Dietary Supplementation with Biobran/MGN-3 Increases Innate Resistance and Reduces the Incidence of Influenza-like Illnesses in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial
Abstract
1. Background
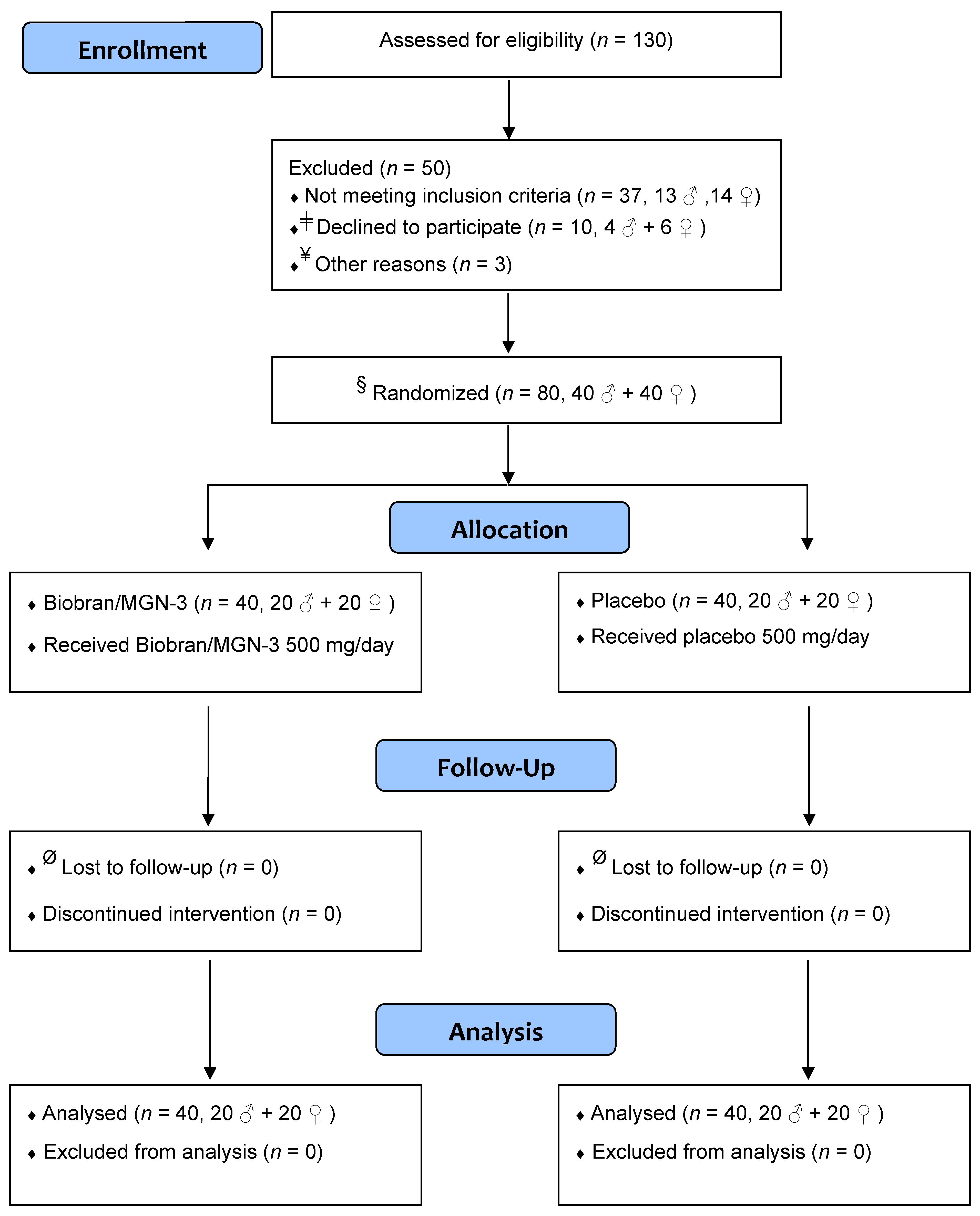
2. Subjects and Methods
2.1. Trial Design
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.4. Consent
2.5. Randomization and Allocation Concealment
2.6. Intervention
2.7. Ingredients of Biobran/MGN-3 Sachets
2.8. Blinding
2.9. Follow-Up
2.10. Outcome
2.11. Sample Size
2.12. Laboratory Investigations
2.13. Degranulation Assay for NK Cell Activity
2.14. Cell Culture and Flow Cytometry
2.15. Statistical Analysis
3. Results
3.1. Biobran/MGN-3 Did Not Adversely Affect the Hematological, Liver, and Kidney Functions
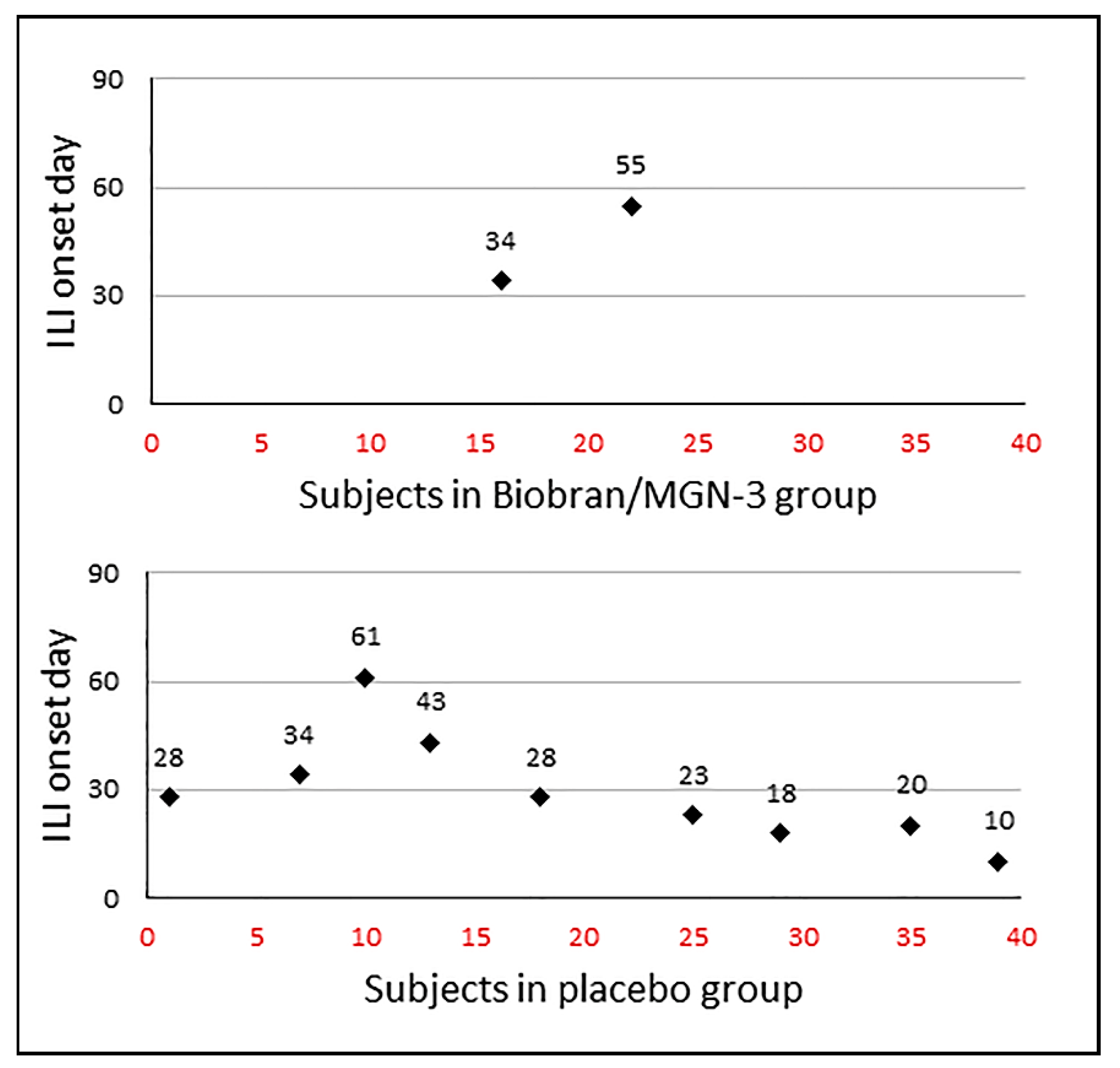
3.2. Biobran/MGN-3 Supplementation Significantly Reduced the ILI Incidence Rate and Density
3.3. Biobran/MGN-3 Specifically Enhanced NK Cell Activity but Not NKT Activity
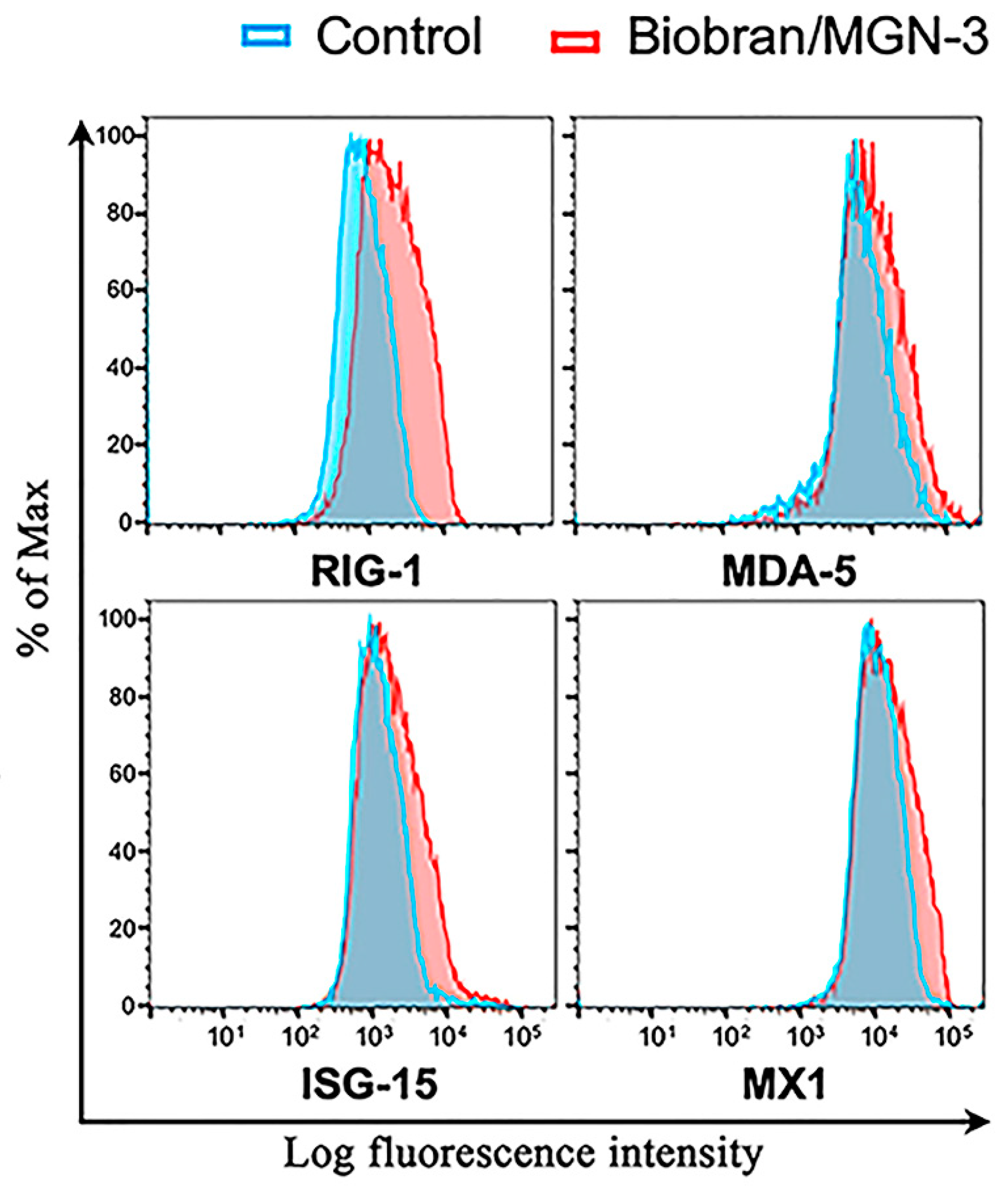
3.4. Biobran/MGN-3 Upregulates the Expression of RIG-1, MDA5, ISG15, and MX1 in Pulmonary Epithelial BEAS-2B Cells
4. Discussion
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stohr, K. Influenza—WHO cares. Lancet Infect. Dis. 2002, 2, 517. [Google Scholar] [CrossRef]
- Lee, N.; Chan, P.K.; Lui, G.C.; Wong, B.C.; Sin, W.W.; Choi, K.W.; Wong, R.Y.; Lee, E.L.; Yeung, A.C.; Ngai, K.L.; et al. Complications and outcomes of pandemic 2009 Influenza A (H1N1) virus infection in hospitalized adults: How do they differ from those in seasonal influenza? J. Infect. Dis. 2011, 203, 1739–1747. [Google Scholar] [CrossRef]
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Health Organ. 2018, 96, 122–128. [Google Scholar] [CrossRef]
- Kammerer, P.E.; Montiel, S.; Kriner, P.; Bojorquez, I.; Ramirez, V.B.; Vazquez-Erlbeck, M. Influenza-like illness surveillance on the California–Mexico border, 2004–2009. Influenza Other Respir. Viruses 2012, 6, 358–366. [Google Scholar] [CrossRef]
