–2 days) before the experiment [161,230,231,232,233]. Since the CHO content of any diet is a determinant factor of the gut absorption capacity, as later studies have shown (due to the intestinal transporters’ upregulation when the amount of CHO intake is increased [201,234,235]), it should have a significant impact on the oxidation rate. In this sense, as the subjects did not experience the same diet routine, it is reasonable to assume that their gut was trained differently, and they would have different CHO absorption rates [234,235], most probably uncorrelated to their BM, and thus different oxidation rates. This alone should call for a re-examination of the notion of expressing CHO dose guidelines in g·h−1 values.
4.7. Methodological Aspects, Strength, Limitations, and Suggestions for Future Research
Though meta-analysis has been widely applied to human performance research [236,237,238,239], its results have been criticized as ‘mixing apples and oranges’ [240]. However, what needs to be considered is whether studies of different internal validity and methodological control (e.g., small sample size, no control group, and non-randomized) should be included, and whether they should be considered as weighty as studies with more appropriate experimental designs. For this reason, we only included controlled intervention studies, whose authors reported that they used a specific experimental method involving a comparison group in a parallel or crossover design. We also introduced a modified version of the Cochrane risk-of-bias assessment tool to assess potential limitations of the eligible studies [24]. Nevertheless, it should be emphasized that a comparison of the more methodologically sound studies is not sufficient on its own. The different methodologies and techniques employed by the different studies could also explain the lack of consistency in the literature. Furthermore, we attempted to adjust the methodological research characteristics employed throughout this review using specific predefined criteria; nonetheless, evidence of overall risk-of-bias in the included studies remains subjective to some extent [24]. In the current meta-analysis, the vast majority of the eligible studies used a crossover design (139 trials). We assume that the ~one week washout period reported by the revised studies was sufficient for subjects to recover from residual fatigue derived from the previous exercise testing session, and that the time of washout between repeated trials did not impact the key meta-analysis findings. Hence, crossover designs are not considered a threat to this study. The effect size has also been criticized in the meta-analysis technique. Hedges (1981), who introduced the unbiased effect size, suggested a solution to this, which was adapted for use in our review [36]. In an effort to further increase the homogeneity of our results and be able to draw conclusions on variant modes, durations, and types of exercise, and different classes of subjects CRF, compositions, concentrations, and administration times of CHO supplements, we also applied subgroup analysis. Although only studies with accessible full-text papers written in English were selected, our outcomes show no potential publication bias. It should also be stated that even though all methodological precautions were taken, the extended period (1975–2021) of studies being analyzed introduces a level of uncertainty in drawing conclusions, due to changes in the experimental methods and designs through time. Similarly, another point of introduced uncertainty in the present meta-analysis deals with the fact that performance was not measured in all studies in a uniform way. Time to exhaustion and time trail tests evaluate different physiological mechanisms and each one entails its own repeatability accuracy [32,33,34]. Despite this, the present study found no effect of the performance test on CHO efficacy as others also did [144,145].
Gut trainability derived from ‘nutritional training’ in order to train the GI tract by improving the rate of emptying and fluid absorption and thus favor exercise performance in endurance events is a known practice among endurance athletes [234,235]. However, no study reported that subjects were checked in advance, as to whether they used to ingest CHO supplements or a high CHO-enriched diet for a long period before the study, which means that we do not know if the results of these studies could have been affected by subjects’ gut trainability effect. To overcome this methodological flaw in future studies, all subjects will need to follow a standardized control diet for many weeks prior to the investigation period.
Most of the review studies were conducted on cycling (100 trials), in laboratory environments (133 trials) and in a male population (111 trials), making it hard to generalize to other natural sporting environments (i.e., aquatic activities), elite athletes, and the female population. Perhaps more applied research is needed to investigate the effect of CHO supplements on endurance exercise of different types, durations, and modes at different phases of a female’s menstrual cycle [241] and in elite athletes. Further, investigation is required to assess whether CHO solution administration is ergogenic in ultra-exercise events of varied duration, and what is the optimal dose, type, and concentration of a CHO drink for enhancing performance. Ambient temperature, exercise mode, and intensity seem to differently influence the energy substrate’s oxidation and metabolism [13,14]. We did not find enough studies on different hypobaric or thermal conditions combined with different CHO solution temperatures to draw safe conclusions; therefore, more research is needed to address this issue. In the last decade, however, there has been an emerging interest in CHO dose (CHO g·h−1) response in endurance performance, and various studies recommend 30–60 g·h−1 to 90 or even a surprising 120 g·h−1 [7,9,242]. As was earlier explained, the CHO dose–response in exercise performance could possibly depend on the athlete’s weight [144]. Since no modern study has investigated individuals’ dose–response control for their BM or lean mass in conjunction with their diet, this is another future research topic that needs to be addressed. Furthermore, any recommended dose that will be derived from these studies should preferably be expressed in CHO g·h−1·kg−1 of BM, as suggested by the American College of Sports Medicine. This will enable global scope organizations to update their recommendations and make them more suitable and accurate for a wider range of athletes’ body sizes [9]. In addition, as more investigation on dose–response is required, more studies are needed to determine the potential action of different CHO doses on exercise performance, especially in different exercise modalities and intermittent events. The validity and reliability of the experimental protocols should be measured and reported by the researchers accordingly.
In this article, we selected and analyzed relevant papers from a specific period (1975–2021) and verified the hypothesis that CHO supplementation during prolonged exercise enhances performance or delay fatigue progression. Maintaining blood glucose concentrations and increasing CHO oxidation rates could be the main reasons behind such performance improvement. Fatigue is not solely located at the peripheral level, as explained by the appearance of a “metabolic endpoint”, in which muscle glycogen levels are exhausted and plasma GL levels are lowered (peripheral factor) [243,244]. There is substantial evidence that mechanisms located at the CNS are also involved in fatigue exhibition (central factor) [245,246] and it seems that the CHO ergogenic effect also has a central component independent of the metabolic one [247]. The drop in central activation during persistent muscular contraction could be caused by substrates’ depletion in the CNS and/or changes in the levels of particular neurotransmitters [244]. Exercise-induced hypoglycemia has been linked to a significant reduction in voluntary activation during persistent muscle contractions [248], as well as a drop in brain GL uptake and the overall cerebral metabolic rate [249]. On the contrary, when euglycemia is maintained, the drop in CNS activation recedes [250]. Moreover, a decrease in brain GL has also been linked to the homeostatic drive to sleep [251], suggesting that it may play a role in fatigue progression, and changes in extracellular GL concentrations have been shown to have a considerable impact on serotonin release and absorption during exercise and recovery, indicating that GL plays a key role in central neurotransmission control [252]. Thus, as exercise activity duration increases (e.g., >2 h) and glycogen depletion becomes apparent, CHO supplementation is also expected to continue to have a significant influence on central fatigue markers [253]. However, the mechanisms that cause performance declines can interact at any level of the brain–muscle circuit, and while the literature normally distinguishes between peripheral and central fatigue, both pathways may be intertwined and the complicated relationship between peripheral and cerebral components could influence fatigue during prolonged exercise [254]. Therefore, as the effects of CHO supplementation on the interactive mechanisms of central and peripheral fatigue during prolonged exercise are still unclear [255], more research is needed on this complex phenomenon impacted by both central and peripheral factors.
4.8. Practical Implications and Guidelines for the Athlete
In the last 45 years, the undiminished interest in research on CHO solutions (sport drinks) has promoted our insights and sport supplementation practices. Gastrointestinal absorption, splanchnic and muscle blood flow, muscle energy uptake, and substrate oxidation have all been established as possible barriers to the potential ergogenic effects of CHO solutions [256]. However, with an ever-growing body of reviews on the influence of ingesting CHO solutions during prolonged exercise on performance, and different recommendations from a different perspective, there is the question of what constitutes substantive advice [7,8,9,10,11]. Moreover, many athletes have reported that is not very clear if the dose should be up to 60 g·h−1 for the first 2.5 h and then be increased to 90 g·h−1, or the dose should be 90 g·h−1 from the beginning of an endurance event when the exercise lasts more than 2.5 h (personal communication, in recent ultra-endurance races). There are also many who are misled by retailers, possibly due to the difficulty of interpreting the scientific data, the existence of a plethora of different guidelines, and various misconceptions [8,257,258].
Therefore, since our study does not cover the whole spectrum of research on CHO solution ingestion during prolonged exercise, our recommendations are inevitably limited by our findings. We also feel that it will be more appropriate to propose a more simplified version of the already existing guidelines to athletes [9], which is likely to have a greater impact on the sport population. Thus, we think that the same or an even better result could be obtained by simply recommending a dose up to 90 g·h−1 of two transportable CHOs (GL:FRU in a ratio of 2:1) in the form of a 6–8% CHO solution during exercise events (intermittent and continuous) lasting from 1 to 4 h (Figure 9). With regard to ‘stop and start’ sports, it seems that a 6–8% concentrated single-source CHO solution (e.g., GL or MD or SUC) is sufficient not only to serve hydration purposes, but also to meet increased demands for the exogenous CHO supply. For exercise lasting 45 min ≤ T ≤ 60 min, a ~6% CHO solution is recommended. Taking supplemental CHO solutions (e.g., 30–50 g·h−1 as proposed by the International Society of Sports Nutrition [177]) in prolonged exercise (T > 240 min) may be beneficial. Nevertheless, the fact that we found only three relevant studies prevents us from making universal recommendations; therefore, athletes should test and rehearse race nutrition strategies, including precise macronutrient/fluid, quality, and amount during training sessions. It should be duly pointed out that the guidelines suggested above are based on our analysis, which mainly derived from mild environmental condition studies, in the male population and non-elite athletes, and so their generalizability is limited to the study population/ambient condition. As perceived, scientific evidence should never be ignored. However, athletes’ CHO supplementation plans could be tailored to their individual preferences, responses, and tolerance to various dose strategies and needs. Athletes should also take into account the provided options for CHO solution ingestion in a competition event or training session [8,9,10,11,12,145,146]. Additionally, athletes should not neglect the benefit of a balanced diet, gut training, and pre-event CHO supplementation, and they should always be well hydrated [8,9,10,11,145,146,234].
