Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management
Abstract
:1. Introduction
2. Pathophysiology of Allergic Asthma
2.1. The Sensitization Phase of Allergic Asthma
2.2. The Efffector Phase of Allergic Asthma
3. The Gut-Lung Axis
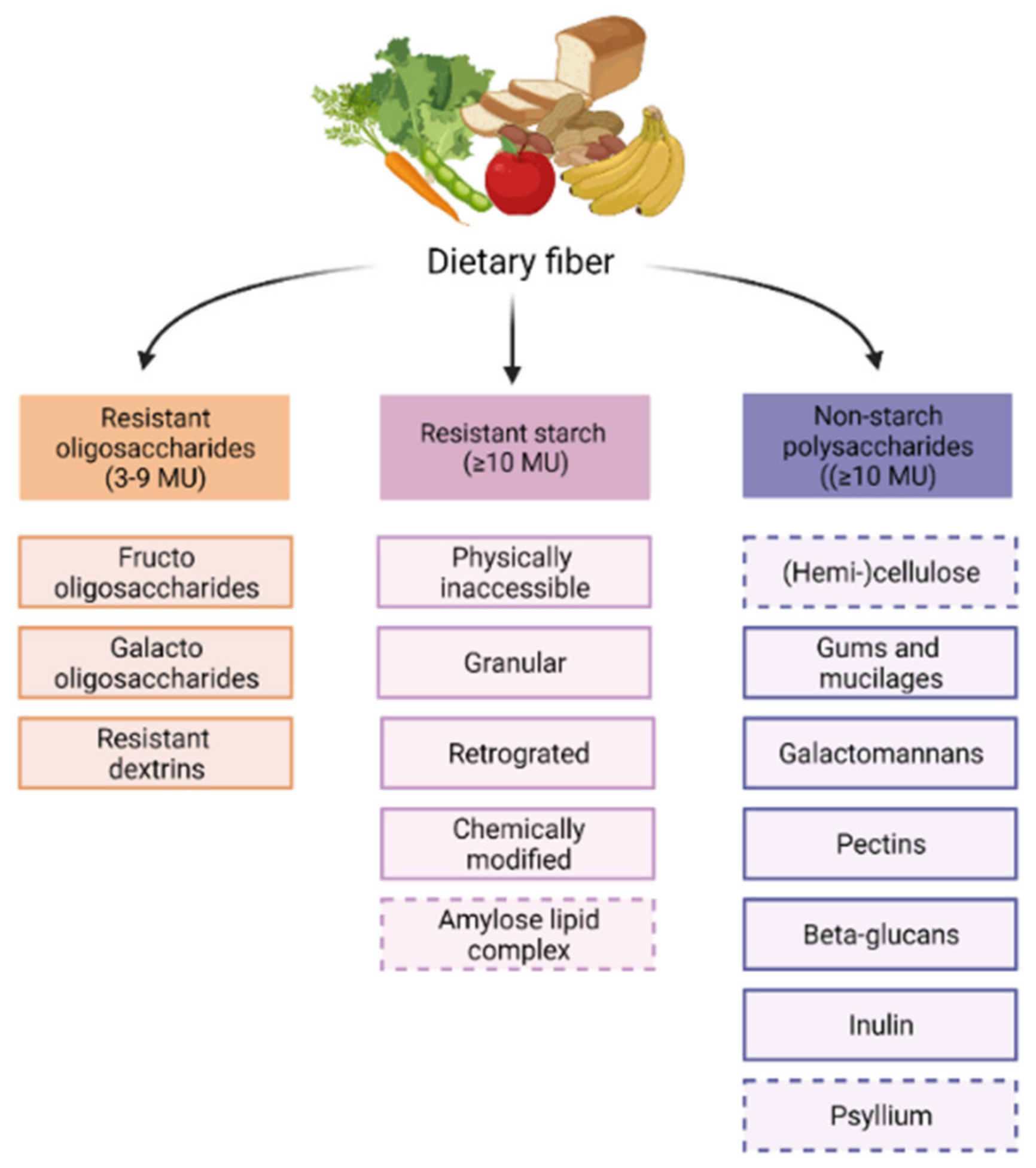
4. Fiber Types, Functions and Metabolites
5. Mechanisms of Action of SCFAs
5.1. GPCRs and Downstream Signaling Pathways
5.2. HDAC Inhibition and Epigenetic Imprinting
6. Immune Effects SCFAs
6.1. Sensitization Phase of Allergic Asthma
6.1.1. (Airway) Epithelium
6.1.2. Dendritic Cells
6.1.3. B Cells and Immunoglobulins
6.2. Effector Phase of Allergic Asthma
6.2.1. Mast Cells
6.2.2. Eosinophils
6.2.3. T Cells and ILC2s
Th2 Cells
ILC2s
Tregs
6.2.4. Macrophages and Neutrophils
7. Preclinical Studies on the Effects of Dietary Fibers on Asthma
7.1. Preventive Effects of Dietary Fibers
| Cells and Mediators | Specific Factor of Interest Increased in AAD | Effect Dietary Fiber Compared to Control Diet | Fiber Type and Dose | Asthma Model | Reference |
|---|---|---|---|---|---|
| Sensitization phase | |||||
| Dendritic cells | Activation (surface expression) | ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] |
| Antibody response | Total IgE | ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] |
| Allergen specific IgE | ↓ | - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| Allergen specific IgG1 | ↓ | - 2.5% FOS | HDM | [133] | |
| Effector phase | |||||
| Total inflammatory cells BAL | ↓ | - 50 g/kg RAF, 50 g/kg GOS | OVA | [135] | |
| - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g) | OVA | [136] | |||
| - 1% v/w GOS | HDM | [127] | |||
| - 1% w/w scFOS/lcFOS (1:1) + 2% w/w Bb | HDM | [129] | |||
| - 1% (w/w) scGOS/lcFOS (9:1), 1% (w/w) (83% scGOS/lcFOS + 17% AOS) | OVA | [134] | |||
| - 30% pectin (in PK diet 3202) | HDM | [78] | |||
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 1 mg/kg SC-MN (i.n.) | OVA | [137] | |||
| - 1% and 2.5% GOS | HDM | [128] | |||
| Degranulating cells | Mast cells | #↓ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g.) | OVA | [136] |
| - 2.5% GOS | HDM | [128] | |||
| Eosinophils | #↓ | - 50 g/kg RAF, 50 g/kg GOS | OVA | [135] | |
| - 1% v/w GOS | HDM | [127] | |||
| - 1% w/w scFOS/lcFOS (1:1) + 2% w/w Bb | HDM | [129] | |||
| - 30% pectin (in PK diet 3202) | HDM | [78] | |||
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 1% and 2.5% GOS | HDM | [128] | |||
| - 30% pectin | IL-33 | [112] | |||
| - 2.5% FOS | HDM | [133] | |||
| %↓ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g) | OVA | [136] | ||
| - 30% pectin | IL-33 | [112] | |||
| Macrophages | #↓ | - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| Neutrophils | %↑ | - 30% pectin | IL-33 | [112] | |
| T cells and related cells | All lymphocytes | #↓ | - 50 g/kg RAF, 50 g/kg GOS | OVA | [135] |
| - 1% w/w scFOS/lcFOS (1:1) + 2% w/w Bb | HDM | [129] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| Overall Th | #↓ | - 200/400 mg/kg/day AO (in water) | OVA | [132] | |
| Th2 | #↓ | - 400 mg/kg/day AO (in water) | OVA | [132] | |
| - 30% pectin | IL-33 | [112] | |||
| ILC2 | #↓ | - 30% pectin | IL-33 | [112] | |
| Th1 | #↓ | - 400 mg/kg/day AO (in water) | OVA | [132] | |
| Treg * | #↑ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g.) | OVA | [136] | |
| Cytokines | IL-33 | ↓ | - 1% v/w GOS | HDM | [126] |
| IL-4 | ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] | |
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 30% pectin | IL-33 | [112] | |||
| - 16.5 mg/kg BW LM-COS | OVA | [138] | |||
| IL-5 | ↓ | - 50 g/kg RAF | OVA | [135] | |
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 16.5 mg/kg BW LM-COS | OVA | [138] | |||
| IL-13 | ↓ | - 1% v/w GOS | HDM | [127] | |
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 30% pectin | IL-33 | [112] | |||
| - 16.5 mg/kg BW LM-COS | OVA | [138] | |||
| IFN-y | ↑ | - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |
| - 30% pectin | IL-33 | [112] | |||
