Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study
Abstract
:1. Introduction
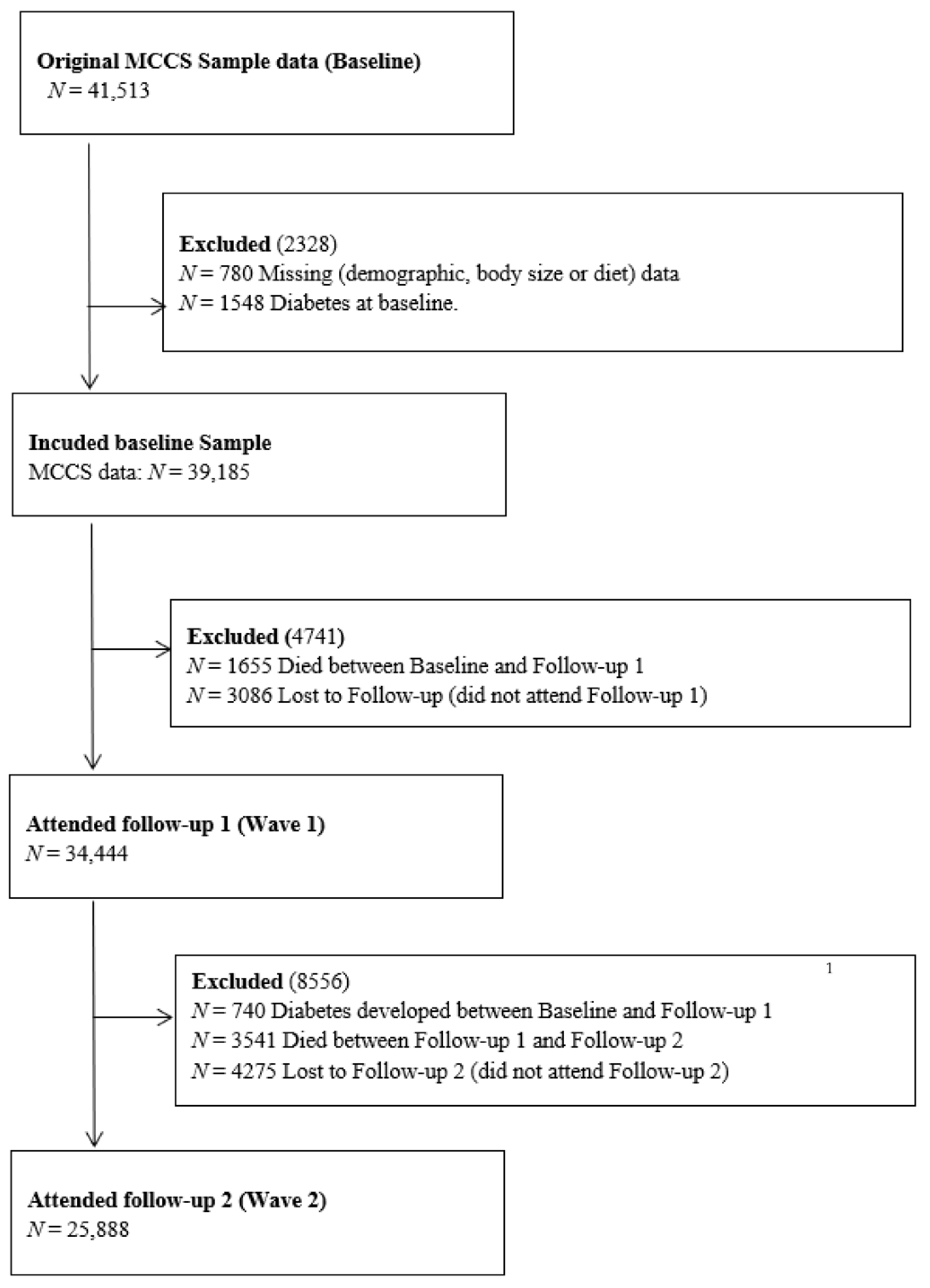
2. Materials and Methods
2.1. The Melbourne Collaborative Cohort Study (MCCS)
2.2. Dietary Assessment
2.3. Socio Demographic and Comorbidity Data
2.4. Anthropometric Assessment Data
2.5. Diabetes Ascertainment
2.6. Statistical Analysis
2.7. Ethics Approval
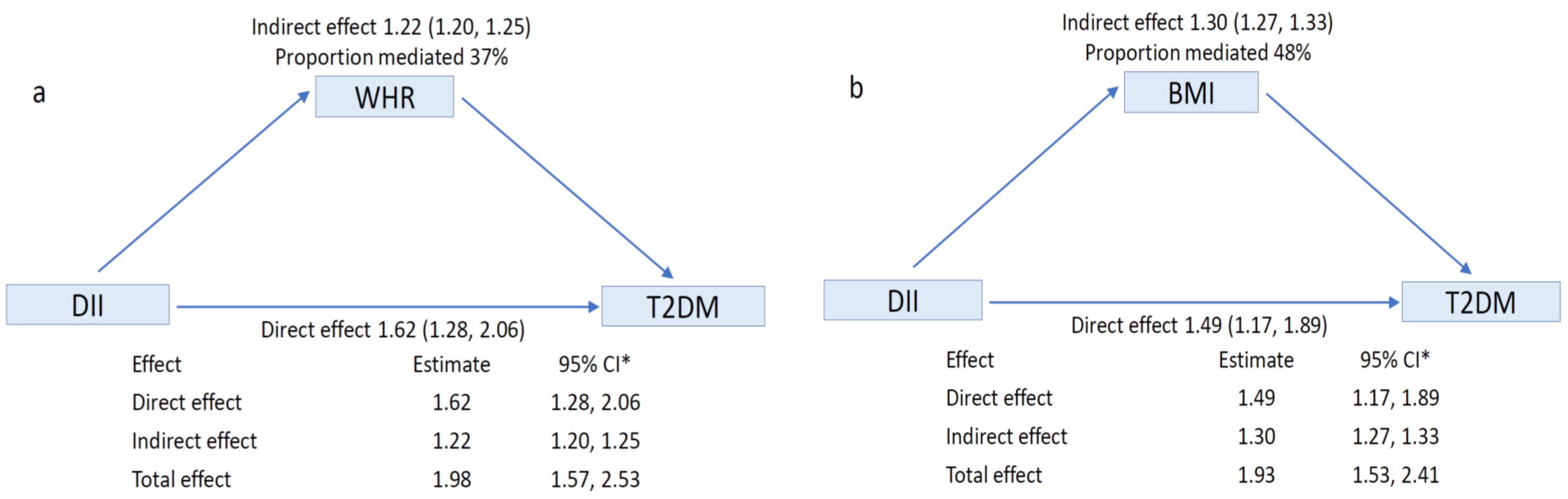
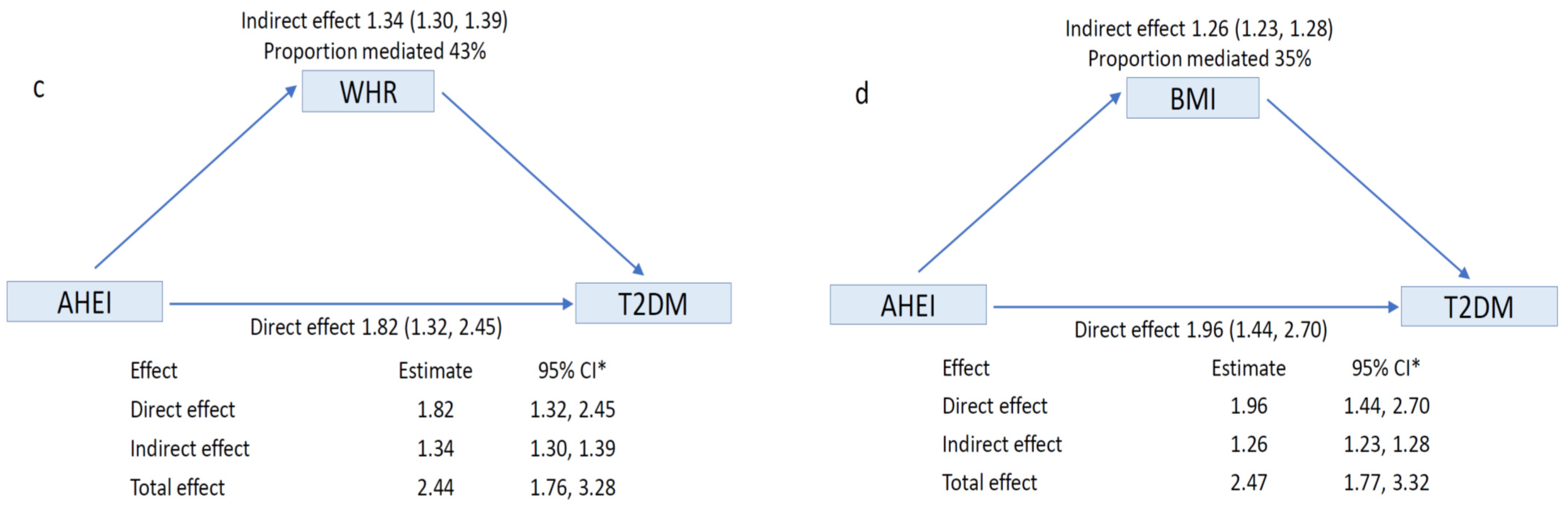
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Foundation. IDF DIABETES ATLAS Ninth Edition 2019; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Australian Institute of Health and Welfare. Impact of Overweight and Obesity as a Risk Factor for Chronic Conditions: Australian Burden of Disease Study; AIHW: Canberra, Australia, 2017. [Google Scholar]
- Hemmingsen, B.; Gimenez-Perez, G.; Mauricio, D.; Roque, I.F.M.; Metzendorf, M.I.; Richter, B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017, 12, CD003054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guess, N.D. Dietary Interventions for the Prevention of Type 2 Diabetes in High-Risk Groups: Current State of Evidence and Future Research Needs. Nutrients 2018, 10, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F.; Kroger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Missbach, B.; Konig, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.C.; Koh, W.P.; Neelakantan, N.; Yuan, J.M.; Qin, L.Q.; van Dam, R.M. Diet Quality Indices and Risk of Type 2 Diabetes Mellitus: The Singapore Chinese Health Study. Am. J. Epidemiol. 2018, 187, 2651–2661. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Lindsay, R.S.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002, 51, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: A meta-analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvencion con DIeta MEDiterranea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef]