- Bollaerts, K.; Antoine, J.; Van Casteren, V.; Ducoffre, G.; Hens, N.; Quoilin, S. Contribution of respiratory pathogens to influenza-like illness consultations. Epidemiol. Infect. 2013, 141, 2196–2204. [Google Scholar] [CrossRef][Green Version]
- Fu, Y.; Pan, L.; Sun, Q.; Zhu, W.; Zhu, L.; Ye, C.; Xue, C.; Wang, Y.; Liu, Q.; Ma, P.; et al. The clinical and etiological characteristics of influenza-like illness (ILI) in outpatients in Shanghai, China, 2011 to 2013. PLoS ONE 2015, 10, e0119513. [Google Scholar] [CrossRef] [PubMed]
- Souty, C.; Masse, S.; Valette, M.; Behillil, S.; Bonmarin, I.; Pino, C. Baseline characteristics and clinical symptoms related to respiratory viruses identified among patients presenting with influenza-like illness in primary care. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 1147–1153. [Google Scholar] [CrossRef]
- Refaey, S.; Amin, M.; Labib, M.; Kandeel, A. Influenza virus positivity and circulating subtypes among cases of influenza-like illness and severe acute respiratory infection, Egypt, 2012–2015. East Mediterr. Health J. 2016, 22, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Pop-Vicas, A.; Gravenstein, S. Influenza in the elderly: A mini-review. Gerontology 2011, 57, 397–404. [Google Scholar] [CrossRef]
- Talbot, H.K. Influenza in older adults. Infect. Dis. Clin. N. Am. 2017, 31, 757–766. [Google Scholar] [CrossRef]
- Takahasi, K.; Yoneyama, M.; Nishihori, T.; Hirai, R.; Kumeta, H.; Narita, R.; Gale, M., Jr.; Inagaki, F.; Fujita, T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell. 2008, 29, 428–440. [Google Scholar] [CrossRef]
- Baum, A.; Sachidanandam, R.; Garcia-Sastre, A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl. Acad. Sci. USA 2010, 108, 3092. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Barral, P.M.; Sarkar, D.; Su, Z.Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: Key regulators of innate immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Lindenmann, J.; Lane, C.A.; Hobson, D. The resistance of A2G mice to myxoviruses. J. Immunol. 1963, 90, 942–951. [Google Scholar]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 1371–1376. [Google Scholar] [CrossRef]