| ↓ | - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | ||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| IL-17 | ↑ | - 30% pectin | IL-33 | [112] | |
| ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] | ||
| IL-10 | ↑ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g.) | OVA | [136] | |
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| ↓ | - 72.7% HAMRS diet (SF11-025) | HDM | [117] | ||
| TNF-α | ↓ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g.) | OVA | [136] | |
| - 50/200/400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 16.5 mg/kg BW LM-COS | OVA | [138] | |||
| IL-1β | ↓ | - 0.2 mL Bb/scFOS/lcFOS/AOS in PBS (i.g.) | OVA | [136] | |
| - 400 mg/kg/day AO (in water) | OVA | [132] |
| Physiological Airway Effects | Specific Factor of Interest Increased in AAD | Effect of Dietary Fiber Compared to Control Diet | Fiber Type and Dose | Asthma Model | Reference |
|---|---|---|---|---|---|
| Airway remodeling | Epithelial denudation | ↓ | - 1 mg/kg SC-MN (i.n.) | OVA | [137] |
| Surface area infiltrated with inflammatory cells | ↓ | - 400 mg/kg/day AO (in water) | OVA | [132] | |
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 1% and 2.5% GOS | HDM | [128] | |||
| - 2.5% FOS | HDM | [133] | |||
| Airway smooth muscle mass | ↓ | - 45 mg/kg SC-MN (i.n.) | OVA | [137] | |
| Goblet cell hyperplasia or metaplasia | ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] | |
| - 400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 0.4%/kg/day pectin (i.g.) (KF chow) | OVA | [131] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 2.5% FOS | HDM | [133] | |||
| Mucus production | ↓ | - 30% pectin (in PK diet 3202) | HDM | [78] | |
| - 400 mg/kg/day AO (in water) | OVA | [132] | |||
| - 1 mg/kg SC-MN (i.n.) | OVA | [137] | |||
| Airway functioning | Airway hyperresponsiveness | ↓ | - 1% v/w GOS | HDM | [127] |
| - 30% pectin (in PK diet 3202) | HDM | [78] | |||
| - 72.7% HAMRS diet (SF11-025) | HDM | [117] | |||
| - 1 mg/kg SC-MN (i.n.) | OVA | [137] | |||
| - 30% pectin | IL-33 | [112] |
7.2. Symptom Control by Dietary Fibers
8. Human Studies on the Effects of Dietary Fibers on Asthma
8.1. Epidemiological Evidence
8.2. Clinical Evidence Early Life
8.3. Clinical Evidence Adults
9. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barcik, W.; Boutin, R.C.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2021 Update); Global Initiative for Asthma: Fontana, WI, USA, 2021. [Google Scholar]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zhang, Z. Allergic asthma: Influence of genetic and environmental factors. J. Biol. Chem. 2011, 286, 32883–32889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2019. [Google Scholar]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 467–485. [Google Scholar] [CrossRef]
- Strachan, D.P. Family size, infection and atopy: The first decade of the “hygiene hypothesis”. Thorax 2000, 55 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef] [Green Version]
- Romagnani, S. Human TH1 and TH2 subsets: Regulation of differentiation and role in protection and immunopathology. Int. Arch. Allergy Immunol. 1992, 98, 279–285. [Google Scholar] [CrossRef]
- Bjorksten, B. Diverse microbial exposure—Consequences for vaccine development. Vaccine 2012, 30, 4336–4340. [Google Scholar] [CrossRef]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozanska, B.; Sikorska-Szaflik, H. Diet Modifications in Primary Prevention of Asthma. Where Do We Stand? Nutrients 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Williams, A.M.; Probert, C.S.; Stepankova, R.; Tlaskalova-Hogenova, H.; Phillips, A.; Bland, P.W. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 2006, 119, 470–478. [Google Scholar] [CrossRef]
- El Aidy, S.; Hooiveld, G.; Tremaroli, V.; Backhed, F.; Kleerebezem, M. The gut microbiota and mucosal homeostasis: Colonized at birth or at adulthood, does it matter? Gut Microbes 2013, 4, 118–124. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.; Skov, T.; Paludan-Muller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Prescott, S.L. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J. Allergy Clin. Immunol. 2013, 131, 23–30. [Google Scholar] [CrossRef]