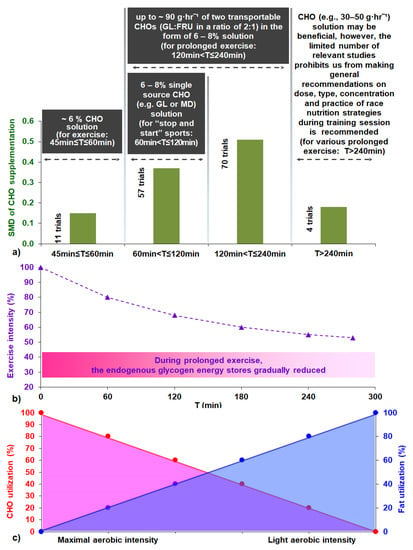
Figure 9.
(a) Suggested key guidelines for carbohydrate supplementation during prolonged exercise and effect size of CHO supplementation as a function of exercise duration, referenced in the present study. (b) Idealized various intensities for constant exercise as a function of exercise time and endogenous glycogen energy stores reduction [170,171,172,173]. (c) Idealized fuels used as a function of exercise effort [170,171,172,173]. Abbreviations: CHO, carbohydrate; FRU, fructose; GL, glucose; MD, maltodextrin; SMD, standardized mean difference; T, exercise time.
5. Conclusions
We examined the efficacy of CHO (≤ 20%) solution compared to controls on prolonged exercise performance in subjects over 18 years old in 96 studies (142 trials). We found that CHO solution favors performance in prolonged exercise, continuous and intermittent, and cyclists tend to have a greater effect size in comparison to runners. As the exercise duration increases from 1 to up to 4 h, so does the effect size of CHO intake on performance. A crossover interaction between SMD and the subjects’ CRF level was also considered, although not between gender classes; however, the number of studies in the female population are limited and the athlete’s gender may be a worthy variable for future consideration. A 6–8% CHO concentrated solution composed of two transportable CHOs (i.e., GL:FRU in a ratio of 2:1) appears to be sufficient to increase the chance of a better performance. An administration schedule during the event seems superior for performance in comparison to administration prior to exercise on its own, although CHO supplementation prior to exercise should not neglected. Additionally, although the effect size on performance seems to be unaffected by different CHO dose schedules, it appears that a CHO dose of 80–100 g·h−1 has a greater effect size on performance in comparison to 60–80 g·h−1 and is significantly more beneficial for endurance performance in comparison to a CHO dose >100 g·h−1. However, all included studies investigating the efficacy of CHO supplements during prolonged exercise have shown some evidence of risk-of-bias, an issue that should be addressed in future research. More research is needed to investigate the impact of CHO supplementation and CHO dose, preferably expressed per kg of BM as suggested [9], on prolonged exercise of different types, duration, modes, in the female population, at different phases of the menstrual cycle, and in elite athletes. We hope that the findings of and questions raised by this review will help set a direction for future applied research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13124223/s1, Figure S1: Forest plot of comparison, experimental carbohydrate supplementation vs. control on exercise outcome including raw data (in mean and SD) and risk-of-bias judgments of all studies (and trials) for 142 interventions. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study; +, low risk-of-bias; ?, some concerns risk-of-bias; −, high risk-of-bias, Figure S2: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to the subjects’ characteristics in three gender groups (Male, Female, Mixed). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S3: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 140 interventions. Subgroup analyses show the results with regard to the subjects’ characteristics in three age groups [Young (18–29 years), Adults I (30–39 years), Adults II (40–49 years)]. The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S4: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 127 interventions. Subgroup analyses show the results with regards to subjects’ characteristics in four CRF groups (Superior, Excellent, Good and Fair). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following fixed effects meta-analyses. Studies or trials that provided not sufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; CRF, cardio-respiratory fitness; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S5: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to exercise task in three exercise mode groups [Cycling, Running, Other (triathlon, duathlon, walking, loaded marching, roller-skiing)]. The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S6: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to exercise task in two exercise protocol test groups (Capacity, Performance). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S7: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to exercise task in three exercise type groups (Intermittent, Continuous, Intermittent shuttle). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S8: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to supplementation in seven carbohydrate concentration groups (0% < CHO ≤ 2%, 2% < CHO ≤ 4%, 4% < CHO ≤ 6%, 6% < CHO ≤ 8%, 8% < CHO ≤ 10%, 10% < CHO ≤ 15%, 15% < CHO ≤ 20%). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S9: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 31 interventions. Subgroup analyses show the results with regard to supplementation in five carbohydrate dose groups (CHO dose ≤ 40 g·hr−1, 40 g·hr−1 < CHO dose ≤ 60 g·hr−1, 60 g·hr−1 < CHO dose ≤ 80 g·hr−1, 80 g·hr−1 < CHO dose ≤ 100 g·hr−1, CHO dose > 100 g·hr−1). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S10: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 53 interventions. Subgroup analyses show the results with regard to supplementation in six single source carbohydrate only groups (GL, MD, SUC, MAL, FRU, GAL). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; FRU, fructose; GAL, galactose; GL, glucose; IV, inverse variance; MD, maltodextrin; MAL, maltose; SD, standard deviation; Std, standardized; SUC, sucrose; i–vi denote different intervention arms (trials) within the same study, Figure S11: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 103 interventions. Subgroup analyses show the results with regard to supplementation in three carbohydrate solution formulation groups (Single-source CHO solution, Double-source CHO solution, Triple-or-more-source CHO solution). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S12: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 142 interventions. Subgroup analyses show the results with regard to supplementation in four carbohydrate administration time groups (Prior to or at the beginning, During, Prior to or at the beginning + during, Late in exercise). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S13: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 21 interventions. Subgroup analyses show the results with regard to supplementation in two temperature of supplement administration groups [Cool (< 18 °C), Neutral (18–26 °C)]. The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S14: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 50 interventions. Subgroup analyses show the results with regards to supplementation in twelve MTC groups (GL:FRU, GL:SUC, GL:MD, MD:FRU, MD:DEX, MD:SUC, GL:MD:FRU, GL:MD:DEX, GL:SUC:FRU, GL:MD:MAL:Saccharides, SUC:MD:IsoMAL, Unclear CHO substances mixture). The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following fixed effects meta-analyses. Studies or trials that provided not sufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate, CI, confi-dence interval; DEX, dextrose; FRU, fructose; GAL, galactose; GL, glucose; IV, inverse variance; MD, maltodextrin; MAL, maltose; MTC, multiple transportable carbohydrate; SD, standard deviation; Std, standardized; SUC, sucrose; i–vi denote different intervention arms (trials) within the same study, Figure S15: Forest plot shows the effects of experimental carbohydrate supplementation as compared to control on exercise outcome for 102 interventions. Subgroup analyses show the results with regard to ambient conditions in two thermal condition groups [Cool (<18 °C), Neutral (18–26 °C), Heat (>26 °C)]. The black diamond symbol at the subgroups and at the bottom of the figure represents the standardized mean difference with the 95% confidence intervals for all interventions following random-effect meta-analyses. Studies or trials that provided insufficient data for subgroup classification were not included. Abbreviations: CHO, carbohydrate; CI, confidence interval; IV, inverse variance; SD, standard deviation; Std, standardized; i–vi denote different intervention arms (trials) within the same study, Figure S16: Funnel plot of comparison: I. in all studies (and trials); and II.–VII. in classes of different subgroups analysis.
Author Contributions
Conceptualization, D.I.B.; methodology, D.I.B.; software, D.I.B.; validation, D.I.B.; A.S.; E.D.Z.; N.D.G. and A.K.T.; formal analysis, D.I.B. and A.K.T.; investigation, D.I.B. and A.K.T.; resources, D.I.B. and A.S.; data curation, D.I.B.; E.D.Z. and A.K.T.; writing—original draft preparation, D.I.B.; writing—review and editing, D.I.B.; A.S.; E.D.Z.; N.D.G. and A.K.T.; visualization, D.I.B.; supervision, D.I.B.; project administration, D.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the reported results can be found in the Supplements section.