- Jin, Q.; Shi, N.; Aroke, D.; Lee, D.H.; Joseph, J.J.; Donneyong, M.; Conwell, D.L.; Hart, P.A.; Zhang, X.; Clinton, S.K.; et al. Insulinemic and Inflammatory Dietary Patterns Show Enhanced Predictive Potential for Type 2 Diabetes Risk in Postmenopausal Women. Diabetes Care 2021, 44, 707–714. [Google Scholar] [CrossRef]
- Lee, D.H.; Li, J.; Li, Y.; Liu, G.; Wu, K.; Bhupathiraju, S.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory and Insulinemic Potential and Risk of Type 2 Diabetes: Results from Three Prospective U.S. Cohort Studies. Diabetes Care 2020, 43, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Karim, M.N.; Hebert, J.R.; Shivappa, N.; Milne, R.L.; de Courten, B. Diet scores and prediction of general and abdominal obesity in the Melbourne collaborative cohort study. Public Health Nutr. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.L.; Fletcher, A.S.; MacInnis, R.J.; Hodge, A.M.; Hopkins, A.H.; Bassett, J.K.; Bruinsma, F.J.; Lynch, B.M.; Dugue, P.A.; Jayasekara, H.; et al. Cohort Profile: The Melbourne Collaborative Cohort Study (Health 2020). Int. J. Epidemiol. 2017, 46, 1757–1757i. [Google Scholar] [CrossRef]
- Ireland, P.; Jolley, D.; Giles, G.; O’Dea, K.; Powles, J.; Rutishauser, I.; Wahlqvist, M.L.; Williams, J. Development of the Melbourne FFQ: A food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac. J. Clin. Nutr. 1994, 3, 19–31. [Google Scholar]
- Lewis, J.; Milligan, G.; Hunt, A. NUTTAB95 Nutrient Data Table for Use in Australia; Australian Government Publishing Service: Canberra, Australia, 1995. [Google Scholar]
- Holland, B.; Welch, A.A.; Unwin, I.D.; Buss, D.H.; Paul, A.; Southgate, D.A.T. McCance and Widdowson’s the Composition of Foods, 5th ed.; Royal Society of Chemistry: Cambridge, UK, 1993. [Google Scholar]
- RMIT Lipid Research Group. Fatty acid Compositional Database; Xyris Software: Brisbane, Australia, 2001. [Google Scholar]
- US Department of Agriculture Agricultural Research Service Nutrient Data Laboratory. USDA-NCC Carotenoid. Database for US Foods—1998. Available online: http://www.nal.usda.gov/fnic/foodcomp/Data/car98/car98.html (accessed on 10 March 2009).
