Orally Induced Hyperthyroidism Regulates Hypothalamic AMP-Activated Protein Kinase
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
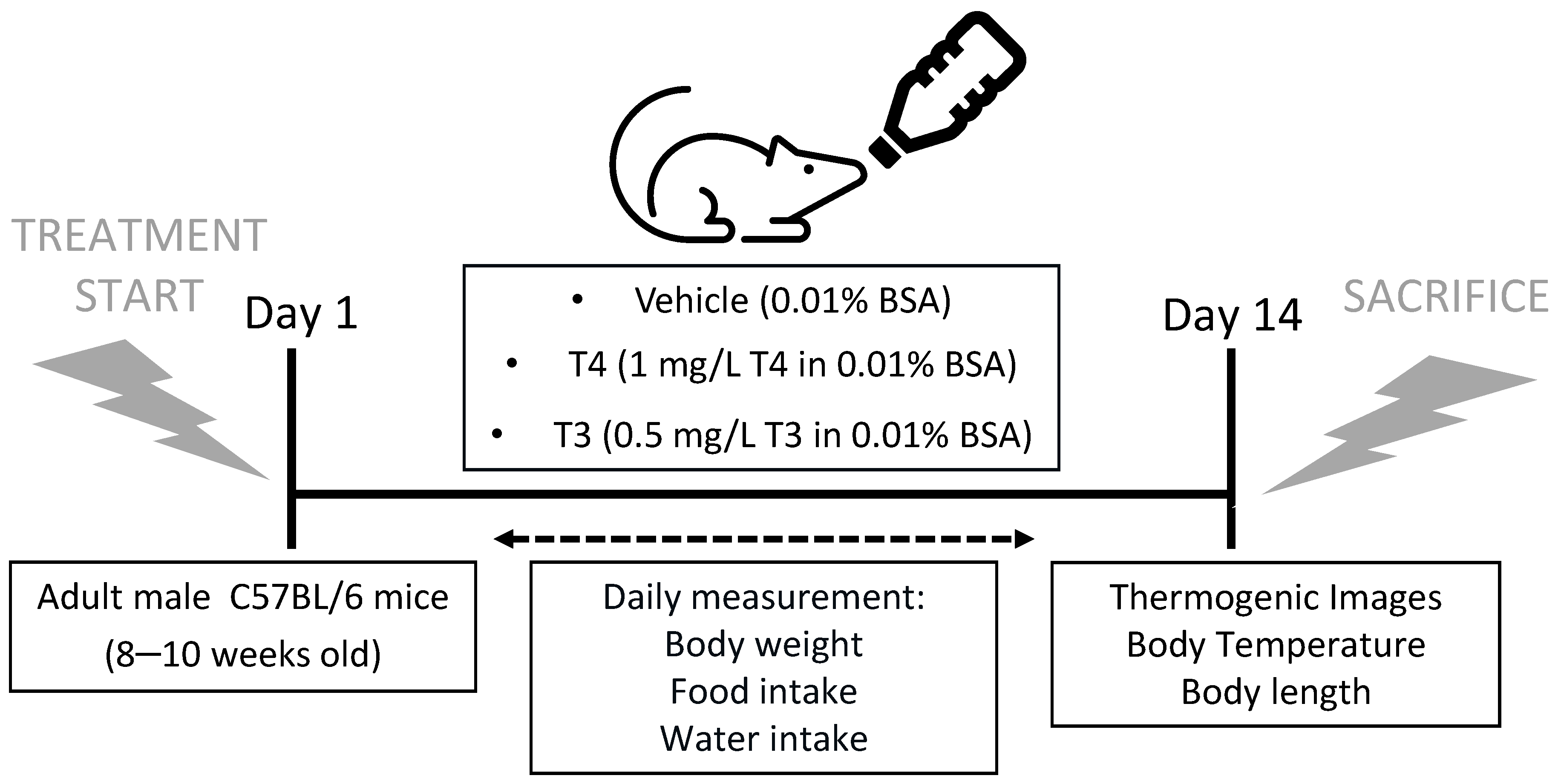
2.2. Induction of Hyperthyroidism and Monitoring
2.3. Core Body Temperature, BAT and Tail Temperature Measurements
2.4. Sample Processing
2.5. Radioimmunoassay
2.6. Quantification of Lipids
2.7. Real-Time Quantitative RT-PCR
2.8. Histology and Immunohistochemistry
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
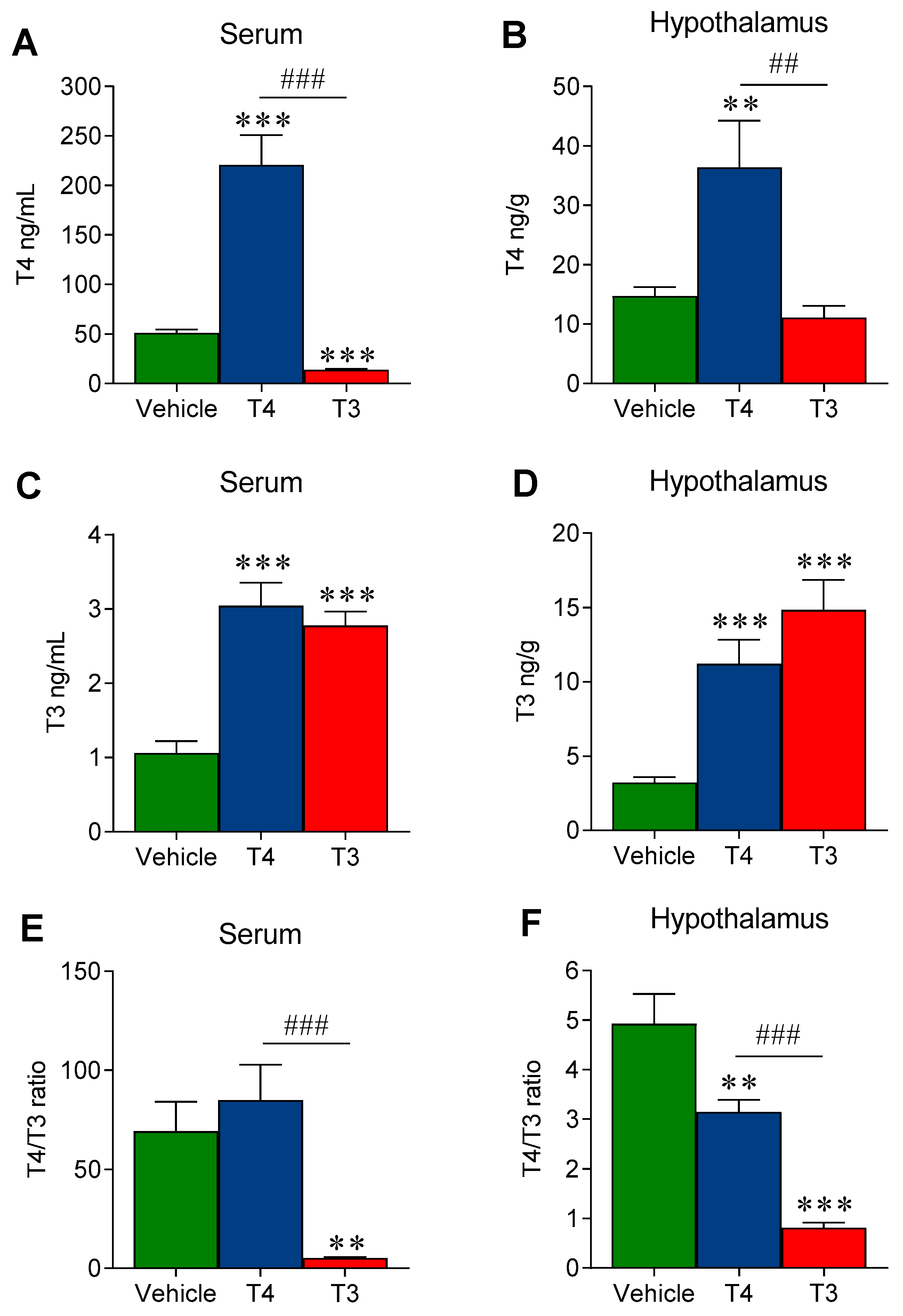
3.1. Oral TH Administration Leads to a Significant Increase in Both Serum and Hypothalamic TH Levels
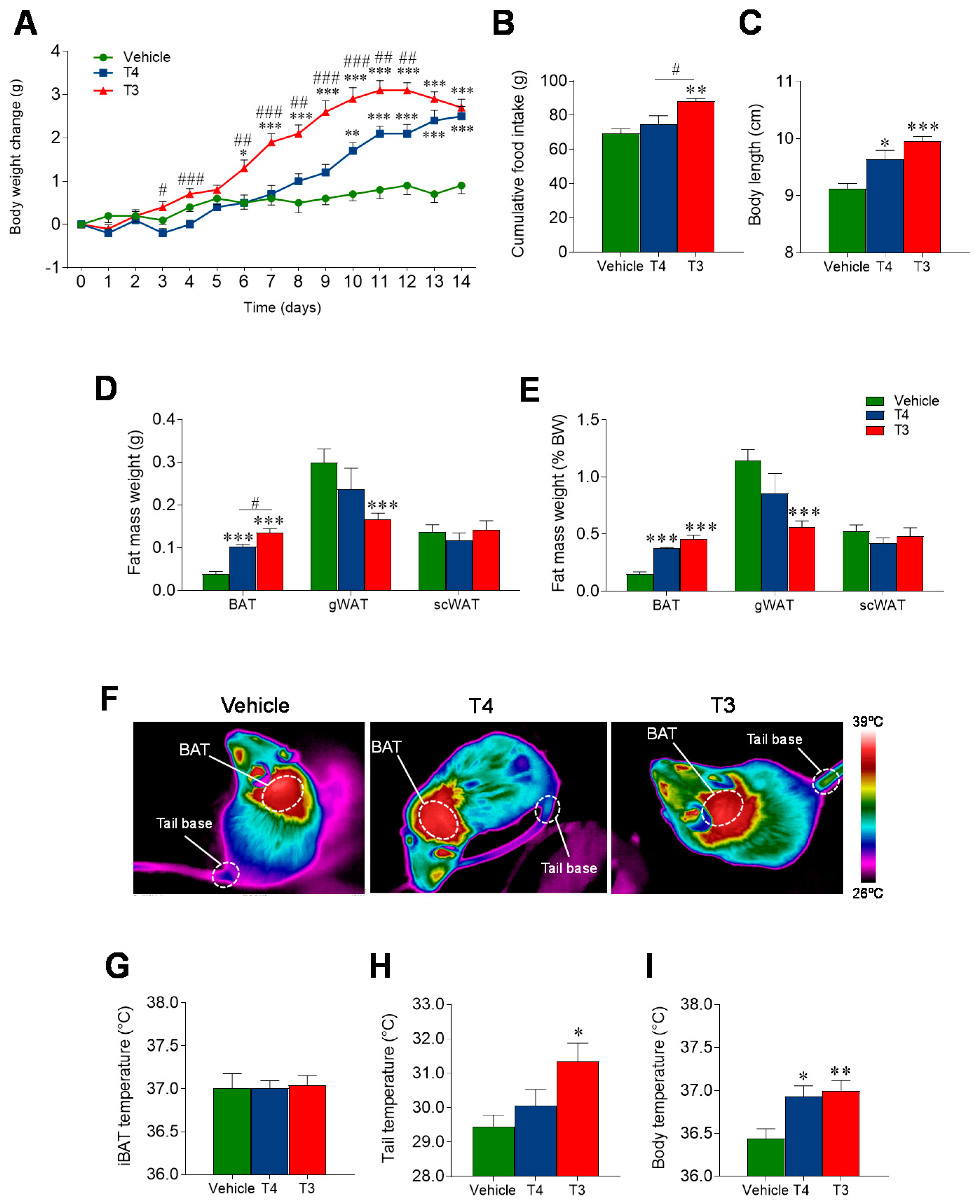
3.2. Orally Induced Hyperthyroidism Leads to Changes in Energy Balance
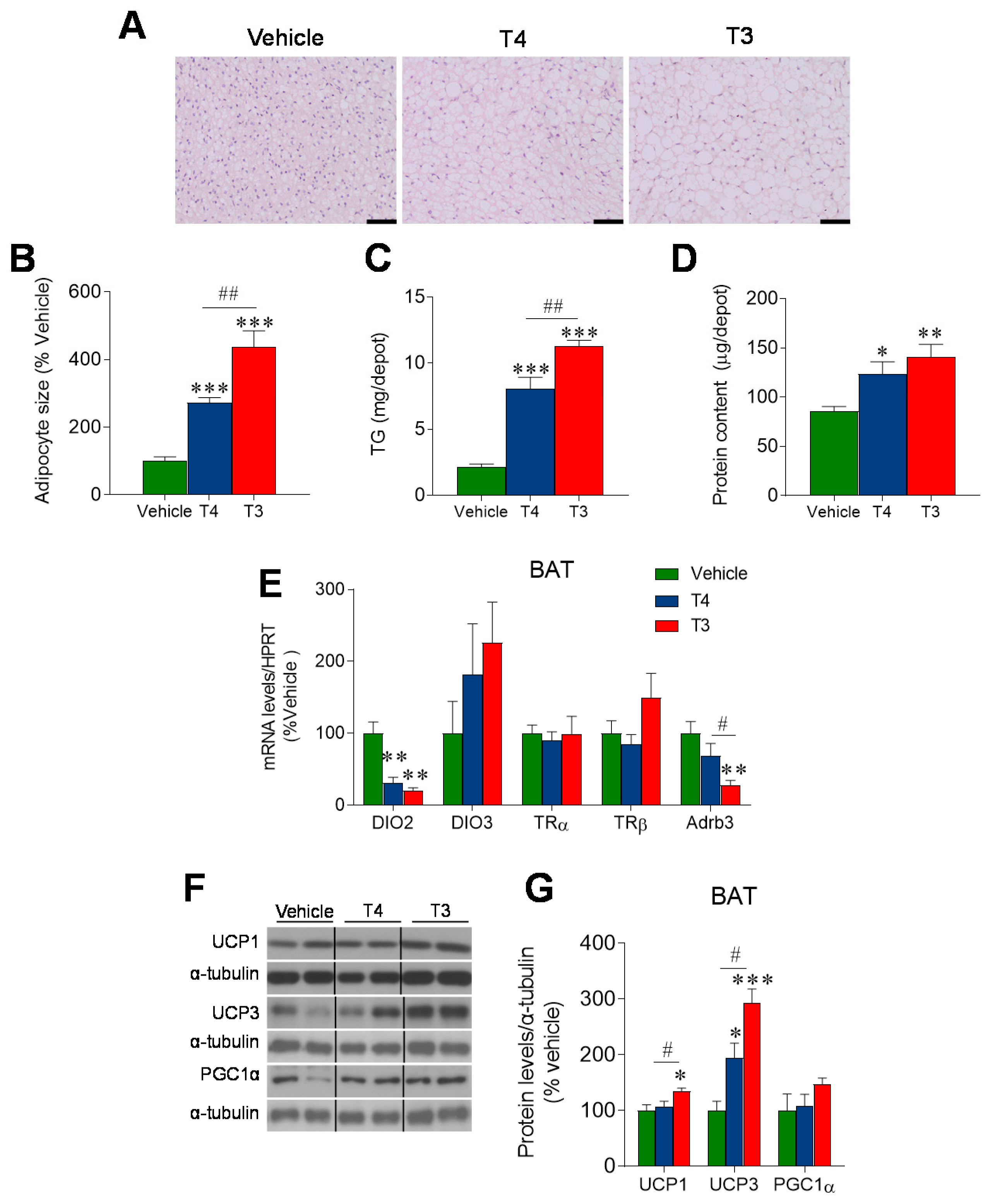
3.3. Orally Induced Hyperthyroidism Impacts BAT Metabolism
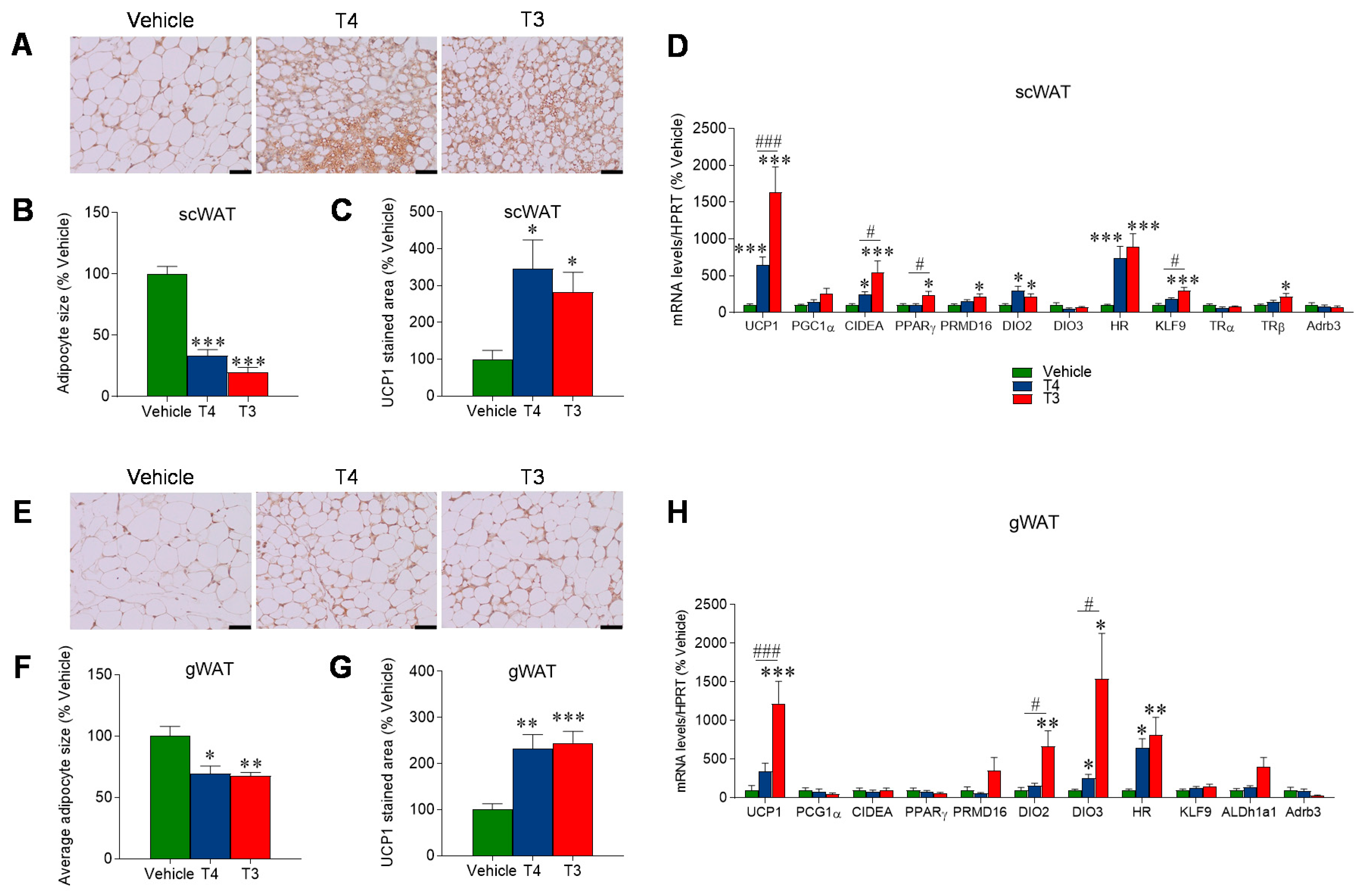
3.4. Orally Induced Hyperthyroidism Promotes Browning of WAT
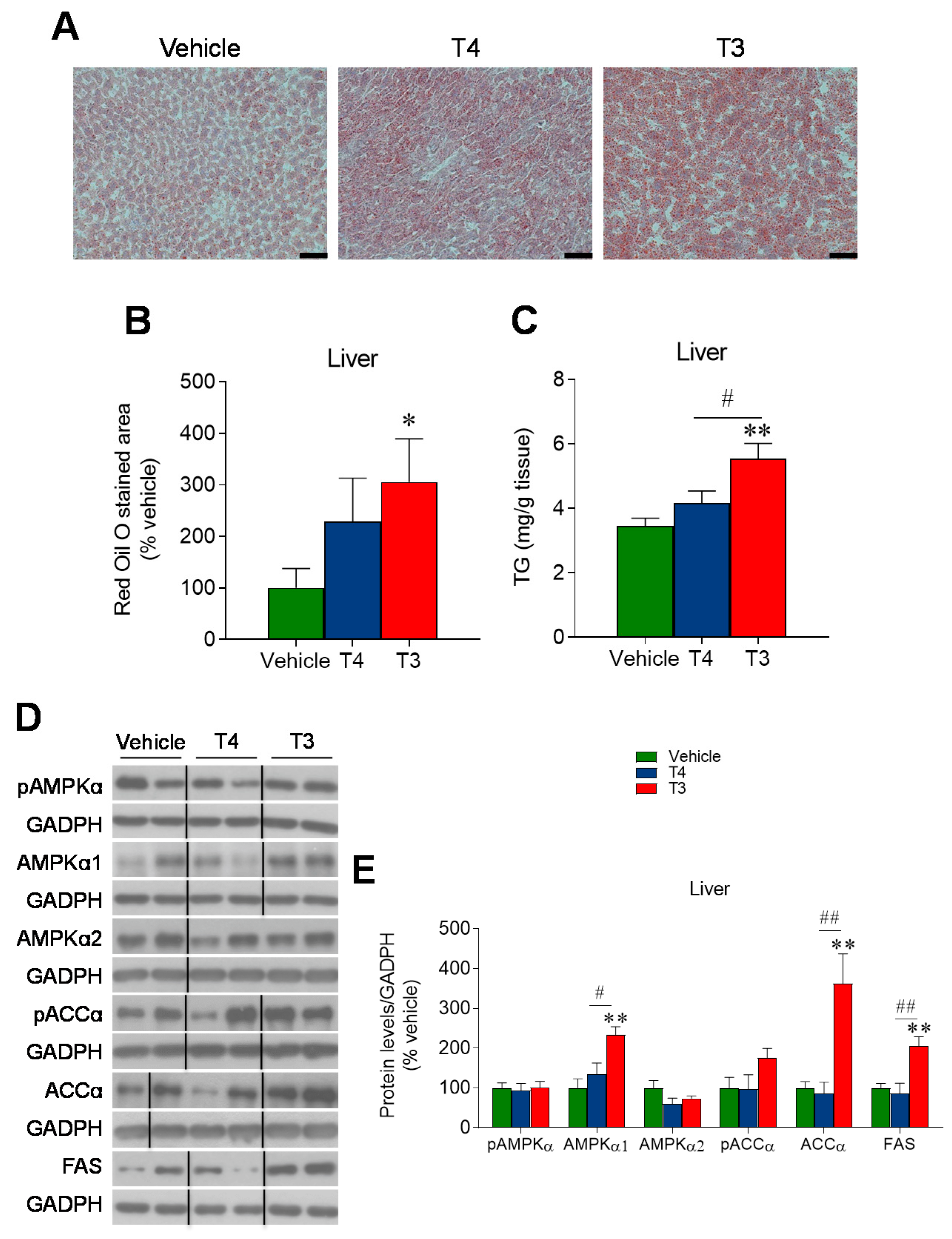
3.5. Orally Induced Hyperthyroidism Promotes Hepatic Lipid Accretion
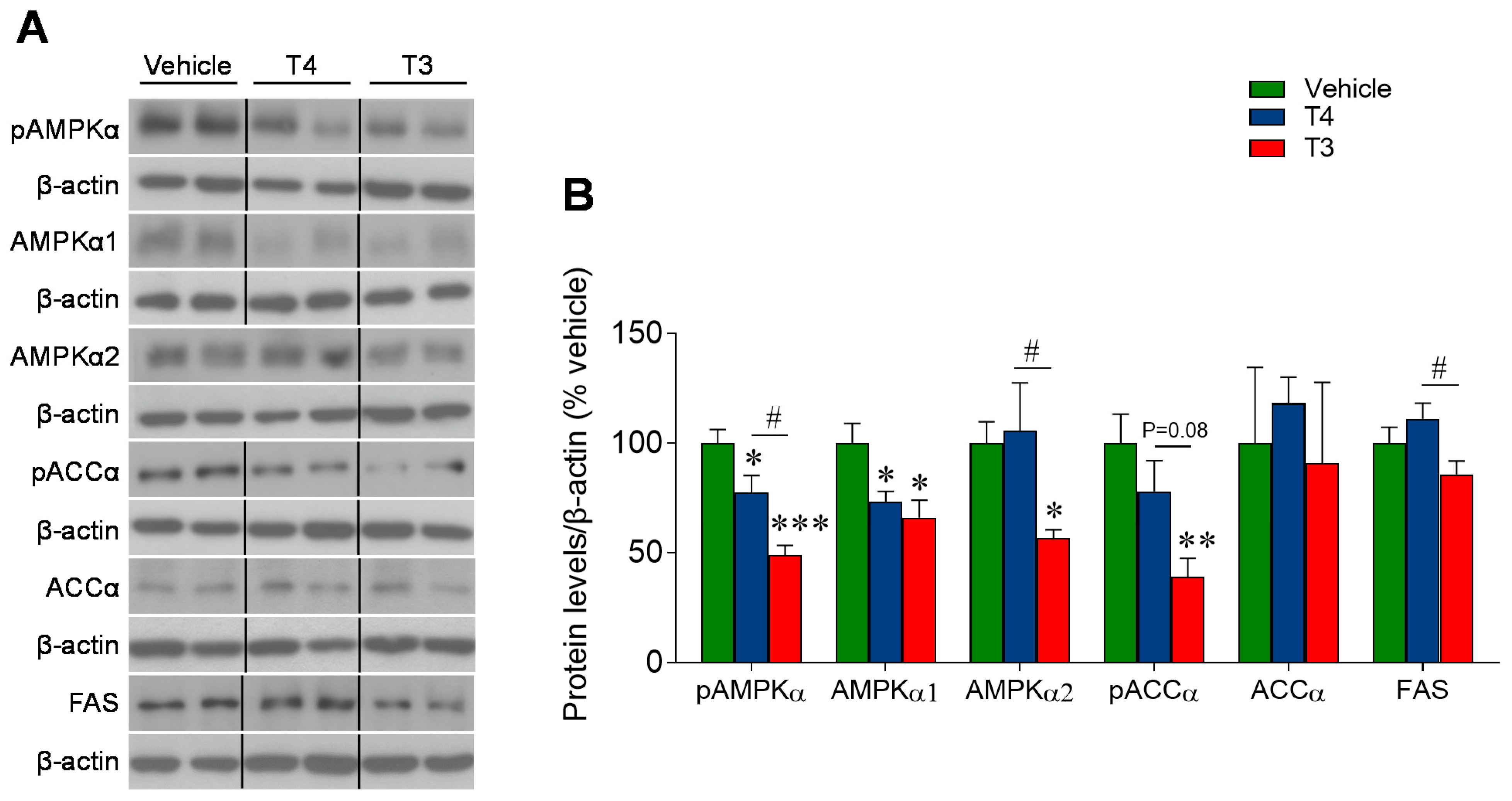
3.6. Orally Induced Hyperthyroidism Inhibits AMPK Signaling in the Ventromedial Hypothalamus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindholm, J.; Laurberg, P. Hypothyroidism and thyroid substitution: Historical aspects. J. Thyroid. Res. 2011, 2011, 809341. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012, 18, 988–1028. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef]
- Brent, G.A. Clinical practice. Graves’ disease. N. Engl. J. Med. 2008, 358, 2594–2605. [Google Scholar] [CrossRef]
- Silva, J.E. The thermogenic effect of thyroid hormone and its clinical implications. Ann. Intern. Med. 2003, 139, 205–213. [Google Scholar] [CrossRef]