Acknowledgments
We are indebted to the independent reviewers (E.Z., G.M., A.K., K.P. and T.T.) for their enthusiastic and consistent assistance in data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuntz, N. Ueber die Bedeutung der verschiedenen Nährstoffe als Erzeuger der Muskelkraft. Pflüg. Arch. Gesammte Physiol. Menschen Thiere 1901, 83, 557–571. [Google Scholar] [CrossRef]
- Christensen, E.H.; Hansen, O., II. Untersuchungen über die Verbrennungsvorgänge bei langdauernder, schwerer Muskelarbeit1. Skand. Arch. Physiol. 1939, 81, 152–159. [Google Scholar] [CrossRef]
- Bergström, J.; Hultman, E. Muscle glycogen synthesis after exercise: An enhancing factor localized to the muscle cells in man. Nature 1966, 210, 309–310. [Google Scholar] [CrossRef]
- Bergström, J.; Hultman, E. A study of the glycogen metabolism during exercise in man. Scand. J. Clin. Lab. Investig. 1967, 19, 218–228. [Google Scholar] [CrossRef]
- Brooke, J.D.; Davies, G.J.; Green, L.F. The effects of Normal and Glucose Syrup Work on the Performance of Racing Cyclists. J Sports Med. Phys. Fit. 1975, 15, 257–265. [Google Scholar]
- Coyle, E.F.; Hagberg, J.M.; Hurley, B.F.; Martin, W.H.; Ehsani, A.A.; Holloszy, J.O. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 230–235. [Google Scholar] [CrossRef]
- Baker, L.B.; Jeukendrup, A.E. Optimal composition of fluid-replacement beverages. Compr. Physiol. 2014, 4, 575–620. [Google Scholar] [CrossRef]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med. 2020, 55, 416. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Mata, F.; Valenzuela, P.L.; Gimenez, J.; Tur, C.; Ferreria, D.; Domínguez, R.; Sanchez-Oliver, A.J.; Sanz, J.M.M. Carbohydrate availability and physical performance: Physiological overview and practical recommendations. Nutrients 2019, 11, 1084. [Google Scholar] [CrossRef]
- Brooks, G.A. The precious few grams of glucose during exercise. Int. J. Mol. Sci. 2020, 21, 5733. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Gagnon, D.D.; Perrier, L.; Dorman, S.C.; Oddson, B.; Larivière, C.; Serresse, O. Ambient temperature influences metabolic substrate oxidation curves during running and cycling in healthy men. Eur. J. Sport Sci. 2020, 20, 90–99. [Google Scholar] [CrossRef]
- Maunder, E.D.; Plews, D.J.; Merien, F.; Kilding, A.E. Exercise intensity regulates the effect of heat stress on substrate oxidation rates during exercise. Eur. J. Sport Sci. 2020, 20, 935–943. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; ISBN 9781119536628. [Google Scholar]
- Phillips, S.M.; Sproule, J.; Turner, A.P. Carbohydrate ingestion during team games exercise: Current knowledge and areas for future investigation. Sports Med. 2011, 41, 559–585. [Google Scholar] [CrossRef]
- Coyle, E.F. Fluid and fuel intake during exercise. J. Sports Sci. 2004, 22, 39–55. [Google Scholar] [CrossRef]
- Hopewell, S.; Dutton, S.; Yu, L.M.; Chan, A.W.; Altman, D.G. The quality of reports of randomised trials in 2000 and 2006: Comparative study of articles indexed in PubMed. BMJ 2010, 340, c723. [Google Scholar] [CrossRef]
- Deltsidou, A.; Zarikas, V.; Mastrogiannis, D.; Kapreli, E.; Bourdas, D.; Raftopoulos, V.; Noula, M.; Lykeridou, K. Data on advanced glycation end-products concentrations and haemodynamic parameters following caffeine and nicotine consumption in nursing students. Data Brief 2020, 32, 106063. [Google Scholar] [CrossRef]
- Havenetidis, K.; Bourdas, D. Creatine supplementation: Effects on urinary excretion and anaerobic performance. J. Sports Med. Phys. Fit. 2003, 43, 347–355. [Google Scholar]
- Stecker, R.A.; Harty, P.S.; Jagim, A.R.; Candow, D.G.; Kerksick, C.M. Timing of ergogenic aids and micronutrients on muscle and exercise performance. J. Int. Soc. Sports Nutr. 2019, 16, 37. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Koes, B.W.; Assendelft, W.J.J.; Van der Heijden, G.J.M.G.; Bouter, L.M.; Knipschild, P.G. Spinal manipulation and mobilisation for back and neck pain: A blinded review. Br. Med. J. 1991, 303, 1298–1303. [Google Scholar] [CrossRef]
- Beckerman, H.; De Bie, R.A.; Bouter, L.M.; De Cuyper, H.J.; Oostendorp, R.A.B. The efficacy of laser therapy for musculoskeletal and skin disorders: A criteria-based meta-analysis of randomized clinical trials. Phys. Ther. 1992, 72, 483–491. [Google Scholar] [CrossRef]
- Wood, L.; Egger, M.; Gluud, L.L.; Schulz, K.F.; Jüni, P.; Altman, D.G.; Gluud, C.; Martin, R.M.; Wood, A.J.G.; Sterne, J.A.C. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ 2008, 336, 601–605. [Google Scholar] [CrossRef]
- Verhagen, A.P.; De Vet, H.C.W.; De Bie, R.A.; Kessels, A.G.H.; Boers, M.; Knipschild, P.G. Taking baths: The efficacy of balneotherapy in patients with arthritis. A systematic review. J. Rheumatol. 1997, 24, 1964–1971. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, 834–840. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Current Version of RoB 2. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 24 November 2021).
- Hopkins, W.G.; Schabort, E.J.; Hawley, J.A. Reliability of power in physical performance tests. Sports Med. 2001, 31, 211–234. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hawley, J.A.; Burke, L.M. Design and analysis of research on sport performance enhancement. Med. Sci. Sports Exerc. 1999, 31, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. How to Interpret Changes in an Athletic Performance Test. Sportscience 2004, 8, 1–7. [Google Scholar]
- Thomas, J.R.; Nelson, J.K.; Silverman, S.J. Research Methods in Physical Activity, 7th ed.; Human Kinetics Publishers Inc.: Champaign, IL, USA, 2015; ISBN 9781450470445. [Google Scholar]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Hedges, L.V.; Stock, W. The Effects of Class Size: An Examination of Rival Hypotheses. Am. Educ. Res. J. 1983, 20, 85. [Google Scholar] [CrossRef]
- Deeks, J.; Higgins, J. Statistical Algorithms in Review Manager 5; Cochrane Collaboration: London, UK, 2010; pp. 1–11. [Google Scholar]
- World Health Organization. World Health Statistics 2019: Monitoring Health for the SDGs, Sustainable Development Goals; WHO/DAD/2019.1: Geneva, Switzerland, 2019; ISBN 9789241565707. [Google Scholar]
- Heyward, V.H.; Gibson, A.L. Assessing Cardiorespiratory Fitness. In Advanced Fitness Assessment and Exercise Prescription; Human Kinetics Publishers Inc.: Champaign, IL, USA, 2014; pp. 79–119. ISBN 78-1-4504-6600-4. [Google Scholar]
- Sedgwick, P. Meta-analyses: What is heterogeneity? BMJ 2015, 350, h1435. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Brockwell, S.E.; Gordon, I.R. A comparison of statistical methods for meta-analysis. Stat. Med. 2001, 20, 825–840. [Google Scholar] [CrossRef]
- Rice, K.; Higgins, J.P.T.; Lumley, T. A re-evaluation of fixed-effect(s) meta-analysis. J. R. Stat. Soc. 2018, 181, 205–227. [Google Scholar] [CrossRef]
- Peto, R.; Collins, R.; Gray, R. “Large-scale randomized evidence: Large, simple trials and overviews of trials”: Discussion. A clinician’s perspective on meta-analyses. J. Clin. Epidemiol. 1995, 48, 23–40. [Google Scholar] [CrossRef]
- Acker-Hewitt, T.L.; Shafer, B.M.; Saunders, M.J.; Goh, Q.; Luden, N.D. Independent and combined effects of carbohydrate and caffeine ingestion on aerobic cycling performance in the fed state tiffany. Appl. Physiol. Nutr. Metab. 2012, 37, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Alghannam, A.F. Carbohydrate-protein ingestion improves subsequent running capacity towards the end of a football-specific intermittent exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Williams, C.; Nicholas, C.W.; Foskett, A. The influence of carbohydrate-electrolyte ingestion on soccer skill performance. Med. Sci. Sports Exerc. 2007, 39, 1969–1976. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Kavouras, S.A.; Koutsari, C.; Georgakakis, C.; Skenderi, K.; Beer, M.; Sidossis, L.S. Effect of maltose-containing sports drinks on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 609–625. [Google Scholar] [CrossRef]
- Angus, D.J.; Hargreaves, M.; Dancey, J.; Febbraio, M.A. Effect of carbohydrate or carbohydrate plus medium-chain triglyceride ingestion on cycling time trial performance. J. Appl. Physiol. 2000, 88, 113–119. [Google Scholar] [CrossRef]
- Bailey, S.P.; Zacher, C.M.; Mittleman, K.D. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J. Appl. Physiol. 2000, 88, 690–697. [Google Scholar] [CrossRef]
- Baldassarre, R.; Ieno, C.; Bonifazi, M.; Di Castro, A.; Gianfelici, A.; Piacentini, M.F. Carbohydrate supplementation during a simulated 10-km open water swimming race: Effects on physiological, perceptual parameters and performance. Eur. J. Sport Sci. 2021. ahead of print. [Google Scholar] [CrossRef]