- Covar, R.A.; Strunk, R.; Zeiger, R.S.; Wilson, L.A.; Liu, A.H.; Weiss, S.; Tonascia, J.; Spahn, J.D.; Szefler, S.J.; Childhood Asthma Management Program Research Group. Predictors of remitting, periodic, and persistent childhood asthma. J. Allergy Clin. Immunol. 2010, 125, 359–366.e353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, M.; Charbonnier, A.S.; Taront, S.; Brichet, A.; Wallaert, B.; Pestel, J.; Tonnel, A.B.; Gosset, P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J. Allergy Clin. Immunol. 2005, 115, 771–778. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Ballesteros-Tato, A.; Randall, T.D.; Lund, F.E.; Spolski, R.; Leonard, W.J.; Leon, B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 2016, 44, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Coquet, J.M.; Schuijs, M.J.; Smyth, M.J.; Deswarte, K.; Beyaert, R.; Braun, H.; Boon, L.; Karlsson Hedestam, G.B.; Nutt, S.L.; Hammad, H.; et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 2015, 43, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Peebles, R.S., Jr.; Aronica, M.A. Proinflammatory Pathways in the Pathogenesis of Asthma. Clin. Chest Med. 2019, 40, 29–50. [Google Scholar] [CrossRef]
- Tindemans, I.; van Schoonhoven, A.; KleinJan, A.; de Bruijn, M.J.; Lukkes, M.; van Nimwegen, M.; van den Branden, A.; Bergen, I.M.; Corneth, O.B.; van, I.W.F.; et al. Notch signaling licenses allergic airway inflammation by promoting Th2 cell lymph node egress. J. Clin. Investig. 2020, 130, 3576–3591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckl-Dorna, J.; Villazala-Merino, S.; Campion, N.J.; Byazrova, M.; Filatov, A.; Kudlay, D.; Karsonova, A.; Riabova, K.; Khaitov, M.; Karaulov, A.; et al. Tracing IgE-Producing Cells in Allergic Patients. Cells 2019, 8, 994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindemans, I.; Serafini, N.; Di Santo, J.P.; Hendriks, R.W. GATA-3 function in innate and adaptive immunity. Immunity 2014, 41, 191–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef]
- Van der Ploeg, E.K.; Carreras Mascaro, A.; Huylebroeck, D.; Hendriks, R.W.; Stadhouders, R. Group 2 Innate Lymphoid Cells in Human Respiratory Disorders. J. Innate. Immun. 2020, 12, 47–62. [Google Scholar] [CrossRef]
- Li, B.W.; de Bruijn, M.J.; Tindemans, I.; Lukkes, M.; KleinJan, A.; Hoogsteden, H.C.; Hendriks, R.W. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur. J. Immunol. 2016, 46, 1392–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Huang, Y.; Chen, X.; Hu-Li, J.; Urban, J.F., Jr.; Paul, W.E. Innate immunological function of TH2 cells in vivo. Nat. Immunol. 2015, 16, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Nobs, S.P.; Kayhan, M.; Kopf, M. GM-CSF intrinsically controls eosinophil accumulation in the setting of allergic airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 1513–1524.e1512. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Al Heialy, S.; Hamid, Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev. Respir. Med. 2019, 13, 1057–1068. [Google Scholar] [CrossRef]
- Koch, S.; Sopel, N.; Finotto, S. Th9 and other IL-9-producing cells in allergic asthma. Semin. Immunopathol. 2017, 39, 55–68. [Google Scholar] [CrossRef]
- Akbari, O.; Stock, P.; DeKruyff, R.H.; Umetsu, D.T. Role of regulatory T cells in allergy and asthma. Curr. Opin. Immunol. 2003, 15, 627–633. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Norte-Munoz, M.; Martinez-Garcia, J. Regulatory T Cells in Allergy and Asthma. Front. Pediatr. 2017, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal. Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Raes, J.; van den Bogert, B.; Arumugam, M.; Booijink, C.C.; Troost, F.J.; Bork, P.; Wels, M.; de Vos, W.M.; Kleerebezem, M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Bierla, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [Green Version]