- Lai, C.; Struckhoff, J.J.; Schneider, J.; Martinez-Sobrido, L.; Wolff, T.; Garcia-Sastre, A.; Zhang, D.E.; Lenschow, D.J. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J. Virol. 2009, 83, 1147–1151. [Google Scholar] [CrossRef]
- Orange, J.S. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002, 4, 1545–1558. [Google Scholar] [CrossRef]
- Lodoen, M.B.; Lanier, L.L. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006, 18, 391–398. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Terunuma, H.; Deng, X.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef]
- Cooley, S.; Burns, L.J.; Repka, T.; Miller, J.S. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp. Hematol. 1999, 27, 1533–1541. [Google Scholar] [CrossRef]
- Hammer, Q.; Romagnani, C. About training and memory: NK-cell adaptation to viral infections. Adv. Immunol. 2017, 133, 171–207. [Google Scholar]
- Peng, H.; Tian, Z. NK cells in liver homeostasis and viral hepatitis. Sci. China Life Sci. 2018, 61, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, J.; Oriolo, F.; Zaghi, E.; Di Vito, C.; Mavilio, D. Natural killer cells in HIV-1 infection and therapy. AIDS 2017, 31, 2317–2330. [Google Scholar] [CrossRef]
- Flórez-Álvarez, L.; Hernandez, J.C.; Zapata, W. NK cells in HIV-1 infection: From basic science to vaccine strategies. Front. Immunol. 2018, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.K.; Kim, T.S.; Hufford, M.M.; Braciale, T.J. Viral infection of the lung: Host response and sequelae. J. Allergy Clin. Immunol. 2013, 132, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Miller, J.S. Biology of Natural Killer Cells in Cancer and Infection. Cancer Investig. 2002, 20, 405–419. [Google Scholar] [CrossRef]