- Baur, D.A.; Schroer, A.B.; Luden, N.D.; Womack, C.J.; Smyth, S.A.; Saunders, M.J. Glucose-fructose enhances performance versus isocaloric, but not moderate, glucose. Med. Sci. Sports Exerc. 2014, 46, 1778–1786. [Google Scholar] [CrossRef]
- Below, P.R.; Mora-Rodríguez, R.; José, G.A.; Coyle, E.F. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med. Sci. Sports Exerc. 1995, 27, 200–210. [Google Scholar] [CrossRef]
- Bishop, N.C.; Blannin, A.K.; Walsh, N.P.; Gleeson, M. Carbohydrate beverage ingestion and neutrophil degranulation responses following cycling to fatigue at 75% VO2 max. Int. J. Sports Med. 2001, 22, 226–231. [Google Scholar] [CrossRef]
- Burgess, W.A.; Davis, J.M.; Bartoli, W.P.; Woods, J.A. Failure of low dose carbohydrate feeding to attenuate glucoregulatory hormone responses and improve endurance performance. Int. J. Sport Nutr. 1991, 1, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Lin, C.L.; Chew, S.A.N.; Ming, E.T.Y. Water versus Carbohydrate-Electrolyte Fluid Replacement during. Mil. Med. 2005, 170, 715–722. [Google Scholar] [CrossRef]
- Campbell, C.; Prince, D.; Braun, M.; Applegate, E.; Casazza, G.A. Carbohydrate-supplement form and exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Jeukendrup, A.E.; Mundel, T.; Jones, D.A. Carbohydrate supplementation improves moderate and high-intensity exercise in the heat. Pflug. Arch. Eur. J. Physiol. 2003, 446, 211–219. [Google Scholar] [CrossRef]
- Carter, J.; Jeukendrup, A.E.; Jones, D.A. The effect of sweetness on the efficacy of carbohydrate supplementation during exercise in the heat. Can. J. Appl. Physiol. 2005, 30, 379–391. [Google Scholar] [CrossRef]
- Clark, V.R.; Hopkins, W.G.; Hawley, J.A.; Burke, L.M. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med. Sci. Sports Exerc. 2000, 32, 1642–1647. [Google Scholar] [CrossRef]
- Clarke, N.D.; Maclaren, D.P.M.; Reilly, T.; Drust, B. Carbohydrate ingestion and pre-cooling improves exercise capacity following soccer-specific intermittent exercise performed in the heat. Eur. J. Appl. Physiol. 2011, 111, 1447–1455. [Google Scholar] [CrossRef]
- Cole, K.J.; Grandjean, P.W.; Sobszak, R.J.; Mitchell, J.B. Effect of carbohydrate composition on fluid balance, gastric emptying, and exercise performance. Int. J. Sport Nutr. 1993, 3, 408–417. [Google Scholar] [CrossRef]
- Cole, M.; Hopker, J.G.; Wiles, J.D.; Coleman, D.A. The effects of acute carbohydrate and caffeine feeding strategies on cycling efficiency. J. Sports Sci. 2018, 36, 817–823. [Google Scholar] [CrossRef]
- Coletta, A.; Thompson, D.L.; Raynor, H.A. The influence of commercially-available carbohydrate and carbohydrate-protein supplements on endurance running performance in recreational athletes during a field trial. J. Int. Soc. Sports Nutr. 2013, 10, 17. [Google Scholar] [CrossRef]
- Cureton, K.J.; Warren, G.L.; Millard-Stafford, M.L.; Wingo, J.E.; Trilk, J.; Buyckx, M. Caffeinated sports drink: Ergogenic effects and possible mechanisms. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Currell, K.; Jeukendrup, A.E. Superior endurance performance with ingestion of multiple transportable carbohydrates. Med. Sci. Sports Exerc. 2008, 40, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Welsh, R.S.; Alderson, N.A. Effects of carbohydrate and chromium ingestion during intermittent high-intensity exercise to fatigue. Int. J. Sport Nutr. 2000, 10, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, B.; Anderson, S.; Barrett, J.; Rao, E.; Hargreaves, M. Carbohydrate-electrolyte feedings and 1h time trial cycling performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 541–549. [Google Scholar] [CrossRef]
- Duhamel, T.A.; Green, H.J.; Stewart, R.D.; Foley, K.P.; Smith, I.C.; Ouyang, J. Muscle metabolic, SR Ca2+-cycling responses to prolonged cycling, with and without glucose supplementation. J. Appl. Physiol. 2007, 103, 1986–1998. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Rattu, A.J.M.; Roberts, I. Effects of carbohydrate feeding before and during prolonged exercise on subsequent maximal exercise performance capacity. Int. J. Sport Nutr. 1995, 5, 215–224. [Google Scholar] [CrossRef][Green Version]
- El-Sayed, M.S.; Balmer, J.; Rattu, A.J.M. Carbohydrate ingestion improves endurance performance during a 1 h simulated cycling time trial. J. Sports Sci. 1997, 15, 223–230. [Google Scholar] [CrossRef]
- Finger, D.; Farinha, J.B.; Brusco, C.M.; Boeno, F.P.; Cadore, E.L.; Pinto, R.S.; Lanferdini, F.J.; Helal, L. Ingestion of carbohydrate or carbohydrate plus protein does not enhance performance during endurance exercise: A randomized crossover placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 2018, 43, 937–944. [Google Scholar] [CrossRef]
- Fulco, C.S.; Zupan, M.; Muza, S.R.; Rock, P.B.; Kambis, K.; Payn, T.; Hannon, M.; Glickman, E.; Cymerman, A. Carbohydrate supplementation and endurance performance of moderate altitude residents at 4300 m. Int. J. Sports Med. 2007, 28, 437–443. [Google Scholar] [CrossRef]
- Funnell, M.P.; Dykes, N.R.; Owen, E.J.; Mears, S.A.; Rollo, I.; James, L.J. Ecologically valid carbohydrate intake during soccer-specific exercise does not affect running performance in a fed state. Nutrients 2017, 9, 39. [Google Scholar] [CrossRef]
- Ganio, M.S.; Klau, J.F.; Lee, E.C.; Yeargin, S.W.; McDermott, B.P.; Buyckx, M.; Maresh, C.M.; Armstrong, L.E. Effect of various carbohydrate-electrolyte fluids on cycling performance and maximal voluntary contraction. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 104–114. [Google Scholar] [CrossRef]
- Glace, B.W.; Kremenic, I.J.; McHugh, M.P. Effect of carbohydrate beverage ingestion on central versus peripheral fatigue: A placebo-controlled, randomized trial in cyclists. Appl. Physiol. Nutr. Metab. 2019, 44, 139–147. [Google Scholar] [CrossRef]
- Goedecke, J.H.; White, N.J.; Chicktay, W.; Mahomed, H.; Durandt, J.; Lambert, M.I. The effect of carbohydrate ingestion on performance during a simulated soccer match. Nutrients 2013, 5, 5193–5204. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Sun, F.; Si, G.; Chen, Y. Effect of protein and carbohydrate solutions on running performance and cognitive function in female recreational runners. PLoS ONE 2017, 12, e0185982. [Google Scholar] [CrossRef] [PubMed]
- Heesch, M.W.S.; Mieras, M.E.; Slivka, D.R. The performance effect of early versus late carbohydrate feedings during prolonged exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 58–63. [Google Scholar] [CrossRef]
- Hulston, C.J.; Jeukendrup, A.E. Substrate metabolism and exercise performance with caffeine and carbohydrate intake. Med. Sci. Sports Exerc. 2008, 40, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Jeukendrup, A.E. No placebo effect from carbohydrate intake during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 275–284. [Google Scholar] [CrossRef]
- Ivy, J.L.; Miller, W.; Dover, V.; Goodyear, L.G.; Sherman, W.M.; Farrell, S.; Williams, H. Endurance improved by ingestion of a glucose polymer supplement. Med. Sci. Sports Exerc. 1983, 15, 466–471. [Google Scholar] [CrossRef]
- Jarvis, A.T.; Felix, S.D.; Sims, S.; Jones, M.T.; Anne, M.; Headley, S.A. Carbohydrate supplementation fails to improve the sprint performance of female cyclists. JEP Online 1999, 2, 16–23. [Google Scholar]
- Jeukendrup, A.; Brouns, F.; Wagenmakers, A.J.M.; Saris, W.H.M. Carbohydrate-electrolyte feedings improve 1 h time trial cycling performance. Int. J. Sports Med. 1997, 18, 125–129. [Google Scholar] [CrossRef]
- Kang, J.; Robertson, R.J.; Denys, B.G.; DaSilva, S.G.; Visich, P.; Suminski, R.R.; Utter, A.C.; Goss, F.L.; Metz, K.F. Effect of carbohydrate ingestion subsequent to carbohydrate supercompensation on endurance performance. Int. J. Sport Nutr. 1995, 5, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Robertson, R.J.; Goss, F.L.; DaSilva, S.G.; Visich, P.; Suminski, R.R.; Utter, A.C.; Denys, B.C. Effect of carbohydrate substrate availability on ratings of perceived exertion during prolonged exercise of moderate intensity. Percept. Mot. Ski. 1996, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Khanna, G.L.; Manna, I. Supplementary effect of carbohydrate-electrolyte drink on sports performance, lactate removal & cardiovascular response of athletes. Indian J. Med. Res. 2005, 121, 665–669. [Google Scholar]