- Solis, G.; de Los Reyes-Gavilan, C.G.; Fernandez, N.; Margolles, A.; Gueimonde, M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010, 16, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B.; Ravel, J. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. MBio 2015, 6, e00037-15. [Google Scholar] [CrossRef] [Green Version]
- Pattaroni, C.; Watzenboeck, M.L.; Schneidegger, S.; Kieser, S.; Wong, N.C.; Bernasconi, E.; Pernot, J.; Mercier, L.; Knapp, S.; Nicod, L.P.; et al. Early-Life Formation of the Microbial and Immunological Environment of the Human Airways. Cell Host Microbe 2018, 24, 857–865.e854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsland, B.J.; Gollwitzer, E.S. Host-microorganism interactions in lung diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, D.R.; Welsh, D.A.; Shellito, J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.B.; Scribner, T.A.; Akei, H.S.; Rothenberg, M.E. Experimental gastrointestinal allergy enhances pulmonary responses to specific and unrelated allergens. J. Allergy Clin. Immunol. 2006, 118, 420–427. [Google Scholar] [CrossRef]
- Navarro, S.; Cossalter, G.; Chiavaroli, C.; Kanda, A.; Fleury, S.; Lazzari, A.; Cazareth, J.; Sparwasser, T.; Dombrowicz, D.; Glaichenhaus, N.; et al. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal. Immunol. 2011, 4, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivniouk, V.; Gimenes-Junior, J.A.; Ezeh, P.; Michael, A.; Pivniouk, O.; Hahn, S.; VanLinden, S.R.; Malone, S.P.; Abidov, A.; Anderson, D.; et al. Airway administration of OM-85, a bacterial lysate, blocks experimental asthma by targeting dendritic cells and the epithelium/IL-33/ILC2 axis. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2011, 127, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Ruane, D.; Brane, L.; Reis, B.S.; Cheong, C.; Poles, J.; Do, Y.; Zhu, H.; Velinzon, K.; Choi, J.H.; Studt, N.; et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 2013, 210, 1871–1888. [Google Scholar] [CrossRef] [Green Version]
- Tulic, M.K.; Piche, T.; Verhasselt, V. Lung-gut cross-talk: Evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy 2016, 46, 519–528. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazici, C.; Soberanes, S.; Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgman, S.L.; Azad, M.B.; Field, C.J.; Haqq, A.M.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Sears, M.R.; Scott, J.A.; et al. Fecal Short-Chain Fatty Acid Variations by Breastfeeding Status in Infants at 4 Months: Differences in Relative versus Absolute Concentrations. Front. Nutr. 2017, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Segal, L.N.; Clemente, J.C.; Li, Y.; Ruan, C.; Cao, J.; Danckers, M.; Morris, A.; Tapyrik, S.; Wu, B.G.; Diaz, P.; et al. Anaerobic Bacterial Fermentation Products Increase Tuberculosis Risk in Antiretroviral-Drug-Treated HIV Patients. Cell Host Microbe 2017, 21, 530–537.e534. [Google Scholar] [CrossRef] [Green Version]
- Mirkovic, B.; Murray, M.A.; Lavelle, G.M.; Molloy, K.; Azim, A.A.; Gunaratnam, C.; Healy, F.; Slattery, D.; McNally, P.; Hatch, J.; et al. The Role of Short-Chain Fatty Acids, Produced by Anaerobic Bacteria, in the Cystic Fibrosis Airway. Am. J. Respir. Crit. Care Med. 2015, 192, 1314–1324. [Google Scholar] [CrossRef]
- Ghorbani, P.; Santhakumar, P.; Hu, Q.; Djiadeu, P.; Wolever, T.M.; Palaniyar, N.; Grasemann, H. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur. Respir. J. 2015, 46, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Blad, C.C.; Tang, C.; Offermanns, S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 2012, 11, 603–619. [Google Scholar] [CrossRef]
- Theiler, A.; Barnthaler, T.; Platzer, W.; Richtig, G.; Peinhaupt, M.; Rittchen, S.; Kargl, J.; Ulven, T.; Marsh, L.M.; Marsche, G.; et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J. Allergy Clin. Immunol. 2019, 144, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Steinmeyer, S.; Lee, K.; Jayaraman, A.; Alaniz, R.C. Microbiota metabolite regulation of host immune homeostasis: A mechanistic missing link. Curr. Allergy Asthma Rep. 2015, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Sealy, L.; Chalkley, R. The effect of sodium butyrate on histone modification. Cell 1978, 14, 115–121. [Google Scholar] [CrossRef]