- Silva, J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Bianco, S.D.; Kaneshige, M.; Schultz, J.J.; Cheng, S.Y.; Bianco, A.C.; Brent, G.A. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology 2010, 151, 432–440. [Google Scholar] [CrossRef]
- Johann, K.; Cremer, A.L.; Fischer, A.W.; Heine, M.; Pensado, E.R.; Resch, J.; Nock, S.; Virtue, S.; Harder, L.; Oelkrug, R.; et al. Thyroid-Hormone-Induced Browning of White Adipose Tissue Does Not Contribute to Thermogenesis and Glucose Consumption. Cell Rep. 2019, 27, 3385–3400.e3. [Google Scholar] [CrossRef]
- Sjogren, M.; Alkemade, A.; Mittag, J.; Nordstrom, K.; Katz, A.; Rozell, B.; Westerblad, H.; Arner, A.; Vennstrom, B. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor alpha1. EMBO J. 2007, 26, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Sentis, S.C.; Oelkrug, R.; Mittag, J. Thyroid hormones in the regulation of brown adipose tissue thermogenesis. Endocr. Connect. 2021, 10, R106–R115. [Google Scholar] [CrossRef] [PubMed]
- Mitrou, P.; Raptis, S.A.; Dimitriadis, G. Insulin action in hyperthyroidism: A focus on muscle and adipose tissue. Endocr. Rev. 2010, 31, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buque, X.; Aurrekoetxea, I.; Delgado, T.C.; Vazquez-Martinez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 Axis Mediates the Central Actions of Thyroid Hormones on Energy Balance. Cell Metab. 2017, 26, 212–229.e12. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Weiner, J.; Hones, S.; Kloting, N.; Rijntjes, E.; Heiker, J.T.; Gebhardt, C.; Kohrle, J.; Fuhrer, D.; Steinhoff, K.; et al. The Effects of Thyroid Hormones on Gene Expression of Acyl-Coenzyme A Thioesterases in Adipose Tissue and Liver of Mice. Eur. Thyroid J. 2015, 4 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varela, L.; Martinez-Sanchez, N.; Gallego, R.; Vazquez, M.J.; Roa, J.; Gandara, M.; Schoenmakers, E.; Nogueiras, R.; Chatterjee, K.; Tena-Sempere, M.; et al. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J. Pathol. 2012, 227, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Crespo, M.; Csikasz, R.I.; Martínez-Sánchez, N.; Diéguez, C.; Cannon, B.; Nedergaard, J.; López, M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metab. 2016, 5, 271–282. [Google Scholar] [CrossRef]
- Martinez-Sanchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Ferno, J.; Nogueiras, R.; Dieguez, C.; Fernandez-Real, J.M.; Lopez, M. Thyroid hormones induce browning of white fat. J. Endocrinol. 2017, 232, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dittner, C.; Lindsund, E.; Cannon, B.; Nedergaard, J. At thermoneutrality, acute thyroxine-induced thermogenesis and pyrexia are independent of UCP1. Mol. Metab. 2019, 25, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Kranz, M.; Kloting, N.; Kunath, A.; Steinhoff, K.; Rijntjes, E.; Kohrle, J.; Zeisig, V.; Hankir, M.; Gebhardt, C.; et al. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep. 2016, 6, 38124. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Varela, L.; Vazquez, M.J.; Rodriguez-Cuenca, S.; Gonzalez, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Klieverik, L.P.; Janssen, S.F.; van Riel, A.; Foppen, E.; Bisschop, P.H.; Serlie, M.J.; Boelen, A.; Ackermans, M.T.; Sauerwein, H.P.; Fliers, E.; et al. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc. Natl. Acad. Sci. USA 2009, 106, 5966–5971. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.; Harder, L.; Oelkrug, R.; Chen, J.; Gachkar, S.; Nock, S.; Resch, J.; Korkowski, M.; Heuer, H.; Mittag, J. Central Hypothyroidism Impairs Heart Rate Stability and Prevents Thyroid Hormone-Induced Cardiac Hypertrophy and Pyrexia. Thyroid 2020, 30, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Gachkar, S.; Oelkrug, R.; Martinez-Sanchez, N.; Rial-Pensado, E.; Warner, A.; Hoefig, C.S.; Lopez, M.; Mittag, J. 3-Iodothyronamine Induces Tail Vasodilation through Central Action in Male Mice. Endocrinology 2017, 158, 1977–1984. [Google Scholar] [CrossRef]
- Zekri, Y.; Flamant, F.; Gauthier, K. Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic. Cells 2021, 10, 1327. [Google Scholar] [CrossRef]
- Capelli, V.; Dieguez, C.; Mittag, J.; Lopez, M. Thyroid wars: The rise of central actions. Trends Endocrinol. Metab. 2021, 32, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Alvarez, C.V.; Nogueiras, R.; Dieguez, C. Energy balance regulation by thyroid hormones at central level. Trends Mol. Med. 2013, 19, 418–427. [Google Scholar] [CrossRef]
- Mendoza, A.; Hollenberg, A.N. New insights into thyroid hormone action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, 146–201. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Kohrle, J. Function of thyroid hormone transporters in the central nervous system. Biochim. Biophys. Acta 2013, 1830, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vazquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Seoane-Collazo, P.; Contreras, C.; Gonzalez-Garcia, I.; Martinez-Sanchez, N.; Gonzalez, F.; Zalvide, J.; Gallego, R.; Dieguez, C.; Nogueiras, R.; et al. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016, 16, 2231–2242. [Google Scholar] [CrossRef]
- Martinez de Morentin, P.B.; Gonzalez-Garcia, I.; Martins, L.; Lage, R.; Fernandez-Mallo, D.; Martinez-Sanchez, N.; Ruiz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, I.; Contreras, C.; Estevez-Salguero, A.; Ruiz-Pino, F.; Colsh, B.; Pensado, I.; Linares-Pose, L.; Rial-Pensado, E.; Martinez de Morentin, P.B.; Ferno, J.; et al. Estradiol Regulates Energy Balance by Ameliorating Hypothalamic Ceramide-Induced ER Stress. Cell Rep. 2018, 25, 413–423.e5. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Roa, J.; Rial-Pensado, E.; Linares-Pose, L.; Beiroa, D.; Ruiz-Pino, F.; Lopez-Gonzalez, T.; Morgan, D.A.; Pardavila, J.A.; Sanchez-Tapia, M.J.; et al. SF1-Specific AMPKalpha1 Deletion Protects against Diet-Induced Obesity. Diabetes 2018, 67, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.; Rahman, A.; Solsjo, P.; Gottschling, K.; Davis, B.; Vennstrom, B.; Arner, A.; Mittag, J. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor alpha1. Proc. Natl. Acad. Sci. USA 2013, 110, 16241–16246. [Google Scholar] [CrossRef] [PubMed]
- Oelkrug, R.; Mittag, J. An improved method for the precise unravelment of non-shivering brown fat thermokinetics. Sci. Rep. 2021, 11, 4799. [Google Scholar] [CrossRef] [PubMed]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Ferno, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Milbank, E.; Dragano, N.R.V.; Gonzalez-Garcia, I.; Garcia, M.R.; Rivas-Limeres, V.; Perdomo, L.; Hilairet, G.; Ruiz-Pino, F.; Mallegol, P.; Morgan, D.A.; et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKalpha1 corrects obesity through BAT activation. Nat. Metab. 2021, 3, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Rial-Pensado, E.; Estevez-Salguero, A.; Milbank, E.; Garcia-Caballero, L.; Rios, M.; Linares-Pose, L.; Scotece, M.; Gallego, R.; Fernandez-Real, J.M.; et al. Activation of hypothalamic AMPK ameliorates metabolic complications of experimental arthritis. Arthritis Rheumatol. 2022. [Google Scholar] [CrossRef]
- Urisarri, A.; Gonzalez-Garcia, I.; Estevez-Salguero, A.; Pata, M.P.; Milbank, E.; Lopez, N.; Mandia, N.; Grijota-Martinez, C.; Salgado, C.A.; Nogueiras, R.; et al. BMP8 and activated brown adipose tissue in human newborns. Nat. Commun. 2021, 12, 5274. [Google Scholar] [CrossRef]