- King, A.J.; O’Hara, J.P.; Morrison, D.J.; Preston, T.; King, R.F.G.J. Carbohydrate dose influences liver and muscle glycogen oxidation and performance during prolonged exercise. Physiol. Rep. 2018, 6, e13555. [Google Scholar] [CrossRef]
- King, A.J.; O’Hara, J.P.; Arjomandkhah, N.C.; Rowe, J.; Morrison, D.J.; Preston, T.; King, R.F.G.J. Liver and muscle glycogen oxidation and performance with dose variation of glucose–fructose ingestion during prolonged (3 h) exercise. Eur. J. Appl. Physiol. 2019, 119, 1157–1169. [Google Scholar] [CrossRef]
- Kingwell, B.; McKenna, M.J.; Sandstrom, E.R.; Hargreaves, M. Effect of glucose polymer ingestion on energy and fluid balance during exercise. J. Sports Sci. 1989, 7, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Learsi, S.K.; Ghiarone, T.; Silva-Cavalcante, M.D.; Andrade-Souza, V.A.; Ataide-Silva, T.; Bertuzzi, R.; de Araujo, G.G.; McConell, G.; Lima-Silva, A.E. Cycling time trial performance is improved by carbohydrate ingestion during exercise regardless of a fed or fasted state. Scand. J. Med. Sci. Sports 2019, 29, 651–662. [Google Scholar] [CrossRef]
- Lugo, M.; Sherman, W.M.; Wimer, G.S.; Garleb, K. Metabolic responses when different forms of carbohydrate energy are consumed during cycling. Int. J. Sport Nutr. 1993, 3, 398–407. [Google Scholar] [CrossRef]
- Martínez-Lagunas, V.; Ding, Z.; Bernard, J.R.; Wang, B.; Ivy, J.L. Added Protein Maintains Efficacy of a Low-Carbohydrate Sports Drink. J. Strength Cond. Res. 2010, 24, 48–59. [Google Scholar] [CrossRef]
- Maughan, R.J.; Fenn, C.E.; Leiper, J.B. Effects of fluid, electrolyte and substrate ingestion on endurance capacity. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 481–486. [Google Scholar] [CrossRef]
- McConell, G.; Kloot, K.; Hargreaves, M. Effect of timing of carbohydrate ingestion on endurance exercise performance. Med. Sci. Sports Exerc. 1996, 28, 1300–1304. [Google Scholar] [CrossRef]
- McConell, G.; Snow, R.J.; Proietto, J.; Hargreaves, M. Muscle metabolism during prolonged exercise in humans: Influence of carbohydrate availability. J. Appl. Physiol. 1999, 87, 1083–1086. [Google Scholar] [CrossRef]
- McConell, G.K.; Canny, B.J.; Daddo, M.C.; Nance, M.J.; Snow, R.J. Effect of carbohydrate ingestion on glucose kinetics and muscle metabolism during intense endurance exercise. J. Appl. Physiol. 2000, 89, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- McGawley, K.; Shannon, O.; Betts, J. Ingesting a high-dose carbohydrate solution during the cycle section of a simulated olympicdistance triathlon improves subsequent run performance. Appl. Physiol. Nutr. Metab. 2012, 37, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Millard-Stafford, M.; Sparling, P.B.; Rosskopf, L.B.; Hinson, B.T.; Dicarlo, L.J. Carbohydrate-electrolyte replacement during a simulated triathlon in the heat. Med. Sci. Sports Exerc. 1990, 22, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Millard-Stafford, M.L.; Sparling, P.B.; Rosskopf, L.B.; Snow, T.K. Should carbohydrate concentration of a sports drink be less than 8% during exercise in the heat? Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 117–130. [Google Scholar] [CrossRef]
- Millard-Stafford, M.L.; Cureton, K.J.; Wingo, J.E.; Trilk, J.; Warren, G.L.; Buyckx, M. Hydration during exercise in warm, humid conditions: Effect of a caffeinated sports drink. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 163–177. [Google Scholar] [CrossRef]
- Morris, J.G.; Nevill, M.E.; Thompson, D.; Collie, J.; Williams, C. The influence of a 6.5% carbohydrate-electrolyte solution on performance of prolonged intermittent high-intensity running at 30 °C. J. Sports Sci. 2003, 21, 371–381. [Google Scholar] [CrossRef]
- Murray, R.; Eddy, D.E.; Murray, T.W.; Seifert, J.G.; Paul, G.L.; Halaby, G.A. The effect of fluid and carbohydrate feedings during intermittent cycling exercise. Med. Sci. Sports Exerc. 1987, 19, 597–604. [Google Scholar] [CrossRef]
- Murray, R.; Seifert, J.G.; Eddy, D.E.; Paul, G.L.; Halaby, G.A. Carbohydrate feeding and exercise: Effect of beverage carbohydrate content. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 152–158. [Google Scholar] [CrossRef]
- Murray, R.; Paul, G.L.; Seifert, J.G.; Eddy, D.E. Responses to varying rates of carbohydrate ingestion during exercise. Med. Sci. Sports Exerc. 1991, 23, 713–718. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Judith, A.; Alkhatib, A.; Baddeley-White, D.; Garcia, S.; Zanotto, T. Effect of a multi-ingredient supplement on intermittent sprint performance, fatigue perception, muscle damage and immunosuppression in recreational athletes. J. Int. Soc. Sports Nutr. 2014, 11, 1151–1158. [Google Scholar] [CrossRef]
- Nassif, C.; Gomes, A.R.; Peixoto, G.H.C.; Chagas, M.H.; Soares, D.D.; Silami-Garcia, E.; Drinkwater, E.J.; Cannon, J.; Marino, F.E. The effect of double—Blind carbohydrate ingestion during 60 km of self-paced exercise in warm ambient conditions. PLoS ONE 2014, 9, e104710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nassis, G.P.; Williams, C.; Chisnall, P. Effect of a carbohydrate-electrolyte drink on endurance capacity during prolonged intermittent high intensity running. Br. J. Sports Med. 1998, 32, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.L.; Hunter, A.M.; Lawrence, C.; Tipton, K.D.; Galloway, S.D.R. The ingestion of 39 or 64 g·hr-1 of carbohydrate is equally effective at improving endurance exercise performance in cyclists. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 285–292. [Google Scholar] [CrossRef]
- Nicholas, C.W.; Williams, C.; Lakomy, H.K.A.; Phillips, G.; Nowitz, A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J. Sports Sci. 1995, 13, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, V.; Arkinstall, M.J.; Hawley, J.A. Reduced Neuromuscular Activity with Carbohydrate Ingestion during Constant Load Cycling. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 161–170. [Google Scholar] [CrossRef]
- Nishibata, I.; Sadamoto, T.; Mutoh, Y.; Miyashita, M. Glucose ingestion before and during exercise does not enhance performance of daily repeated endurance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 65–69. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Carstens, M.; Millen, A.M.E. Ingesting isomaltulose versus fructose-maltodextrin during prolonged moderate-heavy exercise increases fat oxidation but impairs gastrointestinal comfort and cycling performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 427–438. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Carstens, M.; Millen, A.M.E. Whey or Casein Hydrolysate with Carbohydrate for Metabolism and Performance in Cycling. Int. J. Sports Med. 2015, 36, 636–646. [Google Scholar] [CrossRef]
- Osterberg, K.L.; Zachwieja, J.J.; Smith, J.W. Carbohydrate and carbohydrate + protein for cycling time-trial performance. J. Sports Sci. 2008, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.P.; Carroll, S.; Cooke, C.B.; King, R.F.G.J. The effect of pre-exercise galactose and glucose ingestion on high-intensity endurance cycling. J. Strength Cond. Res. 2014, 28, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, E.K.; Poulos, S.P.; Wingo, J.E.; Richardson, M.T.; Bishop, P.A. Post-prandial carbohydrate ingestion during 1-h of moderate-intensity, intermittent cycling does not improve mood, perceived exertion, or subsequent power output in recreationally-active exercisers. J. Int. Soc. Sports Nutr. 2013, 10, 4. [Google Scholar] [CrossRef]
- Pettersson, S.; Edin, F.; Bakkman, L.; McGawley, K. Effects of supplementing with an 18% carbohydrate-hydrogel drink in -5 °C with elite cross-country ski athletes: A crossover study. J. Int. Soc. Sports Nutr. 2019, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Pottier, A.; Bouckaert, J.; Gilis, W.; Roels, T.; Derave, W. Mouth rinse but not ingestion of a carbohydrate solution improves 1-h cycle time trial performance. Scand. J. Med. Sci. Sports 2010, 20, 105–111. [Google Scholar] [CrossRef]
- Riley, M.L.; Israel, R.G.; Holbert, D.; Tapscott, E.B.; Dohm, G.L. Effect of carbohydrate ingestion on exercise endurance and metabolism after a 1-day fast. Int. J. Sports Med. 1988, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Tarpey, M.D.; Kass, L.S.; Tarpey, R.J.; Roberts, M.G. Assessing a commercially available sports drink on exogenous carbohydrate oxidation, fluid delivery and sustained exercise performance. J. Int. Soc. Sports Nutr. 2014, 11, 8. [Google Scholar] [CrossRef]
- Robson-Ansley, P.; Barwood, M.; Eglin, C.; Ansley, L. The effect of carbohydrate ingestion on the interleukin-6 response to a 90-minute run time trial. Int. J. Sports Physiol. Perform. 2009, 4, 186–194. [Google Scholar] [CrossRef]