- Adcock, I.M.; Tsaprouni, L.; Bhavsar, P.; Ito, K. Epigenetic regulation of airway inflammation. Curr. Opin. Immunol. 2007, 19, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Stadhouders, R.; Filion, G.J.; Graf, T. Transcription factors and 3D genome conformation in cell-fate decisions. Nature 2019, 569, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [Green Version]
- Meyers, D.A.; Bleecker, E.R.; Holloway, J.W.; Holgate, S.T. Asthma genetics and personalised medicine. Lancet Respir. Med. 2014, 2, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Willis-Owen, S.A.G.; Laprise, C.; Wong, K.C.C.; Davies, G.A.; Hudson, T.J.; Binia, A.; Hopkin, J.M.; Yang, I.V.; Grundberg, E.; et al. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature 2015, 520, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.V.; Pedersen, B.S.; Liu, A.; O’Connor, G.T.; Teach, S.J.; Kattan, M.; Misiak, R.T.; Gruchalla, R.; Steinbach, S.F.; Szefler, S.J.; et al. DNA methylation and childhood asthma in the inner city. J. Allergy Clin. Immunol. 2015, 136, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Seumois, G.; Chavez, L.; Gerasimova, A.; Lienhard, M.; Omran, N.; Kalinke, L.; Vedanayagam, M.; Ganesan, A.P.; Chawla, A.; Djukanovic, R.; et al. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat. Immunol. 2014, 15, 777–788. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Maleki, M.; Ebrahimi Vargoorani, M.; Amiri, V. A review of epigenetic changes in asthma: Methylation and acetylation. Clin. Epigenet. 2021, 13, 65. [Google Scholar] [CrossRef]
- Stadhouders, R.; Li, B.W.S.; de Bruijn, M.J.W.; Gomez, A.; Rao, T.N.; Fehling, H.J.; van, I.W.F.J.; Lim, A.I.; Di Santo, J.P.; Graf, T.; et al. Epigenome analysis links gene regulatory elements in group 2 innate lymphocytes to asthma susceptibility. J. Allergy Clin. Immunol. 2018, 142, 1793–1807. [Google Scholar] [CrossRef] [Green Version]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.B.; Li, M.; Folkerts, G.; Henricks, P.A.J.; Garssen, J.; van Esch, B. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int. J. Mol. Sci. 2020, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, P.; Wawrzyniak, M.; Wanke, K.; Sokolowska, M.; Bendelja, K.; Ruckert, B.; Globinska, A.; Jakiela, B.; Kast, J.I.; Idzko, M.; et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steelant, B.; Wawrzyniak, P.; Martens, K.; Jonckheere, A.C.; Pugin, B.; Schrijvers, R.; Bullens, D.M.; Vanoirbeek, J.A.; Krawczyk, K.; Dreher, A.; et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2019, 144, 1242–1253.e1247. [Google Scholar] [CrossRef] [Green Version]
- Millard, A.L.; Mertes, P.M.; Ittelet, D.; Villard, F.; Jeannesson, P.; Bernard, J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2002, 130, 245–255. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal. Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Berndt, B.E.; Zhang, M.; Owyang, S.Y.; Cole, T.S.; Wang, T.W.; Luther, J.; Veniaminova, N.A.; Merchant, J.L.; Chen, C.C.; Huffnagle, G.B.; et al. Butyrate increases IL-23 production by stimulated dendritic cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1384–G1392. [Google Scholar] [CrossRef] [Green Version]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Enriquez, E.; Hallgren, J. Mast Cells and Their Progenitors in Allergic Asthma. Front. Immunol. 2019, 10, 821. [Google Scholar] [CrossRef] [Green Version]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van, I.W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcepsilonRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Rada-Iglesias, A.; Enroth, S.; Ameur, A.; Koch, C.M.; Clelland, G.K.; Respuela-Alonso, P.; Wilcox, S.; Dovey, O.M.; Ellis, P.D.; Langford, C.F.; et al. Butyrate mediates decrease of histone acetylation centered on transcription start sites and down-regulation of associated genes. Genome Res. 2007, 17, 708–719. [Google Scholar] [CrossRef] [Green Version]