- Ghoneum, M.; Suzuki, K.; Gollapudi, S. Phorbol myristate acetate corrects impaired NK function of old mice. Scand. J. Immunol. 1991, 34, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Sgobbi, S.; Meneghetti, A.; Tadolini, M.; Tarozzi, A.; Sinoppi, M.; Cattini, L.; Facchini, A. Perforins in human cytolytic cells: The effect of age. Mech. Ageing Dev. 1996, 92, 195–209. [Google Scholar] [CrossRef]
- Rukavina, D.; Laskarin, G.; Rubesa, G.; Strbo, N.; Bedenicki, I.; Manestar, D.; Glavas, M.; Christmas, S.E.; Podack, E.R. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood 1998, 92, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M. Enhancement of human natural killer cell activity by modified arabinoxylan from rice bran (MGN-3). Int. J. Immunother. 1998, XIV, 89–99. [Google Scholar]
- Ghoneum, M.; Jewett, A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect. Prev. 2000, 24, 314–324. [Google Scholar]
- Cholujova, D.; Jakubikova, J.; Czako, B.; Martisova, M.; Hunakova, L.; Duraj, J.; Mistrik, M.; Sedlak, J. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol. Immunother. 2013, 62, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2015, 17, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Abedi, S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J. Pharm. Pharmacol. 2004, 56, 1581–1588. [Google Scholar] [CrossRef]
- Elsaid, A.F.; Shaheen, M.; Ghoneum, M. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: A randomized, double blind, placebo-controlled clinical trial. Exp. Ther. Med. 2018, 15, 2313–2320. [Google Scholar] [CrossRef]
- Ghoneum, M.; Matsuura, M.; Gollapudi, S. Modified arabinoxylan rice bran (MGN3/Biobran) enhances intracellular killing of microbes by human phagocytic cells in vitro. Int. J. Immunopathol. Pharmacol. 2008, 21, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Agrawal, S. Activation of human monocyte-derived dendritic cells in vitro by biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int. J. Immunopathol. Pharmcol. 