- Hodge, A.M.; Simpson, J.A.; Fridman, M.; Rowley, K.; English, D.R.; Giles, G.G.; Su, Q.; O’Dea, K. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr. 2009, 12, 2438–2447. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.M.; Simpson, J.A.; Gibson, R.A.; Sinclair, A.J.; Makrides, M.; O’Dea, K.; English, D.R.; Giles, G.G. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 415–426. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [Green Version]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Emsley, R.; Liu, H. PARAMED: Stata Module to Perform Causal Mediation Analysis Using Parametric Regression Models. Stata J. 2013. Available online: https://econpapers.repec.org/software/bocbocode/s457581.htm (accessed on 12 November 2021).
- Valeri, L.; Vanderweele, T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Ikram, M.A.; VanderWeele, T.J. A proposed clinical and biological interpretation of mediated interaction. Eur. J. Epidemiol. 2015, 30, 1115–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderweele, T.J.; Vansteelandt, S. Conceptual issues concerning mediation, interventions and composition. Stat. Interface 2009, 2, 457–468. [Google Scholar] [CrossRef] [Green Version]
- VanderWeele, T.J. Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Laouali, N.; Mancini, F.R.; Hajji-Louati, M.; El Fatouhi, D.; Balkau, B.; Boutron-Ruault, M.C.; Bonnet, F.; Fagherazzi, G. Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: The mediating role of BMI. Diabetologia 2019, 62, 2222–2232. [Google Scholar] [CrossRef]
- Bassett, J.K.; English, D.R.; Fahey, M.T.; Forbes, A.B.; Gurrin, L.C.; Simpson, J.A.; Brinkman, M.T.; Giles, G.G.; Hodge, A.M. Validity and calibration of the FFQ used in the Melbourne Collaborative Cohort Study. Public Health Nutr. 2016, 19, 2357–2368. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Mayr, H.L.; Thomas, C.J.; Tierney, A.C.; Kucianski, T.; George, E.S.; Ruiz-Canela, M.; Hebert, J.R.; Shivappa, N.; Itsiopoulos, C. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: The AUSMED Heart Trial. Nutr. Res. 2018, 55, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Lee, K.W.; Brann, L.; Shivappa, N.; Hebert, J.R. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition 2019, 63–64, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.; Shivappa, N.; Hebert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hebert, J.R. Construct Validation of the Dietary Inflammatory Index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef] [Green Version]
- ABS. Australian Health Survey: Biomedical Results for Chronic Diseases; Australian Bureau of Statistics: Canberra, Australia, 2013. [Google Scholar]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Increased diabetes incidence in Greek and Italian migrants to Australia: How much can be explained by known risk factors? Diabetes Care 2004, 27, 2330–2334. [Google Scholar] [CrossRef] [Green Version]
| (a) | |||||||
| DII Q1 | DII Q2 | DII Q3 | DII Q4 | DII Q5 | Total | Test Statistics | |
| DII median (IQR) | −2.9 (−3.3, −2.7) | −1.9 (−2.2, −1.7) | −1.0 (−1.2, −0.7) | −0.1 (−0.2, 0.4) | 1.8 (1.3, 2.6) | −1.0 (−2.2, 0.4) | |
| Age | |||||||
| <50 Years | 2367 (30.1) | 2512 (31.8) | 2561 (32.4) | 2645 (33.9) | 2584 (33.4) | 12,669 (32.3) | χ2 = 86.4; df = 8 |
| 50–59 Years | 2436 (31.0) | 2583 (32.7) | 2576 (32.6) | 2612 (33.5) | 2586 (33.5) | 12,793 (33.7) | p < 0.001 |