- Krajewski, D.; Kaczenski, E.; Rovatti, J.; Polukort, S.; Thompson, C.; Dollard, C.; Ser-Dolansky, J.; Schneider, S.S.; Kinney, S.R.M.; Mathias, C.B. Epigenetic Regulation via Altered Histone Acetylation Results in Suppression of Mast Cell Function and Mast Cell-Mediated Food Allergic Responses. Front. Immunol. 2018, 9, 2414. [Google Scholar] [CrossRef] [PubMed]
- Richon, V.M.; Sandhoff, T.W.; Rifkind, R.A.; Marks, P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. USA 2000, 97, 10014–10019. [Google Scholar] [CrossRef] [Green Version]
- Thio, C.L.; Chi, P.Y.; Lai, A.C.; Chang, Y.J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 2018, 142, 1867–1883.e1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal. Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, R.S.; Castoldi, A.; Basso, P.J.; Hiyane, M.I.; Camara, N.O.S.; Almeida, R.R. Butyrate Attenuates Lung Inflammation by Negatively Modulating Th9 Cells. Front. Immunol. 2019, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Aronow, B.J.; Rochman, Y.; Rochman, M.; Kc, K.; Dexheimer, P.J.; Putnam, P.; Mukkada, V.; Foote, H.; Rehn, K.; et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J. Clin. Investig. 2019, 129, 2014–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051. [Google Scholar] [CrossRef] [Green Version]
- Toki, S.; Goleniewska, K.; Reiss, S.; Zhou, W.; Newcomb, D.C.; Bloodworth, M.H.; Stier, M.T.; Boyd, K.L.; Polosukhin, V.V.; Subramaniam, S.; et al. The histone deacetylase inhibitor trichostatin A suppresses murine innate allergic inflammation by blocking group 2 innate lymphoid cell (ILC2) activation. Thorax 2016, 71, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepahi, A.; Liu, Q.; Friesen, L.; Kim, C.H. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal. Immunol. 2021, 14, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Kasal, D.N.; Liang, Z.; Hollinger, M.K.; O’Leary, C.Y.; Lisicka, W.; Sperling, A.I.; Bendelac, A. A Gata3 enhancer necessary for ILC2 development and function. Proc. Natl. Acad. Sci. USA 2021, 118, e2106311118. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Faith, J.J.; Ahern, P.P.; Ridaura, V.K.; Cheng, J.; Gordon, J.I. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci. Transl. Med. 2014, 6, 220ra211. [Google Scholar] [CrossRef] [Green Version]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 2009, 27, 48–55. [Google Scholar] [CrossRef]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364, fnx075. [Google Scholar] [CrossRef] [PubMed]
- Nials, A.T.; Uddin, S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis. Model. Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.L.; Yildirim, A.O.; Sonar, S.S.; Kilic, A.; Sudowe, S.; Lunow, M.; Teich, R.; Renz, H.; Garn, H. Comparison of adjuvant and adjuvant-free murine experimental asthma models. Clin. Exp. Allergy 2009, 39, 1246–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheijden, K.A.; Akbari, P.; Willemsen, L.E.; Kraneveld, A.D.; Folkerts, G.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Inflammation-induced expression of the alarmin interleukin 33 can be suppressed by galacto-oligosaccharides. Int. Arch. Allergy Immunol. 2015, 167, 127–136. [Google Scholar] [CrossRef]
- Verheijden, K.A.; Willemsen, L.E.; Braber, S.; Leusink-Muis, T.; Delsing, D.J.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model. Respir. Res. 2015, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Verheijden, K.A.T.; Braber, S.; Leusink-Muis, T.; Jeurink, P.V.; Thijssen, S.; Kraneveld, A.D.; Garssen, J.; Folkerts, G.; Willemsen, L.E.M. The Combination Therapy of Dietary Galacto-Oligosaccharides With Budesonide Reduces Pulmonary Th2 Driving Mediators and Mast Cell Degranulation in a Murine Model of House Dust Mite Induced Asthma. Front. Immunol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheijden, K.A.; Willemsen, L.E.; Braber, S.; Leusink-Muis, T.; Jeurink, P.V.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V. Eur. J. Nutr. 2016, 55, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Verheijden, K.A.; Braber, S.; Leusink-Muis, T.; Thijssen, S.; Boon, L.; Kraneveld, A.D.; Garssen, J.; Folkerts, G.; Willemsen, L.E. Regulatory T Cell Depletion Abolishes the Protective Effect of Dietary Galacto-Oligosaccharides on Eosinophilic Airway Inflammation in House Dust Mite-Induced Asthma in Mice. J. Nutr. 2015, 146, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef] [Green Version]