- Weeke, J.; Orskov, H. Synthesis of 125I monolabelled 3, 5, 3’-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand. J. Clin. Lab Investig. 1973, 32, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Obregon, M.J.; Morreale de Escobar, G.; del Rey, F.E. Concentrations of triiodo-L-thyronine in the plasma and tissues of normal rats, as determined by radioimmunoassay: Comparison with results obtained by an isotopic equilibrium technique. Endocrinology 1978, 103, 2145–2153. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Linares-Pose, L.; Rial-Pensado, E.; Romero-Pico, A.; Moreno-Navarrete, J.M.; Martinez-Sanchez, N.; Garrido-Gil, P.; Iglesias-Rey, R.; Morgan, D.A.; Tomasini, N.; et al. Central nicotine induces browning through hypothalamic kappa opioid receptor. Nat. Commun. 2019, 10, 4037. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Romero-Pico, A.; Rial-Pensado, E.; Linares-Pose, L.; Estevez-Salguero, A.; Ferno, J.; Nogueiras, R.; Dieguez, C.; Lopez, M. kappa-Opioid Signaling in the Lateral Hypothalamic Area Modulates Nicotine-Induced Negative Energy Balance. Int. J. Mol. Sci. 2021, 22, 1515. [Google Scholar] [CrossRef]
- Contreras, C.; Gonzalez-Garcia, I.; Seoane-Collazo, P.; Martinez-Sanchez, N.; Linares-Pose, L.; Rial-Pensado, E.; Ferno, J.; Tena-Sempere, M.; Casals, N.; Dieguez, C.; et al. Reduction of Hypothalamic Endoplasmic Reticulum Stress Activates Browning of White Fat and Ameliorates Obesity. Diabetes 2017, 66, 87–99. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 2017, 13, 458–465. [Google Scholar] [CrossRef]