| >=60 Years | 3053 (38.9) | 2803 (35.5) | 2769 (35.0) | 2539 (32.6) | 2559 (32.6) | 13,723 (35.0) | |
| Mean ± SD | 55.9 ± 8.8 | 54.3 ± 8.7 | 55.1 ± 8.7 | 54.8 ± 8.6 | 54.8 ± 8.6 | 54.8 ± 8.5 | p < 0.001 |
| Gender | |||||||
| Male | 3461 (44.1) | 3234 (41.0) | 3113 (39.4) | 3083 (39.6) | 2900 (37.5) | 15,791 (40.3) | χ2 = 76.2; df = 4 |
| Female | 4395 (55.9) | 4664 (59.1) | 4793 (60.6) | 4713 (60.5) | 4829 (62.5) | 23,394 (59.7) | p < 0.001 |
| SEIFA 1 Quintiles | |||||||
| SEIFA Q1 | 1250 (15.9) | 1236 (15.7) | 1343 (17.0) | 1499 (19.2) | 1748 (22.6) | 7076 (18.1) | χ2 = 529; df = 16 p < 0.001 |
| SEIFA Q2 | 1382 (17.6) | 1543 (19.5) | 1604 (20.3) | 1732 (22.2) | 1888 (24.4) | 8149 (20.8) | |
| SEIFA Q3 | 1207 (15.4) | 1224 (15.5) | 1213 (15.3) | 1223 (15.7) | 1304 (16.9) | 6171 (15.8) | |
| SEIFA Q4 | 1584 (20.2) | 1569 (19.9) | 1473 (18.6) | 1370 (17.6) | 1258 (16.3) | 7254 (18.5) | |
| SEIFA Q5 | 2433 (31.0) | 2326 (29.5) | 2273 (28.8) | 1972 (25.3) | 1531 (19.8) | 10,535 (26.9) | |
| Region of Origin | |||||||
| AUS/NZ 2/Other | 6262 (79.7) | 5936 (75.2) | 5764 (72.9) | 5160 (66.2) | 4209 (54.5) | 27,331 (69.8) | χ2 = 1900; df = 8 p < 0.001 |
| Northern Europe | 584 (7.4) | 555 (7.0) | 487 (6.2) | 471 (6.0) | 431 (5.6) | 2528 (6.5) | |
| Southern Europe | 1010 (12.9) | 1407 (17.8) | 1655 (20.9) | 2165 (27.8) | 3089 (40.0) | 9326 (23.8) | |
| Smoking status | |||||||
| Non-smoker | 4690 (59.7) | 4775 (60.5) | 4598 (58.2) | 4414 (56.6) | 4237 (54.8) | 22,714 (58.0) | χ2 = 67.2; df = 4 |
| Smoker | 3166 (40.3) | 3123 (39.5) | 3308 (41.8) | 3382 (43.4) | 3492 (45.2) | 16,471 (42.0) | p <0.001 |
| Alcohol dinking status | |||||||
| Never | 2289 (29.1) | 2161 (27.4) | 2136 (27.0) | 2169 (27.8) | 2386 (30.9) | 11,141 (28.4) | χ2 = 40; df = 8 |
| Former | 808 (10.3) | 819 (10.4) | 846 (10.7) | 813 (10.4) | 813 (10.5) | 4099 (10.5) | p < 0.001 |
| Current | 4759 (60.6) | 4918 (62.3) | 4924 (62.3) | 4814 (61.8) | 4530 (58.6) | 23,945 (61.1) | |
| Physical activity score | |||||||
| 0 | 1185 (15.1) | 1462 (18.5) | 1637 (20.7) | 1943 (24.9) | 2343 (30.3) | 8570 (21.9) | χ2 = 999; df = 12 |
| >0 and <4 | 1399 (17.8) | 1508 (19.01) | 1634 (20.7) | 1672 (21.5) | 1672 (21.6) | 7885 (20.1) | p < 0.0001 |
| >=4 and <6 | 2939 (37.4) | 2921 (37.0) | 2829 (35.8) | 2663 (34.2) | 2577 (33.3) | 13,929 (35.6) | |
| >=6 | 2333 (29.7) | 2007 (25.4) | 1806 (22.8) | 1518 (19.5) | 1137 (14.7) | 8801 (22.5) | |
| WHR 3 at baseline | |||||||
| Normal WHR | 5290 (67.3) | 5295 (76.0) | 5248 (66.4) | 4980 (69.3) | 4696 (60.8) | 25,509 (65.1) | χ2 = 105; df = 4 |
| Raised WHR | 2566 (32.7) | 2603 (33.0) | 2658 (33.6) | 2816 (36.1) | 3033 (39.2) | 13,676 (34.9) | p < 0.001 |
| BMI 4 at baseline | |||||||
| <25 | 3305 (42.1) | 3180 (40.3) | 2910 (38.8) | 2709 (34.8) | 2357 (30.5) | 14,461 (36.9) | χ2 = 432; df = 8 |
| 25–29.9 | 3296 (42.0) | 3396 (40.30 | 3450 (43.6) | 3401 (43.6) | 3371 (43.6) | 16,914 (43.2) | p < 0.001 |
| >=30.0 | 1255 (16.0) | 1322 (16.7) | 1546 (19.6) | 1686 (21.6) | 2001 (25.9) | 7810 (19.9) | |
| Mean ± SD | 26.3 ± 4.1 | 26.5 ± 4.2 | 26.7 ± 4.3 | 27.1 ± 4.4 | 27.6 ± 4.7 | 26.8 ± 4.0 | p < 0.001 |
| Family history of diabetes | |||||||
| No | 6560 (83.5) | 6507 (82.4) | 6495 (82.2) | 6415 (82.3) | 6268 (81.1) | 32,245 (82.3) | χ2 = 15.6; df = 4 |
| Yes | 1296 (16.5) | 1391 (17.6) | 1411 (17.9) | 1381 (17.7) | 1461 (18.9) | 6940 (17.7) | p 0.004 |
| Comorbidity | |||||||
| No | 3443 (43.8) | 3563 (45.1) | 3514 (44.5) | 3622 (46.5) | 3481 (45.0) | 17,623 (45.0) | χ2 = 12.1; df = 4 |