- Robson-Ansley, P.; Walshe, I.; Ward, D. The effect of carbohydrate ingestion on plasma interleukin-6, hepcidin and iron concentrations following prolonged exercise. Cytokine 2011, 53, 196–200. [Google Scholar] [CrossRef]
- Rollo, I.; Williams, C. Influence of ingesting a carbohydrate-electrolyte solution before and during a 1-hr running performance test. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 645–658. [Google Scholar] [CrossRef]
- Rollo, I.; Williams, C. Influence of ingesting a carbohydrate-electrolyte solution before and during a 1-hour run in fed endurance-trained runners. J. Sports Sci. 2010, 28, 593–601. [Google Scholar] [CrossRef]
- Rollo, I.; Williams, C.; Nevill, M. Influence of ingesting versus mouth rinsing a carbohydrate solution during a 1-h Run. Med. Sci. Sports Exerc. 2011, 43, 468–475. [Google Scholar] [CrossRef]
- Smith, J.W.; Zachwieja, J.J.; Péronnet, F.; Passe, D.H.; Massicotte, D.; Lavoie, C.; Pascoe, D.D. Fuel selection and cycling endurance performance with ingestion of [13C]glucose: Evidence for a carbohydrate dose response. J. Appl. Physiol. 2010, 108, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Curmaci, A.; Patrie, J.T.; Gaesser, G.A.; Weltman, A. Effects of carbohydrate supplementation on the RPE-blood lactate relationship. Med. Sci. Sports Exerc. 2009, 41, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.H.; Wong, S.H.S.; Chen, S.H.; Poon, T.C. Carbohydrate electrolyte solutions enhance endurance capacity in active females. Nutrients 2015, 7, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Tokmakidis, S.P.; Karamanolis, I.A. Effects of carbohydrate ingestion 15 min before exercise on endurance running capacity. Appl. Physiol. Nutr. Metab. 2008, 33, 441–449. [Google Scholar] [CrossRef]
- Tsintzas, K.; Liu, R.; Williams, C.; Campbell, I.; Gaitanos, G. The effect of carbohydrate ingestion on performance during a 30-km race. Int. J. Sport Nutr. 1993, 3, 127–139. [Google Scholar] [CrossRef]
- Tsintzas, O.K.; Willia, C.; Singh, R.; Wilson, W.; Burrin, J. Influence of carbohydrate-electrolyte drinks on marathon running performance. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 154–160. [Google Scholar] [CrossRef]
- Tsintzas, O.K.; Williams, C.; Boobis, L.; Greenhaff, P. Carbohydrate ingestion and single muscle fiber glycogen metabolism during prolonged running in men. J. Appl. Physiol. 1996, 81, 801–809. [Google Scholar] [CrossRef]
- Tsintzas, O.K.; Williams, C.; Wilson, W.; Burrin, J. Influence of carbohydrate supplementation early in exercise on endurance running capacity. Med. Sci. Sports Exerc. 1996, 28, 1373–1379. [Google Scholar] [CrossRef]
- Utter, A.C.; Kang, J.; Robertson, R.J.; Nieman, D.C.; Chaloupka, E.C.; Suminski, R.R.; Piccinni, C.R. Effect of carbohydrate ingestion on ratings of perceived exertion during a marathon. Med. Sci. Sports Exerc. 2002, 34, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, M.; Gibala, M.J. Failure of protein to improve time trial performance when added to a sports drink. Med. Sci. Sports Exerc. 2006, 38, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Walton, P.T.; Rhodes, E.C. The effects of solid and liquid carbohydrate ingestion on high-intensity intermittent exercise performance. Biol. Sport 1997, 14, 45–54. [Google Scholar]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. Effect of dilute CHO beverages on performance in cool and warm environments. Med. Sci. Sports Exerc. 2012, 44, 336–343. [Google Scholar] [CrossRef]
- Wilber, R.L.; Moffatt, R.J. Influence of carbohydrate ingestion on blood glucose and performance in runners. Int. J. Sport Nutr. 1992, 2, 317–327. [Google Scholar] [CrossRef]
- Williams, C.; Nute, M.G.; Broadbank, L.; Vinall, S. Influence of fluid intake on endurance running performance—A comparison between water, glucose and fructose solutions. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 112–119. [Google Scholar] [CrossRef]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef]
- Temesi, J.; Johnson, N.A.; Raymond, J.; Burdon, C.A.; O’Connor, H.T. Carbohydrate ingestion during endurance exercise improves performance in adults. J. Nutr. 2011, 141, 890–897. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef]
- Wilson, P.B. Does carbohydrate intake during endurance running improve performance? A critical review. J. Strength Cond. Res. 2016, 30, 3539–3559. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Costill, D.L.; Houmard, J.A.; Fink, W.J.; Robergs, R.A.; Davis, J.A. Gastric emptying influence of prolonged exercise and carbohydrate concentration. Med. Sci. Sports Exerc. 1989, 21, 269–374. [Google Scholar] [PubMed]
- Shi, X.; Summers, R.W.; Schedl, H.P.; Flanagan, S.W.; Chang, R.; Gisolfi, C.V. Effects of carbohydrate type and concentration and solution osmolality on water absorption. Med. Sci. Sports Exerc. 1995, 27, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Rehrer, N.J.; Beckers, E.; Brouns, F.; Hoor ten, F.; Saris, W.H. Exercise and training effects on gastric emptying of carbohydrate beverages. Med. Sci. Sports Exerc. 1989, 21, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Senden, J.; Beckers, E.J.; Saris, W.H.M. Osmolarity does not affect the gastric emptying rate of oral rehydration solutions. J. Parenter. Enter. Nutr. 1995, 19, 403–406. [Google Scholar] [CrossRef]
- Murray, R.; Bartoli, W.; Stofan, J.; Horn, M.; Eddy, D. A comparison of the gastric emptying characteristics of selected sports drinks. Int. J. Sport Nutr. 1999, 9, 263–274. [Google Scholar] [CrossRef]
- Shi, X.; Horn, M.K.; Osterberg, K.L.; Stofan, J.R.; Zachwieja, J.J.; Horswill, C.A.; Passe, D.H.; Murray, R. Gastrointestinal discomfort during intermittent high-intensity exercise: Effect of carbohydrate-electrolyte beverage. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 673–683. [Google Scholar] [CrossRef]
- Peters, H.P.; van Schelven, F.W.; Verstappen, P.A.; de Boer, R.W.; Bol, E.; Erich, W.B.; van der Togt, C.R.; de Vries, W.R. Gastrointestinal problems as a function of carbohydrate supplements and mode of exercise. Med. Sci. Sports Exerc. 1993, 25, 1211–1224. [Google Scholar] [CrossRef]
- Peters, H.P.F.; Akkermans, L.M.A.; Bol, E.; Mosterd, W.L. Gastrointestinal Symptoms During Exercise: The Effect of Fluid Supplementation. Sports Med. 1995, 20, 65–76. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C. Carbohydrate-dependent, exercise-induced gastrointestinal distress. Nutrients 2014, 6, 4191–4199. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef]
- Williams, M.; Rawson, E.; Branch, D. Nutrition for Health, Fitness and Sport, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2017; ISBN 9780078021350. [Google Scholar]
- Stellingwerff, T. Contemporary nutrition approaches to optimize elite marathon performance. Int. J. Sports Physiol. Perform. 2013, 8, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Wahrenberg, V.; Carlsson, M.S.; Andersson, R.; Carlsson, T. Gross and delta efficiencies during uphill running and cycling among elite triathletes. Eur. J. Appl. Physiol. 2020, 120, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Tsintzas, K.; Williams, C. Human muscle glycogen metabolism during exercise. Effect of carbohydrate supplementation. Sports Med. 1998, 25, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Zaltas, E.; Hodgson, A.B.; Jeukendrup, A.E. Carbohydrate oxidation from a drink during running compared with cycling exercise. Med. Sci. Sports Exerc. 2011, 43, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Coyle, E.F. Carbohydrate ingestion during prolonged exercise: Effects on metabolism and performance. Exerc. Sport Sci. Rev. 1991, 19, 1–40. [Google Scholar] [CrossRef]
- Coyle, E.F.; Montain, S.J. Carbohydrate and fluid ingestion during exercise: Are there trade-offs? Med. Sci. Sports Exerc 1992, 24, 671–678. [Google Scholar] [CrossRef]
- Coyle, E.F. Carbohydrate feeding during exercise. Int. J. Sports Med. 1992, 13, 126–128. [Google Scholar] [CrossRef]
- Coyle, E.F. Carbohydrate supplementation during exercise. J. Nutr. 1992, 122, 788–795. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Piehl, K.; Saltin, B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J. Physiol. 1974, 241, 45–57. [Google Scholar] [CrossRef]
- Currell, K.; Jeukendrup, A. Validity, Reliability and Sensitivity of Measures of Sporting Performance. Sports Med. 2008, 38, 297–316. [Google Scholar] [CrossRef]