2011, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Agrawal, S. MGN-3/Biobran enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on dendritic cells. Int. J. Immunopathol. Pharmacol. 2014, 27, 523–530. [Google Scholar] [CrossRef]
- Ghoneum, M. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem. Biophys. Res. Commun. 1998, 243, 25–29. [Google Scholar] [CrossRef]
- Tazawa, K.; Ichihashi, K.; Fujii, T.; Omura, K.; Anazawa, M.; Maeda, H. The orally administration of the Hydrolysis Rice Bran prevents a common cold syndrome for the elderly people based on immunomodulatory function. J. Trad. Med. 2003, 20, 132–141. [Google Scholar]
- Salama, H.; Medhat, E.; Shaheen, M.; Zekri, A.N.; Darwish, T.; Ghoneum, M. Arabinoxylan rice bran (Biobran) suppresses the viremia level in patients with chronic HCV infection: A randomized trial. Int. J. Immunopathol. Pharmacol. 2016, 29, 647–653. [Google Scholar] [CrossRef]
- Elsaid, A.F.; Fahmi, R.M.; Shaheen, M.; Ghoneum, M. The enhancing effects of Biobran/MGN-3, an arabinoxylan rice bran, on healthy old adults’ health-related quality of life: A randomized, double-blind, placebo-controlled clinical trial. Qual. Life Res. 2020, 29, 357–367. [Google Scholar] [CrossRef]
- Ghoneum, M.; Brown, J. NK immunorestoration of cancer patients by MGN-3, a modified arabinoxylan rice bran (study of 32 patients followed for up to 4 years). Anti-Aging Med. Ther. 1999, 3, 217–226. [Google Scholar]
- Takahara, K.; Sano, K. The life prolongation and QOL improvement effect of rice bran arabinoxylan derivative (MGN-3. Bio-Bran) for progressive cancer. Clin. Pharmacol. Ther. 2004, 14, 267–271. [Google Scholar]
- World Health Organization. Proposed Working Definition of an Older Person in Africa for the MDS Project. Available online: http://www.who.int/healthinfo/survey/ageingdefnolder/en/index.html (accessed on 6 May 2020).