- Neilsen, E.L. Studies on hereditary dwarfism in mice. XIV. Effect of thyroxin and growth hormone on growth. Acta Pathol. Microbiol. Scand. 1953, 32, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Rakov, H.; Engels, K.; Hones, G.S.; Strucksberg, K.H.; Moeller, L.C.; Kohrle, J.; Zwanziger, D.; Fuhrer, D. Sex-specific phenotypes of hyperthyroidism and hypothyroidism in mice. Biol. Sex Differ. 2016, 7, 36. [Google Scholar] [CrossRef]
- Coleman, D.K.N.D.C.P.; Russell, E.S.; Fuller, J.L.; Staats, J.; Green, M.C. Biology of the Laboratory Mouse; Dover Publications: New York, NY, USA, 2007. [Google Scholar]
- Kong, W.M.; Martin, N.M.; Smith, K.L.; Gardiner, J.V.; Connoley, I.P.; Stephens, D.A.; Dhillo, W.S.; Ghatei, M.A.; Small, C.J.; Bloom, S.R. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology 2004, 145, 5252–5258. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Walsh, K. The Whitening of Brown Fat and Its Implications for Weight Management in Obesity. Curr. Obes. Rep. 2015, 4, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Cannon, B. UCP1 mRNA does not produce heat. Biochim. Biophys. Acta 2013, 1831, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Araki, O.; Ying, H.; Zhu, X.G.; Willingham, M.C.; Cheng, S.Y. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol. Endocrinol. 2009, 23, 308–315. [Google Scholar] [CrossRef]
- Martinez-Sanchez, N.; Alvarez, C.V.; Ferno, J.; Nogueiras, R.; Dieguez, C.; Lopez, M. Hypothalamic effects of thyroid hormones on metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Nogueiras, R.; Tena-Sempere, M.; Dieguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Morte, B.; Belinchon, M.M.; Ceballos, A.; Bernal, J. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T3 in the mouse cerebral cortex. Endocrinology 2012, 153, 2919–2928. [Google Scholar] [CrossRef]