- Bang, M.A.; Seo, J.H.; Seo, J.W.; Jo, G.H.; Jung, S.K.; Yu, R.; Park, D.H.; Park, S.J. Bacillus subtilis KCTC 11782BP-produced alginate oligosaccharide effectively suppresses asthma via T-helper cell type 2-related cytokines. PLoS ONE 2015, 10, e0117524. [Google Scholar] [CrossRef]
- Yasuda, A.; Inoue, K.I.; Sanbongi, C.; Yanagisawa, R.; Ichinose, T.; Yoshikawa, T.; Takano, H. Dietary supplementation with fructooligosaccharides attenuates airway inflammation related to house dust mite allergen in mice. Int. J. Immunopathol. Pharmacol. 2010, 23, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, A.P.; van Esch, B.C.; Stahl, B.; M’Rabet, L.; Folkerts, G.; Nijkamp, F.P.; Garssen, J. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int. Immunopharmacol. 2007, 7, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, K.; Watanabe, H.; Watanabe, J.; Yamaguchi, N.; Yamashita, A.; Hashimoto, H.; Kishino, E.; Fujita, K.; Okada, M.; Mori, S.; et al. Allergic airway eosinophilia is suppressed in ovalbumin-sensitized Brown Norway rats fed raffinose and alpha-linked galactooligosaccharide. J. Nutr. 2005, 135, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, S.; Vos, A.P.; Morgan, M.E.; Garssen, J.; Georgiou, N.A.; Boon, L.; Kraneveld, A.D.; Folkerts, G. The combination of Bifidobacterium breve with non-digestible oligosaccharides suppresses airway inflammation in a murine model for chronic asthma. Biochim. Biophys. Acta 2014, 1842, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Lew, D.B.; Michael, C.F.; Overbeck, T.; Robinson, W.S.; Rohman, E.L.; Lehman, J.M.; Patel, J.K.; Eiseman, B.; LeMessurier, K.S.; Samarasinghe, A.E.; et al. Beneficial Effects of Prebiotic Saccharomyces cerevisiae Mannan on Allergic Asthma Mouse Models. J. Immunol. Res. 2017, 2017, 3432701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef]
- Vaccaro, J.A.; Niego, J.; Huffman, F.G. Dietary factors, body weight, and screen time in U.S. children with and without asthma. Child. Health Care 2016, 45, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.A.; Gribben, K.C.; Alam, M.; Lyden, E.R.; Hanson, C.K.; LeVan, T.D. Association of Dietary Fiber on Asthma, Respiratory Symptoms, and Inflammation in the Adult National Health and Nutrition Examination Survey Population. Ann. Am. Thorac. Soc. 2020, 17, 1062–1068. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Son, S.; Kim, Y.C.; Kwak, J.W.; Kim, H.G.; Lee, S.H.; Kim, T.H. Association of Allergic Diseases and Related Conditions with Dietary Fiber Intake in Korean Adults. Int. J. Environ. Res. Public Health 2021, 18, 2889. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fibre intake and asthma (symptoms and control): Results from the French national e-cohort NutriNet-Sante. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Lagleva, M.; Shah, S.; Berthon, B.S.; Galbraith, S.; Henry, R.; Kepreotes, H.; Gibson, P.G. Dietary changes in migrant adolescents with increasing length of stay in Australia and associated risk of wheeze—A retrospective, cross sectional study. BMC Pediatr. 2015, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Gabryszewski, S.J.; Hill, D.A. One march, many paths: Insights into allergic march trajectories. Ann. Allergy Asthma Immunol. 2021, 127, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J. Biol. Regul. Homeost. Agents 2012, 26, 49–59. [Google Scholar]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [Green Version]