| Yes | 4413 (56.2) | 4335 (54.9) | 4392 (55.6) | 4174 (53.5) | 4248 (55.0) | 21,562 (55.0) | p 0.017 |
| (b) | |||||||
| AHEI Q1 | AHEI Q2 | AHEI Q3 | AHEI Q4 | AHEI Q5 | Total | Test Statistics | |
| AHEI median (IQR) | 50.5 (46, 53) | 59 (57, 61) | 65 (64, 66) | 70.5 (69, 72) | 78 (75.5, 82) | 64.5 (57, 72) | |
| Age | |||||||
| <50 Years | 2789 (34.9) | 2790 (32.8) | 2329 (30.9) | 2331 (31.3) | 2430 (31.7) | 12,669 (32.3) | χ2 = 41.9; df = 8 |
| 50–59 Years | 2458 (30.8) | 2746 (32.3) | 2513 (33.4) | 2505 (33.5) | 2571 (33.5) | 12,793 (32.6) | p < 0.001 |
| >=60 Years | 2747 (34.4) | 2960 (34.8) | 2692 (35.7) | 2651 (35.4) | 2673 (34.8) | 13,723 (35.0) | |
| Mean ± SD | 54.8 ± 8.8 | 55.1 ± 8.7 | 55.5 ± 8.6 | 55.4 ± 8.6 | 54.3 ± 8.5 | 55.2 ± 8.7 | p < 0.001 * |
| Gender (Sex) | |||||||
| Male | 4592 (57.4) | 3782 (44.5) | 2751 (36.5) | 2457 (32.8) | 2209 (28.8) | 15,791 (40.3) | χ2 = 1700; df = 4 |
| Female | 3402 (42.6) | 4714 (55.5) | 4783 (63.5) | 5030 (67.2) | 5465 (71.2) | 23,394 (59.7) | p < 0.001 |
| SEIFA 1 Quintiles | |||||||
| SEIFA Q1 | 1739 (21.7) | 1700 (20.0) | 1334 (17.7) | 1192 (15.9) | 1111 (14.5) | 7076 (18.0) | χ2 = 396; df = 16 p < 0.001 |
| SEIFA Q2 | 1803 (22.6) | 1791 (21.1) | 1595 (21.2) | 1529 (20.4) | 1431 (18.7) | 8149 (20.8) | |
| SEIFA Q3 | 1297 (16.2) | 1367 (16.1) | 1149 (15.2) | 1228 (16.4) | 1130 (14.7) | 6171 (15.8) | |
| SEIFA Q4 | 1400 (17.5) | 1543 (18.2) | 1431 (19.0) | 1389 (18.6) | 1491 (19.4) | 7254 (18.5) | |
| SEIFA Q5 | 1755 (22.0) | 2095 (24.6) | 2025 (26.9) | 2149 (28.7) | 2511 (32.7) | 10,535 (26.9) | |
| Region of Origin | |||||||
| AUS/NZ 2/Other | 5835 (73.0) | 5800 (68.3) | 5008 (66.5) | 5120 (68.4) | 5568 (72.6) | 27,331 (69.8) | χ2 = 191; df = 8 |
| Northern Europe | 446 (5.6) | 524 (6.2) | 477 (6.3) | 489 (6.5) | 592 (7.7) | 2528 (6.4) | p < 0.001 |
| Southern Europe | 1713 (21.4) | 2172 (25.6) | 2049 (27.2) | 1878 (25.1) | 1514 (19.7) | 9326 (23.8) | |
| Smoking status | |||||||
| Non-smoker | 3972 (49.7) | 4838 (56.9) | 4573 (60.7) | 4691 (62.7) | 4640 (60.5) | 22,714 (58.0) | χ2 = 338; df = 4 |
| Smoker | 4022 (50.3) | 3658 (43.1) | 2961 (39.3) | 2796 (37.3) | 3034 (39.5) | 16,471 (42.0) | p < 0.001 |
| Alcohol dinking status | |||||||
| Never | 2332 (29.2) | 2636 (31.0) | 2532 (33.6) | 2047 (27.3) | 1594 (20.8) | 11,141 (28.4) | χ2 = 394; df = 8 |
| Former | 944 (11.8) | 870 (10.2) | 757 (10.1) | 736 (9.8) | 792 (10.3) | 4099 (10.5) | p < 0.001 |
| Current | 4718 (59.0) | 4990 (58.7) | 4245 (56.3) | 4704 (62.8) | 5288 (68.9) | 23,945 (61.1) | |
| Physical activity score | |||||||
| 0 | 2251 (28.2) | 2048 (24.1) | 1687 (2.4) | 1440 (19.2) | 1144 (14.9) | 8570 (21.9) | χ2 = 674; df = 12 |
| >0 and <4 | 1650 (20.6) | 1823 (21.5) | 1500 (19.9) | 1461 (19.5) | 1451 (18.9) | 7885 (20.1) | p < 0.0001 |
| >=4 and <6 | 2637 (33.0) | 2949 (34.7) | 2759 (36.6) | 2757 (36.8) | 2827 (36.8) | 13,929 (35.5) | |
| >=6 | 1456 (18.2) | 1676 (19.7) | 1588 (21.1) | 1829 (24.4) | 2252 (29.4) | 8801 (22.5) | |
| WHR 3 at baseline | |||||||
| Normal WHR | 4149 (51.9) | 5201 (61.2) | 5026 (66.7) | 5297 (70.8) | 5836 (76.1) | 25,509 (65.1) | χ2 = 1200; df = 4 |
| Raised WHR | 3845 (48.1) | 3295 (38.8) | 2508 (33.3) | 2190 (29.3) | 1838 (23.9) | 13,676 (34.9) | p < 0.001 |
| BMI 4 at baseline | |||||||
| <25 | 2461 (30.8) | 2883 (33.9) | 2640 (35.0) | 2955 (39.5) | 3522 (45.9) | 14,461 (36.9) | χ2 = 489; df = 8 |