- Centers for Disease Control and Prevention. Principles of Epidemiology in Public Health Practice, Third Edition. An Introduction to Applied Epidemiology and Biostatistics. Lesson 3: Measures of Risk; Section 2: Morbidity Frequency Measures. Available online: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section2.html (accessed on 3 September 2020).
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3.01. Available online: www.OpenEpi.com (accessed on 29 January 2014).
- Corp IBM. IBM SPSS Statistics for Windows, Version 20; IBM Corp.: Armonk, NY, USA, 2010. [Google Scholar]
- Tazawa, K. BioBran/MGN-3 (Rice Bran Arabinoxylan Deritive): Basic and Clinical Application to Integrative Medicine; Iyakushuppan Co., Publishers: Tokyo, Japan, 2006; pp. 18–22. [Google Scholar]
- Daiwa Pharmaceutical Co., Ltd. BioBran. Available online: https://www.daiwa-pharm.com/english/product/biobran.html (accessed on 10 November 2020).
- Laue, C.; Stevens, Y.; van Erp, M.; Papazova, E.; Soeth, E.; Pannenbeckers, A.; Stolte, E.; Böhm, R.; Gall, S.L.; Falourd, X.; et al. Adjuvant Effect of Orally Applied Preparations Containing Non-Digestible Polysaccharides on Influenza Vaccination in Healthy Seniors: A Double-Blind, Randomised, Controlled Pilot Trial. Nutrients 2021, 13, 2683. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Hsu, K.C. Natural killer cell education in human health and disease. Curr. Opin. Immunol. 2018, 50, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Mao, H.; Yu, M.; Yang, F.; Feng, T.; Fan, Y.; Lu, Q.; Shen, C.; Yin, Z.; et al. Impaired NK cell antiviral cytokine response against influenza virus in small-for-gestational-age neonates. Cell. Mol. Immunol. 2013, 10, 437–443. [Google Scholar] [CrossRef]
- Sun, C.; Sun, H.Y.; Xiao, W.H.; Zhang, C.; Tian, Z.G. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol. Sin. 2015, 36, 1191–1199. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, L.; Barchet, W.; Gilfillan, S.; Cella, M.; Beutler, B.; Flavell, R.A.; Diamond, M.S.; Colonna, M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 2006, 103, 8459–8464. [Google Scholar] [CrossRef]
- van Kasteren, P.B.; Beugeling, C.; Ninaber, D.K.; Frias-Staheli, N.; van Boheemen, S.; Garcia-Sastre, A.; Snijder, E.J.; Kikkert, M. Arterivirus and nairovirus ovarian tumor domaincontaining deubiquitinases target activated RIG-I to control innate immune signaling. J. Virol. 2012, 86, 773–785. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Dunker, W.; Song, Y.; Karijolich, J. RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection. Nat. Commun. 2018, 9, 4841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Filzmayer, C.; Ni, Y.; Sültmann, H.; Mutz, P.; Hiet, M.-S.; Vondran, F.W.; Bartenschlager, R.; Urban, S. Hepatitis D virus replication is sensed by MDA5 and induces IFN-b/l responses in hepatocytes. J. Hepatol. 2018, 69, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.J.; Lenschow, D.J. The antiviral activities of ISG15. J. Mol. Biol. 2013, 425, 4995–5008. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.G.; Chauveau, L.; Hertzog, J.; Bridgeman, A.; Fowler, G.; Moonen, J.P.; Dupont, M.; Russell, R.A.; Noerenberg, M.; Rehwinkel, J. The RNA sensor MDA5 detects SARS-CoV-2 infection. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Parameter | Baseline | Post-Treatment | p-Value § |