- Williams, C.; Rollo, I. Carbohydrate Nutrition and Team Sport Performance. Sports Med. 2015, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Rollo, I.; Stein, K.W.; Jeukendrup, A.E. Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients 2015, 7, 5733–5763. [Google Scholar] [CrossRef]
- Berg, J.M.; Stryer, L.; Tymoczko, J.; Gatto, G. Biochemistry, 9th ed.; W.H. Freeman & Co. Ltd.: New York, NY, USA, 2019; ISBN 9781319114657. [Google Scholar]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J.F. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol.-Endocrinol. Metab. 1999, 277. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol.-Endocrinol. Metab. 1993, 265, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Uchiyama, N.; Kizaki, S.; Mori, E.; Nonaka, T.; Oneda, H. Application of Continuous Glucose Monitoring for Assessment of Individual Carbohydrate Requirement during Ultramarathon Race. Nutrients 2020, 12, 1121. [Google Scholar] [CrossRef]
- Alfageme, R.J.; López, L.A.; Ayuso, J.M.; Martínez-Sanz, J.M. Analysis of nutritional intake in trail runners during competition. Nutr. Hosp. 2020, 38, 2. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Knechtle, B.; Tarnopolsky, M.; Hoffman, M.D. Nutrition for ultramarathon running: Trail, track, and road. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 130–140. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional considerations for single-stage ultra-marathon training and racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- Martinez, S.; Aguilo, A.; Rodas, L.; Lozano, L.; Moreno, C.; Tauler, P. Energy, macronutrient and water intake during a mountain ultramarathon event: The influence of distance. J. Sports Sci. 2018, 36, 333–339. [Google Scholar] [CrossRef]
- Friedlander, A.L.; Casazza, G.A.; Horning, M.A.; Huie, M.J.; Brooks, G.A. Training-induced alterations of glucose flux in men. J. Appl. Physiol. 1997, 82, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Kohrt, W.M.; Spina, R.J.; Bier, D.M.; Holloszy, J.O. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J. Appl. Physiol. 1990, 68, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Hopkins, W.G. Skeletal Muscle Glycogen Content at Rest and During Endurance Exercise in Humans: A Meta-Analysis. Sports Med. 2018, 48, 2091–2102. [Google Scholar] [CrossRef]
- Bergman, B.C.; Butterfield, G.E.; Wolfel, E.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol.-Endocrinol. Metab. 1999, 277, 81–92. [Google Scholar] [CrossRef]
- Saunders, M.J.; Luden, N.D.; Herrick, J.E. Consumption of an oral carbohydrate-protein gel improves cycling endurance and prevents postexercise muscle damage. J. Strength Cond. Res. 2007, 21, 678–684. [Google Scholar] [CrossRef]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp. Physiol. 2016, 101, 243–249. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, 898–907. [Google Scholar] [CrossRef]
- Riddell, M.C.; Partington, S.L.; Stupka, N.; Armstrong, D.; Rennie, C.; Tarnopolsky, M.A. Substrate Utilization during Exercise Performed with and Without Glucose Ingestion in Female and Male Endurance-Trained Athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 407–421. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, 1120–1128. [Google Scholar] [CrossRef]
- Hargreaves, M. Exercise, muscle, and CHO metabolism. Scand. J. Med. Sci. Sports 2015, 25, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Smith-ryan, A.E.; Hirsch, K.R.; Roberts, M.D.; Vandusseldorp, T.A.; Stratton, M.T.; Kaviani, M.; Little, J.P. Supplements and nutritional interventions to augment high-intensity interval training physiological and performance adaptations—A narrative review. Nutrients 2020, 12, 390. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Diamond, J. Regulation of intestinal sugar transport. Physiol. Rev. 1997, 77, 257–302. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L. The facilitated component of intestinal glucose absorption. J. Physiol. 2001, 531, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Vitavska, O.; Wieczorek, H. Identification of an animal sucrose transporter. J. Cell Sci. 2011, 124, 1984–1991. [Google Scholar] [CrossRef]
- Koivisto, V.A. Fructose as a Dietary Sweetener in Diabetes Mellitus. Diabetes Care 1978, 1, 241–246. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Karonen, S.L.; Nikkilä, E.A. Carbohydrate ingestion before exercise: Comparison between glucose and fructose. J. Appl. Physiol. Respir. Env. Exerc. Physiol. 1981, 40, 783–787. [Google Scholar] [CrossRef]
- Massicotte, D.; Péronnet, F.; Allah, C.; Hillaire-Marcel, C.; Ledoux, M.; Brisson, G. Metabolic response to [13C] glucose and [13C] fructose ingestion during exercise. J. Appl. Physiol. 1986, 61, 1180–1184. [Google Scholar] [CrossRef]
- Massicotte, D.; Peronnet, F.; Brisson, G.; Bakkouch, K.; Hillaire-Marcel, C. Oxidation of a glucose polymer during exercise: Comparison with glucose and fructose. J. Appl. Physiol. 1989, 66, 179–183. [Google Scholar] [CrossRef]
- Jandrain, B.J.; Pallikarakis, N.; Normand, S.; Pirnay, F.; Lacroix, M.; Mosora, F.; Pachiaudi, C.; Gautier, J.F.; Scheen, A.J.; Riou, J.P.; et al. Fructose utilization during exercise in men: Rapid conversion of ingested fructose to circulating glucose. J. Appl. Physiol. 1993, 74, 2146–2154. [Google Scholar] [CrossRef]
- Adopo, E.; Peronnet, F.; Massicotte, D.; Brisson, G.R.; Hillaire-Marcel, C. Respective oxidation of exogenous glucose and fructose given in the same drink during exercise. J. Appl. Physiol. 1994, 76, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate and exercise performance: The role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Burelle, Y.; Peronnet, F.; Massicotte, D.; Brison, G.R.; Hillaire-Marcel, C. Oxidation of 13C-glucose and 13C-fructose ingested as a preexercise meal: Effect of carbohydrate ingestion during exercise. Int. J. Sport Nutr. 1997, 7, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Burke, L.M. Effect of meal frequency and timing on physical performance. Br. J. Nutr. 1997, 77, S91–S103. [Google Scholar] [CrossRef]
- Hawley, J.A.; Dennis, S.C.; Noakes, T.D. Oxidation of Carbohydrate Ingested During Prolonged Endurance Exercise. Sports Med. 1992, 14, 27–42. [Google Scholar] [CrossRef]
- Wagenmakers, A.J.M.; Brouns, F.; Saris, W.H.M.; Halliday, D. Oxidation rates of orally ingested carbohydrates during prolonged exercise in men. J. Appl. Physiol. 1993, 75, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Seiple, R.S.; Vivian, V.M.; Fox, L.; Bartels, R.L. Gastric emptying characteristics of two glucose polymer-electrolyte solutions. Med. Sci. Sports Exerc. 1983, 15, 366–369. [Google Scholar] [CrossRef]
- Sole, C.C.; Noakes, T.D. Faster gastric emptying for glucose-polymer and fructose solutions than for glucose in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 605–612. [Google Scholar] [CrossRef]
- Moodley, D.; Noakes, T.D.; Bosch, A.N.; Hawley, J.A.; Schall, R.; Dennis, S.C. Oxidation of exogenous carbohydrate during prolonged exercise: The effects of the carbohydrate type and its concentration. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 328–334. [Google Scholar] [CrossRef]
- Neufer, P.D.; Costill, D.L.; Fink, W.J.; Kirwan, J.P.; Fielding, R.A.; Flynn, M.G. Effects of exercise and carbohydrate composition on gastric emptying. Med. Sci. Sports Exerc. 1986, 18, 658–662. [Google Scholar] [CrossRef]
- Foster, C.; Costill, D.L.; Fink, W.J. Gastric emptying characteristics of glucose and glucose polymer solutions. Res. Q. Exerc. Sport 1980, 51, 299–305. [Google Scholar] [CrossRef]
- Jentjens, R.L.P.G.; Moseley, L.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and sucrose during exercise. J. Appl. Physiol. 2004, 96, 1277–1284. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate feeding during exercise. Eur. J. Sport Sci. 2008, 8, 77–86. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Moseley, L.; Mainwaring, G.I.; Samuels, S.; Perry, S.; Mann, C.H. Exogenous carbohydrate oxidation during ultraendurance exercise. J. Appl. Physiol. 2006, 100, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Swift, M.; Ros, M.; Green, J.G. Composite versus single transportable carbohydrate solution enhances race and laboratory cycling performance. Appl. Physiol. Nutr. Metab. 2012, 37, 425–436. [Google Scholar] [CrossRef]