| 25–29.9 | 3758 (47.0) | 3769 (44.4) | 3274 (43.5) | 3132 (41.8) | 2981 (38.9) | 16,914 (43.2) | p < 0.001 |
| >=30.0 | 1775 (22.2) | 1844 (21.7) | 1620 (21.5) | 1400 (18.7) | 1171 (15.3) | 7810 (19.9) | |
| Mean ± SD | 27.3 ± 4.4 | 27.1± 4.4 | 27.0 ± 4.4 | 26.6± 4.3 | 26.0 ± 4.5 | 26.8 ± 4.4 | p < 0.001 * |
| Family history of diabetes | |||||||
| No | 6587 (82.4) | 6980 (82.2) | 6217 (82.5) | 6168 (82.4) | 6293 (82.0) | 32,245 (82.3) | χ2 = 0.92; df = 4 |
| Yes | 1407 (17.6) | 1516 (17.8) | 1317 (17.5) | 1319 (17.6) | 1381 (18.0) | 6940 (17.7) | p 0.922 |
| Comorbidity | |||||||
| No | 3616 (45.2) | 3782 (44.5) | 3360 (44.6) | 3311 (44.2) | 3554 (46.3) | 17,623 (45.0) | χ2 = 8.6; df = 4 |
| Yes | 4378 (54.8) | 4714 (55.5) | 4174 (55.4) | 4176 (55.8) | 4120 (53.7) | 21,562 (55.0) | p 0.071 |
| (c) | |||||||
| MDS 1 | MDS 2 | MDS 3 | Total | Test Statistics | |||
| MDS median (IQR) | 3 (2, 3) | 5 (4, 6) | 7 (7, 8) | 4 (3, 6) | |||
| Age | |||||||
| <50 Years | 4411 (33.6) | 6796 (32.0) | 1462 (30.3) | 12,669 (32.3) | χ2 = 20.1; df = 4 | ||
| 50–59 Years | 4187 (31.9) | 7009 (33.0) | 1597 (33.1) | 12,793 (32.7) | p < 0.001 | ||
| >=60 Years | 4538 (34.5) | 7420 (35.0) | 1765 (36.6) | 13,723 (35.0) | |||
| Mean ± SD | 55.0 ± 8.7 | 55.2 ± 8.6 | 55.6 ± 8.6 | 55.2 ± 8.7 | p < 0.001 * | ||
| Gender | |||||||
| Male | 5389 (41.0) | 8451 (39.8) | 1951 (40.4) | 15,791 (40.3) | χ2 = 4.97; df = 2 | ||
| Female | 7747 (59.0) | 12,774 (60.2) | 2873 (59.6) | 23,394 (59.7) | p 0.083 | ||
| SEIFA 1 Quintiles | |||||||
| SEIFA Q1 | 2712 (20.7) | 3690 (17.4) | 674 (14.0) | 7076 (18.10 | χ2 = 272; df = 8 | ||
| SEIFA Q2 | 2931 (22.3) | 4353 (20.5) | 865(17.9) | 8149 (20.8) | p < 0.001 | ||
| SEIFA Q3 | 2074 (15.8) | 3384 (15.9) | 713 (14.8) | 6171 (15.8) | |||
| SEIFA Q4 | 2323 (17.7) | 3943 (15.6) | 988 (20.50 | 7254 (18.5) | |||
| SEIFA Q5 | 3096 (23.6) | 5855 (27.6) | 1584 (32.8) | 10,535 (26.9) | |||
| Region of Origin | |||||||
| AUS/NZ 2/Other | 9196 (70.0) | 14,807 (69.8) | 3328 (69.0) | 27,331 (69.8) | χ2 = 43.8; df = 4 | ||
| Northern Europe | 754 (5.7) | 1368 (6.5) | 406 (8.4) | 2528 (6.5) | p < 0.001 | ||
| Southern Europe | 3186 (24.3) | 5050 (23.8) | 1090 (22.6) | 9326 (23.8) | |||
| Smoking status | |||||||
| Non-smoker | 7552 (57.5) | 12,392 (58.4) | 2770 (57.4) | 22,714 (58.0) | χ2 = 3.33; df = 2 | ||
| Smoker | 5584 (42.5) | 8833 (41.6) | 2054 (42.6) | 16,471 (42.0) | p 0.190 | ||
| Alcohol dinking status | |||||||
| Never | 4374 (33.3) | 5945 (28.0) | 822 (17.0) | 11,141 (28.4) | χ2 = 496; df = 4 | ||
| Former | 1396 (10.6) | 2217 (10.5) | 486 (10.1) | 4099 (10.5) | p < 0.001 | ||
| Current | 7366 (56.1) | 13,063 (61.6) | 3516 (72.9) | 23,945 (61.1) | |||
| Physical activity score | |||||||
| 0 | 3294 (25.1) | 4486 (21.1) | 790 (16.4) | 8570 (21.9) | χ2 = 265; df = 6 | ||
| >0 and <4 | 2708 (20.6) | 4244 (20.0) | 933 (19.3) | 7885 (20.1) | p < 0.001 | ||
| >=4 and <6 | 4621 (35.2) | 7531 (35.5) | 1777 (36.8) | 13,929 (35.6) | |||
| >=6 | 2513 (19.1) | 4964 (23.4) | 1324 (27.5) | 8801 (22.5) | |||
| WHR 3 at baseline | |||||||
| Normal WHR | 8275 (63.0) | 13,967 (65.8) | 3267 (67.7) | 25,509 (65.1) | χ2 = 44.9; df = 2 | ||
| Raised WHR | 4861 (37.0) | 7258 (34.2) | 1557 (32.3) | 13,676 (34.9) | p <0.001 | ||
| BMI 4 at baseline | |||||||
| <25 | 4617 (35.2) | 7979 (37.6) | 1865 (38.7) | 14,461 (36.9) | χ2 = 57.3; df = 4 | ||
| 25–29.9 | 5675 (43.2) | 9115 (42.9) | 2124 (44.0) | 16,914 (43.2) | p < 0.001 | ||