|---|---|---|---|---|
| Placebo ǂ | RBCs (×106/µL) | 4.3 (0.5) | 4.1 (0.5) | 0.58 |
| HB (g/dL) | 11.3 (1.5) | 13.8 (1.4) | 0.40 | |
| HCT % | 35.7 (4.0) | 36.7 (3.5) | 0.51 | |
| MCHC (g/dL) | 27.3 (2.7) | 37.6 (0.7) | 0.80 | |
| WBC (×103/µL) | 5.7 (0.9) | 6.1 (1.2) | 0.76 | |
| Neutrophils % | 56.1 (14.5) | 57.3 (11.6) | 0.32 | |
| Lymphocytes % | 33.3 (10.2) | 39.5 (9.8) | 0.23 | |
| Platelets (×103/µL) | 218.8 (48.4) | 231.5 (48.0) | 0.19 | |
| ALP (U/L) | 185.5 (51.3) | 183.2 (50.1) | 0.66 | |
| ALT (U/L) | 22.0 (9.3) | 20.3 (8.4) | 0.15 | |
| AST (U/L) | 17.7 (4.6) | 18.3 (5.8) | 0.07 | |
| UA (mg/dL) | 7.4 (2.9) | 8.2 (3.8) | 0.51 | |
| Biobran/MGN-3 ǂ (500 mg/day) for 3 months | RBCs (×106/µL) | 4.8 (0.5) | 4.7 (0.6) | 0.18 |
| HB (g/dL) | 12.1 (1.7) | 13.9 (1.6) | 0.93 | |
| HCT % | 37.4 (4.4) | 36.9 (3.1) | 0.6 | |
| MCHC (g/dL) | 32.3 (0.9) | 37.4 (1.5) | 0.46 | |
| WBC (×103/µL) | 6.8 (1.8) | 6.7 (1.4) | 0.68 | |
| Neutrophils % | 57.3 (14.3) | 60.7 (7.3) | 0.37 | |
| Lymphocytes % | 32.5 (7.9) | 38.8 (8.5) | 0.24 | |
| Platelets (×103/µL) | 187.3 (37.1) | 208.8 (16.9) | 0.12 | |
| ALP (U/L) | 213.0 (43.0) | 195.2 (35.1) | 0.58 | |
| ALT (U/L) | 22.0 (7.2) | 19.3 (6.6) | 0.38 | |
| AST (U/L) | 23.8 (11.0) | 20.2 (8.8) | 0.26 | |
| UA (mg/dL) | 5.9 (2.5) | 6.7 (1.8) | 0.33 |
| Treatment | Parameter | Baseline | Post-Treatment | p-Value § |
|---|---|---|---|---|
| Placebo | NK | 5.3 (1.9) ǂ | 6.0 (1.7) ǂ | 0.62 |
| NKT | 4.5 (1.6) ǂ | 5.6 (2.4) ǂ | 0.44 | |
| NK CD-107 a | 45.3 (12.0) ǂ | 50.8 (19.5) ¥ | 0.38 | |
| NKT CD-107 a | 67.9 (15.6) ǂ | 75.5 (22.3) ǂ | 0.23 | |
| Biobran/MGN-3 (500 mg/day) for 3 months | NK | 6.1 (2.6) ǂ | 6.7 (1.7) ǂ | 0.60 |
| NKT | 3.1 (0.6) ǂ | 4.6 (2.5) ǂ | 0.25 | |
| NK CD-107 a | 49.5 (10.4) ǂ | 75.2 (6.6) ¥ | 0.004 * | |
| NKT CD-107 a | 70.6 (10.1) ǂ | 76.9 (9.8) ǂ | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaid, A.F.; Agrawal, S.; Agrawal, A.; Ghoneum, M. Dietary Supplementation with Biobran/MGN-3 Increases Innate Resistance and Reduces the Incidence of Influenza-like Illnesses in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial. Nutrients 2021, 13, 4133. https://doi.org/10.3390/nu13114133
Elsaid AF, Agrawal S, Agrawal A, Ghoneum M. Dietary Supplementation with Biobran/MGN-3 Increases Innate Resistance and Reduces the Incidence of Influenza-like Illnesses in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial. Nutrients. 2021; 13(11):4133. https://doi.org/10.3390/nu13114133
Chicago/Turabian StyleElsaid, Ahmed F., Sudhanshu Agrawal, Anshu Agrawal, and Mamdooh Ghoneum. 2021. "Dietary Supplementation with Biobran/MGN-3 Increases Innate Resistance and Reduces the Incidence of Influenza-like Illnesses in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial" Nutrients 13, no. 11: 4133. https://doi.org/10.3390/nu13114133
APA StyleElsaid, A. F., Agrawal, S., Agrawal, A., & Ghoneum, M. (2021). Dietary Supplementation with Biobran/MGN-3 Increases Innate Resistance and Reduces the Incidence of Influenza-like Illnesses in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial. Nutrients, 13(11), 4133. https://doi.org/10.3390/nu13114133







