Clinical Presentation of Celiac Disease and Diagnosis Accuracy in a Single-Center European Pediatric Cohort over 10 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographics and Clinical Data
2.2. Serological Tests and EGD Biopsy
2.3. CD Diagnosis
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Clinical Presentation
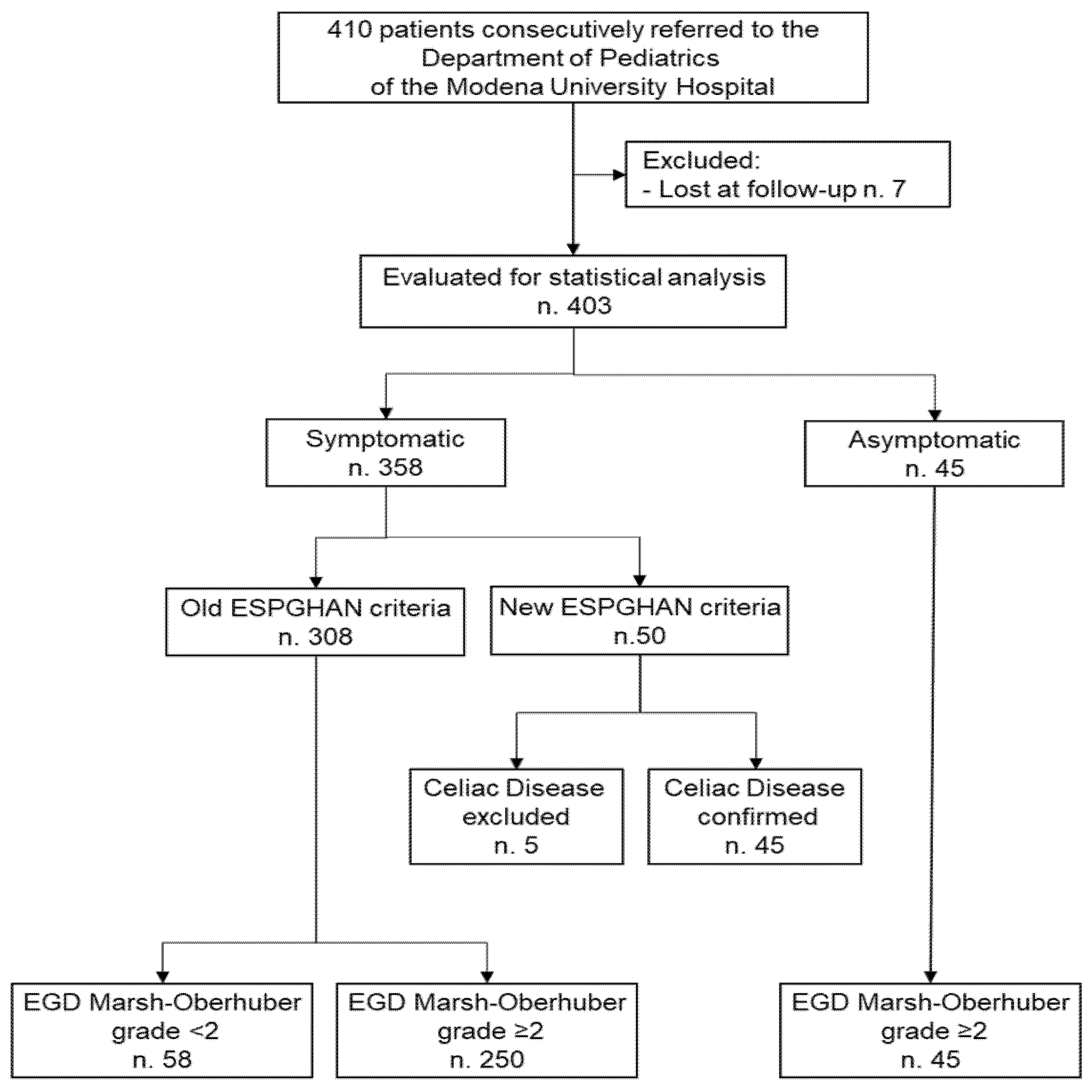
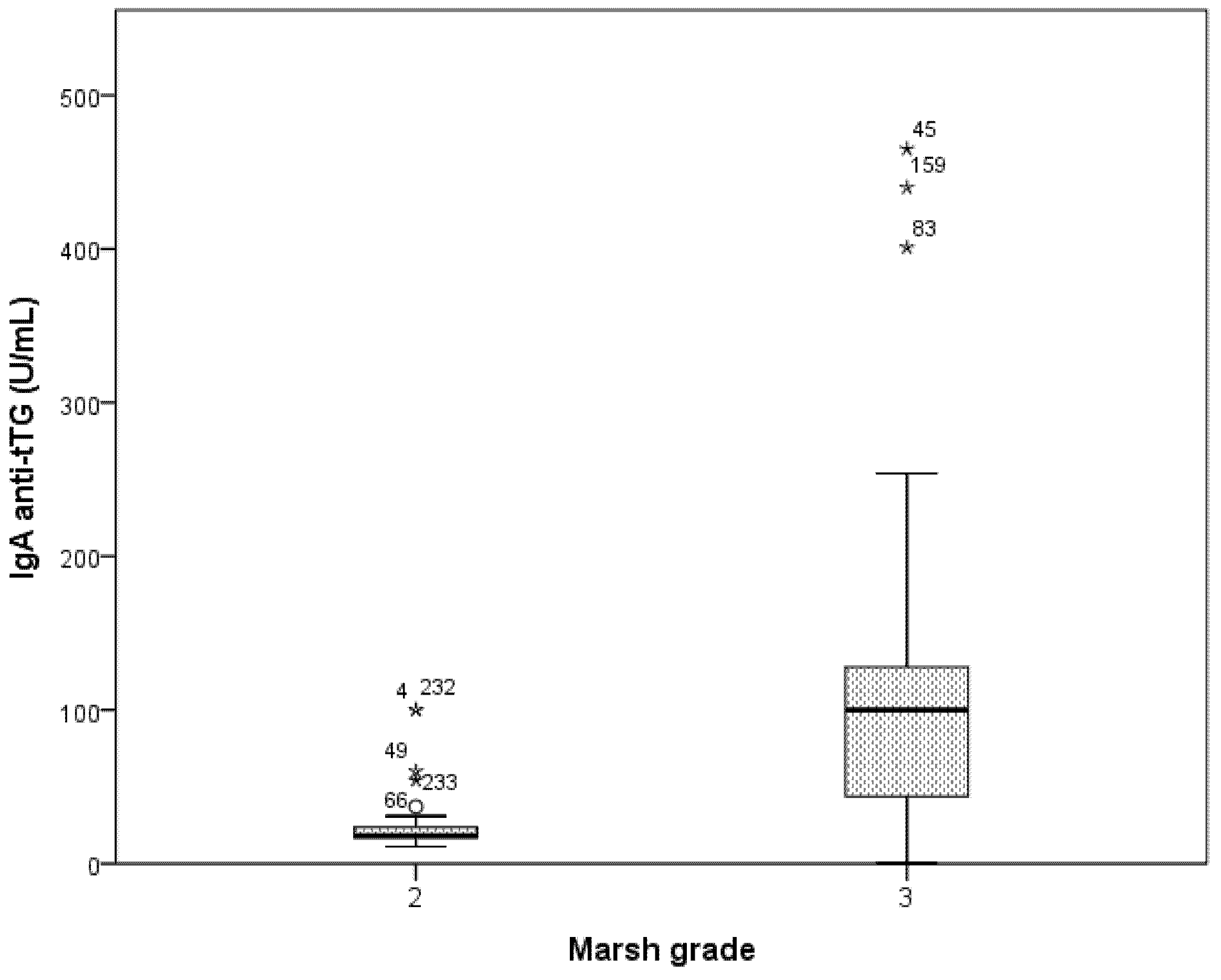
3.3. Accuracy of ESPGHAN Criteria in Symptomatic Patients
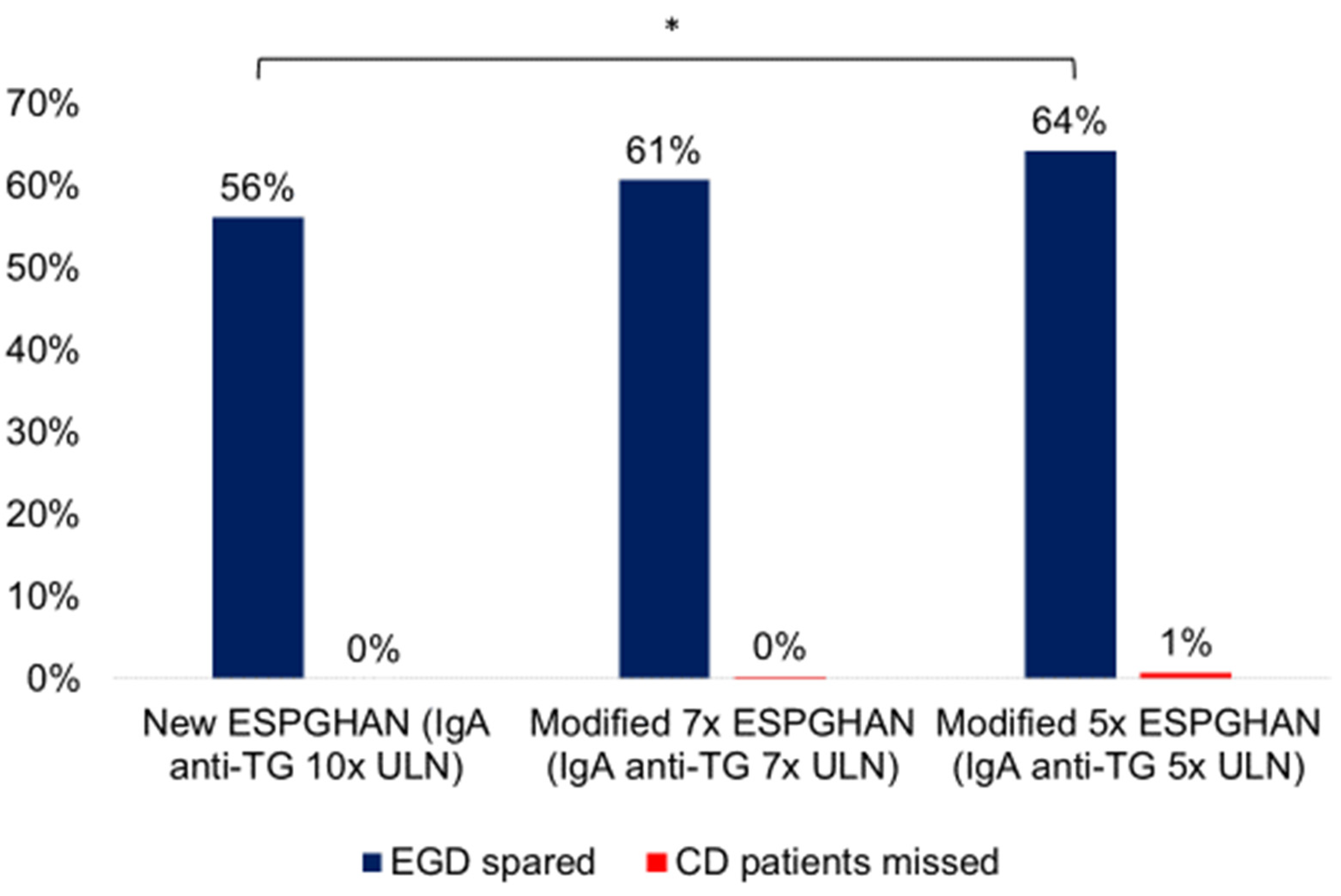
3.4. Accuracy of Modified ESPGHAN Criteria in Symptomatic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- McGowan, K.E.; Castiglione, D.A.; Butzner, J.D. The changing face of childhood celiac disease in North