| >=30.0 | 2844 (21.7) | 4131 (19.5) | 835 (17.3) | 7810 (19.9) | |||
| Mean ± SD | 27.0 ± 4.5 | 26.8± 4.4 | 26.5 ± 4.1 | 26.8± 4.4 | p < 0.001 * | ||
| Family history of diabetes | |||||||
| No | 10,777 (82.0) | 17,448 (82.2) | 4020 (83.3) | 32,245 (82.3) | χ2 = 4. 26; df = 2 | ||
| Yes | 2359 (18.0) | 3777 (17.8) | 804 (16.7) | 6940 (17.7) | p 0.119 | ||
| Comorbidity | |||||||
| No | 5894 (44.9) | 9541 (45.0) | 2188 (45.4) | 17,623 (45.0) | χ2 = 0.348; df = 2 | ||
| Yes | 7242 (55.1) | 11,684 (55.0) | 2636 (54.6) | 21,562 (55.0) | p 0.084 | ||
| Variables | Category | Wave 1 | Wave 2 | ||
|---|---|---|---|---|---|
| n (%) | p Value | n (%) | p Value | ||
| Age | <50 years | 122 (1.1) | <0.001 | 276 (3.4) | <0.001 |
| 50–59 years | 263 (2.4) | 499 (4.5) | |||
| >=60 years | 355 (3.0) | 474 (7.2) | |||
| Sex | Male | 376 (2.8) | <0.001 | 591 (6.9) | <0.001 |
| Female | 356 (1.8) | 658 (4.8) | |||
| SEIFA 1 | Q1 | 214 (3.6) | <0.001 | 282 (8.4) | <0.001 |
| Q2 | 188 (2.8) | 289 (7.2) | |||
| Q3 | 111 (2.1) | 198 (5.6) | |||
| Q4 | 98 (1.6) | 221 (4.9) | |||
| Q5 | 129 (1.4) | 268 (3.8) | |||
| Region of Origin | AUS/NZ 2/Other | 344 (1.5) | <0.001 | 734 (4.5) | <0.001 |
| Northern Europe | 41 (1.9) | 61 (4.0) | |||
| Southern Europe | 355 (4.6) | 454 (10.2) | |||
| WHR 3 group | Normal | 238 (1.1) | <0.001 | 503 (3.3) | <0.001 |
| High | 502 (4.4) | 746 (10.7) | |||
| BMI 4 group | <25 | 63 (0.5) | <0.001 | 133 (1.5) | <0.001 |
| 25–29.9 | 297 (2.1) | 541 (5,7) | |||
| >=30 | 380 (5.8) | 575 (14.5) | |||
| Comorbidity | No | 191 (1.2) | <0.001 | 403 (3.8) | <0.001 |
| Yes | 549 (3.0) | 846 (7.3) | |||
| Family History | No | 493 (1.8) | <0.001 | 865 (4.7) | <0.001 |
| Yes | 247 (4.1) | 384 (10.0) | |||
| Smoking status | No | 379 (1.9) | <0.001 | 699 (5.1) | <0.001 |
| Yes | 361 (2.6) | 550 (6.3) | |||
| Drinking status | Never | 276 (2.9) | <0.001 | 417 (7.0) | <0.001 |
| Former | 91 (2.6) | 150 (6.4) | |||
| Current | 373 (1.8) | 682 (4.8) | |||
| Physical activity | 0 | 266 (3.2) | <0.001 | 356 (7.7) | <0.001 |
| 1–4 | 171 (2.5) | 273 (5.9) | |||
| 4–6 | 243 (2.0) | 430 (5.6) | |||
| >=6 | 100 (1.3) | 190 (3.5) | |||
| DII 5 | Q1 | 124 (1.8) | <0.001 | 199 (4.2) | <0.001 |
| Q2 | 133 (1.9) | 246 (5.2) | |||
| Q3 | 144 (2.1) | 251 (5.4) | |||
| Q4 | 154 (2.3) | 262 (5.9) | |||
| Q5 | 185 (2.9) | 291 (7.5) | |||
| AEHI 6 | Q1 | 175 (2.6) | <0.001 | 313 (7.4) | <0.001 |
| Q2 | 189 (2.6) | 294 (6.2) | |||
| Q3 | 160 (2.5) | 242 (5.6) | |||
| Q4 | 122 (1.9) | 232 (5.3) | |||
| Q5 | 94 (1.4) | 168 (3.6) | |||
| MDS 7 | 0–3 | 268 (2.4) | 0.118 | 444 (6.2) | 0.003 |
| 4–6 | 376 (2.1) | 672 (5.5) | |||
| 7–9 | 96 (2.3) | 133 (5.8) | |||
| Adjusted 1 IRR (95% CI) | p Value | Adjusted 2 IRR (95% CI) | p Value | Adjusted 3 IRR (95% CI) | p Value | Adjusted 4 IRR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|---|
| DII 5 Quintile | ||||||||
| DII Q1 | Reference | Reference | Reference | Reference | ||||
| DII Q2 | 1.17 (1.01, 1.36) | 0.03 | 1.14 (0.98, 1.31) | 0.08 | 1.13 (0.98, 1.30) | 0.11 | 1.10 (0.95, 1.27) | 0.19 |
| DII Q3 | 1.20 (1.05, 1.40) | 0.01 | 1.11 (0.96, 1.27) | 0.16 | 1.09 (0.95, 1.26) | 0.23 | 1.06 (0.92, 1.22) | 0.43 |
| DII Q4 | 1.29 (1.11, 1.48) | 0.001 | 1.14 (0.99, 1.31) | 0.07 | 1.08 (0.96, 1.28) | 0.15 | 1.05 (0.91, 1.21) | 0.47 |
| DII Q5 | 1.49 (1.30, 1.72) | <0.001 | 1.25 (1.08, 1.43) | 0.002 | 1.21 (1.05, 1.39) | 0.008 | 1.10 (0.95, 1.26) | 0.21 |