- Beigelman, A.; Bacharier, L.B. Early-life respiratory infections and asthma development: Role in disease pathogenesis and potential targets for disease prevention. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Ivakhnenko, O.S.; Nyankovskyy, S.L. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: Randomized study. Pediatr. Polska 2013, 88, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Garcia, C.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Morgano, G.P.; Zhang, Y.; Agarwal, A.; Gandhi, S.; Terracciano, L.; Schunemann, H.J.; et al. Prebiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef]
- Van der Aa, L.B.; Heymans, H.S.; van Aalderen, W.M.; Sillevis Smitt, J.H.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B.; Synbad Study, G. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef]
- Van der Aa, L.B.; van Aalderen, W.M.; Heymans, H.S.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.; Ben Amor, K.; Sprikkelman, A.B.; Synbad Study, G. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Candy, D.C.A.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance development in cow’s milk-allergic infants receiving amino acid-based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Wopereis, H.; van Ampting, M.T.J.; Cetinyurek-Yavuz, A.; Slump, R.; Candy, D.C.A.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; et al. A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow’s milk allergic infants: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- NederlandsTrialRegister. Identifier NTR3567, a Prospective Double Blind Randomised Controlled Study to Evaluate the Immunological Benefits and Clinical Effects of an Elimination Diet Using an Amino Acid Formula (AAF) with an Added Pre-probiotic Blend in Infants with Cow’s Milk Allergy (CMA). Available online: https://www.trialregister.nl/trial/3567 (accessed on 13 September 2021).
- van de Pol, M.A.; Lutter, R.; Smids, B.S.; Weersink, E.J.; van der Zee, J.S. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy 2011, 66, 39–47. [Google Scholar] [CrossRef]
- Williams, N.C.; Johnson, M.A.; Shaw, D.E.; Spendlove, I.; Vulevic, J.; Sharpe, G.R.; Hunter, K.A. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. Br. J. Nutr. 2016, 116, 798–804. [Google Scholar] [CrossRef] [Green Version]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.X.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Fransen, F.; Sahasrabudhe, N.M.; Elderman, M.; Bosveld, M.; El Aidy, S.; Hugenholtz, F.; Borghuis, T.; Kousemaker, B.; Winkel, S.; van der Gaast-de Jongh, C.; et al. beta2-->1-Fructans Modulate the Immune System In Vivo in a Microbiota-Dependent and -Independent Fashion. Front. Immunol. 2017, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, Z.; Molla, A.M.; Al-Habashi, H.; Muawad, W.M.; Molla, A.M.; Sharma, P.N. Intestinal permeability is increased in bronchial asthma. Arch. Dis. Child. 2004, 89, 227–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benard, A.; Desreumeaux, P.; Huglo, D.; Hoorelbeke, A.; Tonnel, A.B.; Wallaert, B. Increased intestinal permeability in bronchial asthma. J. Allergy Clin. Immunol. 1996, 97, 1173–1178. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verstegen, R.E.M.; Kostadinova, A.I.; Merenciana, Z.; Garssen, J.; Folkerts, G.; Hendriks, R.W.; Willemsen, L.E.M. Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management. Nutrients 2021, 13, 4153. https://doi.org/10.3390/nu13114153
Verstegen REM, Kostadinova AI, Merenciana Z, Garssen J, Folkerts G, Hendriks RW, Willemsen LEM. Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management. Nutrients. 2021; 13(11):4153. https://doi.org/10.3390/nu13114153
Chicago/Turabian StyleVerstegen, Roos E. M., Atanaska I. Kostadinova, Zenebech Merenciana, Johan Garssen, Gert Folkerts, Rudi W. Hendriks, and Linette E. M. Willemsen. 2021. "Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management" Nutrients 13, no. 11: 4153. https://doi.org/10.3390/nu13114153
APA StyleVerstegen, R. E. M., Kostadinova, A. I., Merenciana, Z., Garssen, J., Folkerts, G., Hendriks, R. W., & Willemsen, L. E. M. (2021). Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management. Nutrients, 13(11), 4153. https://doi.org/10.3390/nu13114153