- Triplett, D.; Doyle, J.A.; Rupp, J.C.; Benardot, D. An isocaloric glucose-fructose beverage’s effect on simulated 100-km cycling performance compared with a glucose-only beverage. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 122–131. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose–Glucose Composite Carbohydrates and Endurance Performance: Critical Review and Future Perspectives. Sports Med. 2015, 45, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Gisolfi, C.V. Use of electrolytes in fluid replacement solutions: What have we learned from intestinal absorption studies? In Fluid Replacement and Heat Stress; Marriott, B.M., Ed.; Institute of Medicine (US) Committee on Military Nutrition Research: Washington, DC, USA, 1994; pp. 11–22. ISBN 0309573459. [Google Scholar]
- Gisolfi, C.V.; Duchman, S.M. Guidelines for optimal replacement beverages for different athletic events. Med. Sci. Sports Exerc. 1992, 24, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef]
- Burke, L.M.; Jones, A.M.; Jeukendrup, A.E.; Mooses, M. Contemporary nutrition strategies to optimize performance in distance runners and race walkers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.W.; Zachwieja, J.J.; Horswill, C.A.; Pascoe, D.D.; Passe, D.; Ruby, B.C.; Stewart, L.K. Evidence of a Carbohydrate Dose and Prolonged Exercise Performance Relationship. Med. Sci. Sports Exerc. 2010, 42, 84. [Google Scholar] [CrossRef]
- Jeukendrup, A. Carbohydrate during exercise: Research of last 10 years turned into new recommendations. Apunt. Educ. Física Deport. 2013, 113, 7–22. [Google Scholar] [CrossRef]
- Smith, J.W.; Pascoe, D.D.; Passe, D.H.; Ruby, B.C.; Stewart, L.K.; Baker, L.B.; Zachwieja, J.J. Curvilinear dose-response relationship of carbohydrate (0–120 g·h-1) and performance. Med. Sci. Sports Exerc. 2013, 45, 336–341. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. J. Sports Sci. 2011, 29, S91–S99. [Google Scholar] [CrossRef]
- Jentjens, R.L.P.G.; Shaw, C.; Birtles, T.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and sucrose during exercise. Metabolism 2005, 54, 610–618. [Google Scholar] [CrossRef]
- Currell, K.; Urch, J.; Cerri, E.; Jentjens, R.L.P.; Blannin, A.K.; Jeukendrup, A.E. Plasma deuterium oxide accumulation following ingestion of different carbohydrate beverages. Appl. Physiol. Nutr. Metab. 2008, 33, 1067–1072. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef]
- Lavoué, C.; Siracusa, J.; Chalchat, É.; Bourrilhon, C.; Charlot, K. Analysis of food and fluid intake in elite ultra-endurance runners during a 24-h world championship. J. Int. Soc. Sports Nutr. 2020, 17, 36. [Google Scholar] [CrossRef]
- Yeo, S.E.; Jentjens, R.L.P.G.; Wallis, G.A.; Jeukendrup, A.E. Caffeine increases exogenous carbohydrate oxidation during exercise. J. Appl. Physiol. 2005, 99, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Zaltas, E.; Jeukendrup, A.E. CHO oxidation from a CHO Gel compared with a drink during exercise. Med. Sci. Sports Exerc. 2010, 42, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.P.G.; Underwood, K.; Achten, J.; Currell, K.; Mann, C.H.; Jeukendrup, A.E. Exogenous carbohydrate oxidation rates are elevated after combined ingestion of glucose and fructose during exercise in the heat. J. Appl. Physiol. 2006, 100, 807–816. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Stellingwerff, T.; Zaltas, E.; Jeukendrup, A.E. Oxidation of solid versus liquid CHO sources during exercise. Med. Sci. Sports Exerc. 2010, 42, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; McLaughlin, J. Carbohydrate ingestion during exercise: Effects on performance, training adaptations and trainability of the gut. Nestle Nutr. Inst. Workshop Ser. 2011, 69, 1–17. [Google Scholar] [CrossRef]
- Lesinski, M.; Hortobágyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Dose-Response Relationships of Balance Training in Healthy Young Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 557–576. [Google Scholar] [CrossRef]
- Goulet, E.D.B.; Hoffman, M.D. Impact of Ad Libitum Versus Programmed Drinking on Endurance Performance: A Systematic Review with Meta-Analysis. Sports Med. 2019, 49, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Pöchmüller, M.; Schwingshackl, L.; Colombani, P.C.; Hoffmann, G. A systematic review and meta-analysis of carbohydrate benefits associated with randomized controlled competition-based performance trials. J. Int. Soc. Sports Nutr. 2016, 13, 27. [Google Scholar] [CrossRef]
- Brietzke, C.; Franco-Alvarenga, P.E.; Coelho-Júnior, H.J.; Silveira, R.; Asano, R.Y.; Pires, F.O. Effects of Carbohydrate Mouth Rinse on Cycling Time Trial Performance: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 57–66. [Google Scholar] [CrossRef]
- Thomas, J.R.; French, K.E. The use of meta-analysis in exercise and sport: A tutorial. Res. Q. Exerc. Sport 1986, 57, 196–204. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Viribay, A.; Arribalzaga, S.; Mielgo-Ayuso, J.; Castañeda-Babarro, A.; Seco-Calvo, J.; Urdampilleta, A. Effects of 120 g/h of carbohydrates intake during a mountain marathon on exercise-induced muscle damage in elite runners. Nutrients 2020, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- St. Clair Gibson, A.; Baden, D.A.; Lambert, M.I.; Lambert, E.V.; Harley, Y.X.R.; Hampson, D.; Russell, V.A.; Noakes, T.D. The conscious perception of the sensation of fatigue. Sports Med. 2003, 33, 167–176. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue: The serotonin hypothesis and beyond. Sports Med. 2006, 36, 881–909. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Piacentini, M.F. Exercise, fatigue, neurotransmission and the influence of the neuroendocrine axis. In Developments in Tryptophan and Serotonin Metabolism; Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H., Varesio, L., Eds.; Springer: Boston, MA, USA, 2003; Volume 527, pp. 521–525. ISBN 9781461501350. [Google Scholar]
- Nybo, L.; Secher, N.H. Cerebral perturbations provoked by prolonged exercise. Prog. Neurobiol. 2004, 72, 223–261. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.L.; Fulton, T.J.; Chapman, R.F.; Fly, A.D.; Koceja, D.M.; Mickleborough, T.D. Effect of carbohydrate ingestion on central fatigue during prolonged running exercise in moderate hypoxia. J. Appl. Physiol. 2019, 126, 141–151. [Google Scholar] [CrossRef]
- Nybo, L. CNS fatigue and prolonged exercise: Effect of glucose supplementation. Med. Sci. Sports Exerc. 2003, 35, 589–594. [Google Scholar] [CrossRef]
- Nybo, L.; Møller, K.; Pedersen, B.K.; Nielsen, B.; Secher, N.H. Association between fatigue and failure to preserve cerebral energy turnover during prolonged exercise. Acta Physiol. Scand. 2003, 179, 67–74. [Google Scholar] [CrossRef]
- Winnick, J.J.; Davis, J.M.; Welsh, R.S.; Carmichael, M.D.; Murphy, E.A.; Blackmon, J.A. Carbohydrates feedings during team sport exercise preserve physical and CNS function. Med. Sci. Sports Exerc. 2005, 37, 306–315. [Google Scholar] [CrossRef]
- Kong, J.; Shepel, P.N.; Holden, C.P.; Mackiewicz, M.; Pack, A.I.; Geiger, J.D. Brain glycogen decreases with increased periods of wakefulness: Implications for homeostatic drive to sleep. J. Neurosci. 2002, 22, 5581–5587. [Google Scholar] [CrossRef] [PubMed]
- Béquet, F.; Gomez-Merino, D.; Berthelot, M.; Guezennec, C.Y. Evidence that brain glucose availability influences exercise-enhanced extracellular 5-HT level in hippocampus: A microdialysis study in exercising rats. Acta Physiol. Scand. 2002, 176, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, R.; Shave, R.; Ross, E.; Stevenson, E.J.; Goodall, S. The effect of a carbohydrate mouth-rinse on neuromuscular fatigue following cycling exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.K.; Selvanayagam, V.S.; Sidhu, S.K.; Yusof, A. Role of carbohydrate in central fatigue: A systematic review. Scand. J. Med. Sci. Sports 2017, 27, 376–384. [Google Scholar] [CrossRef]
- Paris, H.L.; Sinai, E.C.; Shei, R.J.; Keller, A.M.; Mickleborough, T.D. The influence of carbohydrate ingestion on peripheral and central fatigue during exercise in hypoxia: A narrative review. Eur. J. Sport Sci. 2020, 21, 1423–1435. [Google Scholar] [CrossRef]
- Malone, J.J.; Hulton, A.T.; MacLaren, D.P.M. Exogenous carbohydrate and regulation of muscle carbohydrate utilisation during exercise. Eur. J. Appl. Physiol. 2021, 121, 1255–1269. [Google Scholar] [CrossRef]
- Manore, M.M.; Patton-Lopez, M.M.; Meng, Y.; Wong, S.S. Sport nutrition knowledge, behaviors and beliefs of high school soccer players. Nutrients 2017, 9, 350. [Google Scholar] [CrossRef]
- Herriman, M.; Fletcher, L.; Tchaconas, A.; Adesman, A.; Milanaik, R. Dietary supplements and young teens: Misinformation and access provided by retailers. Pediatrics 2017, 139, e20161257. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).