| p trend | <0.001 | 0.005 | 0.02 | 0.40 | ||||
| AHEI 6 Quintile | ||||||||
| AHEI Q1 | Reference | Reference | Reference | Reference | ||||
| AHEI Q2 | 0.97 (0.86, 1.10) | 0.65 | 0.99 (0.88, 1.12) | 0.87 | 1.02 (0.90, 1.15) | 0.75 | 0.98 (0.87, 1.11) | 0.77 |
| AHEI Q3 | 0.94 (0.83, 1.07) | 0.38 | 0.95 (0.84, 1.09) | 0.59 | 0.99 (0.87, 1.13) | 0.88 | 0.94 (0.83, 1.07) | 0.34 |
| AHEI Q4 | 0.87 (0.76, 1.00) | 0.05 | 0.93 (0.81, 1.06) | 0.28 | 0.96 (0.84, 1.10) | 0.59 | 0.91 (0.80, 1.04) | 0.18 |
| AHEI Q5 | 0.67 (0.58, 0.78) | <0.001 | 0.75 (0.65, 0.87) | <0.001 | 0.78 (0.67, 0.91) | 0.001 | 0.73 (0.63, 0.85) | <0.001 |
| p trend | <0.001 | <0.001 | 0.003 | <0.001 | ||||
| MDS 7 Category | ||||||||
| Score 0–3 | Reference | Reference | Reference | Reference | ||||
| Score 4–6 | 0.93 (0.85, 1.02) | 0.18 | 0.95 (0.86, 1.04) | 0.24 | 0.95 (0.87, 1.04) | 0.291 | 0.94 (0.86, 1.03) | 0.20 |
| Score 7–9 | 0.97 (0.84, 1.13) | 0.69 | 0.99 (0.86, 1.15) | 0.90 | 1.02 (0.88, 1.18) | 0.836 | 0.98 (0.85, 1.13) | 0.77 |
| p trend | 0.28 | 0.47 | 0.65 | 0.37 | ||||
| Australia and New Zealand | Northern Europe | Southern Europe | ||||
|---|---|---|---|---|---|---|
| Adjusted IRR (95% CI) 1 | p Value | Adjusted IRR (95% CI) 1 | p Value | Adjusted IRR (95% CI) 1 | p Value | |
| DII 2 Quintile | ||||||
| DII Q1 | Reference | Reference | Reference | |||
| DII Q2 | 1.06 (0.92, 1.32) | 0.27 | 1.63 (0.89, 2.99) | 0.12 | 1.05 (0.80, 1.37) | 0.74 |
| DII Q3 | 1.11 (0.92, 1.33) | 0.29 | 1.37 (0.71, 2.66) | 0.35 | 1.12 (0.86, 1.45) | 0.38 |
| DII Q4 | 1.19 (0.99, 1.43) | <0.001 | 1.77 (0.96, 3.26) | 0.07 | 1.02 (0.79, 1.31) | 0.91 |
| DII Q5 | 1.49 (1.22, 1.80) | <0.001 | 1.66 (0.87, 1.17) | 0.13 | 1.02 (0.80, 1.30) | 0.87 |
| p trend | <0.001 | 0.13 | 0.75 | |||
| AHEI 3 Quintile | ||||||
| AHEI Q1 | Reference | Reference | Reference | |||
| AHEI Q2 | 0.91 (0.77, 1.07) | 0.25 | 1.39 (0.78, 2.47) | 0.27 | 0.93 (0.76, 1.14) | 0.50 |
| AHEI Q3 | 0.75 (0.62, 0.89) | 0.001 | 0.94 (0.49, 1.80) | 0.84 | 1.10 (0.89, 1.36) | 0.35 |
| AHEI Q4 | 0.67 (0.55, 0.81) | 0.05 | 0.87 (0.49, 1.71) | 0.70 | 1.07 (0.87, 1.32) | 0.55 |
| AHEI Q5 | 0.51 (0.41, 0.62) | <0.001 * | 0.90 (0.46, 1.79) | 0.78 | 0.83 (0.65, 1.05) | 0.13 |
| p trend | <0.001 | 0.37 | 0.48 | |||
| MDS 4 Category | ||||||
| Score 0–3 | Reference | Reference | Reference | |||
| Score 4–6 | 0.88 (0.77, 1.00) | 0.047 | 1.05 (0.68, 1.62) | 0.82 | 0.95 (0.82, 1.10) | 0.47 |
| Score 7–9 | 0.79 (0.64, 0.98) | 0.033 | 0.91 (0.50, 1.69) | 0.78 | 1.11 (0.90, 1.39) | 0.33 |
| p trend | 0.011 | 0.94 | 0.80 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodge, A.M.; Karim, M.N.; Hébert, J.R.; Shivappa, N.; de Courten, B. Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study. Nutrients 2021, 13, 4162. https://doi.org/10.3390/nu13114162
Hodge AM, Karim MN, Hébert JR, Shivappa N, de Courten B. Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study. Nutrients. 2021; 13(11):4162. https://doi.org/10.3390/nu13114162
Chicago/Turabian StyleHodge, Allison M., Md Nazmul Karim, James R. Hébert, Nitin Shivappa, and Barbora de Courten. 2021. "Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study" Nutrients 13, no. 11: 4162. https://doi.org/10.3390/nu13114162
APA StyleHodge, A. M., Karim, M. N., Hébert, J. R., Shivappa, N., & de Courten, B. (2021). Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study. Nutrients, 13(11), 4162. https://doi.org/10.3390/nu13114162