- Silva, J.E. The multiple contributions of thyroid hormone to heat production. J. Clin. Investig. 2001, 108, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E. Thyroid hormone and the energetic cost of keeping body temperature. Biosci. Rep. 2005, 25, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Wartofsky, L. Combination treatment with T4 and T3: Toward personalized replacement therapy in hypothyroidism? J. Clin. Endocrinol. Metab 2012, 97, 2256–2271. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Bray, G.A.; Qi, L.; Hu, F.B.; Rood, J.; Sacks, F.M.; Sun, Q. Thyroid hormones and changes in body weight and metabolic parameters in response to weight loss diets: The POUNDS LOST trial. Int. J. Obes. 2017, 41, 878–886. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capelli, V.; Grijota-Martínez, C.; Dragano, N.R.V.; Rial-Pensado, E.; Fernø, J.; Nogueiras, R.; Mittag, J.; Diéguez, C.; López, M. Orally Induced Hyperthyroidism Regulates Hypothalamic AMP-Activated Protein Kinase. Nutrients 2021, 13, 4204. https://doi.org/10.3390/nu13124204
Capelli V, Grijota-Martínez C, Dragano NRV, Rial-Pensado E, Fernø J, Nogueiras R, Mittag J, Diéguez C, López M. Orally Induced Hyperthyroidism Regulates Hypothalamic AMP-Activated Protein Kinase. Nutrients. 2021; 13(12):4204. https://doi.org/10.3390/nu13124204
Chicago/Turabian StyleCapelli, Valentina, Carmen Grijota-Martínez, Nathalia R. V. Dragano, Eval Rial-Pensado, Johan Fernø, Rubén Nogueiras, Jens Mittag, Carlos Diéguez, and Miguel López. 2021. "Orally Induced Hyperthyroidism Regulates Hypothalamic AMP-Activated Protein Kinase" Nutrients 13, no. 12: 4204. https://doi.org/10.3390/nu13124204
APA StyleCapelli, V., Grijota-Martínez, C., Dragano, N. R. V., Rial-Pensado, E., Fernø, J., Nogueiras, R., Mittag, J., Diéguez, C., & López, M. (2021). Orally Induced Hyperthyroidism Regulates Hypothalamic AMP-Activated Protein Kinase. Nutrients, 13(12), 4204. https://doi.org/10.3390/nu13124204