America: Impact of serological testing. Pediatrics 2009, 124, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- White, L.E.; Merrick, V.M.; Bannerman, E.; Russell, R.K.; Basude, D.; Henderson, P.; Wilson, D.C.; Gillett, P.M. The rising incidence of celiac disease in Scotland. Pediatrics 2013, 132, e924–e931. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Rubio-Tapia, A.; van Dyke, C.T.; Melton, L.J.; Zinsmeister, A.R.; Lahr, B.D.; Murray, J.A. Increasing incidence of celiac disease in a North American population. Am. J. Gastroenterol. 2013, 108, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Gatti, S.; Fasano, A. The New epidemiology of celiac disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, S7–S9. [Google Scholar] [CrossRef]
- Mäki, M.; Mustalahti, K.; Kokkonen, J.; Kulmala, P.; Haapalahti, M.; Karttunen, T.; Ilonen, J.; Laurila, K.; Dahlbom, I.; Hansson, T.; et al. Prevalence of celiac disease among children in Finland. N. Engl. J. Med. 2003, 348, 2517–2524. [Google Scholar] [CrossRef]
- Myléus, A.; Ivarsson, A.; Webb, C.; Danielsson, L.; Hernell, O.; Högberg, L.; Karlsson, E.; Lagerqvist, C.; Norström, F.; Rosén, A.; et al. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 170–176. [Google Scholar] [CrossRef]
- Maki, M.; Kallonen, K.; Lahdeaho, M.L.; Visakorpi, J.K. Changing pattern of childhood coeliac disease in Finland. Acta Paediatr. Scand. 1988, 77, 408–412. [Google Scholar] [CrossRef]
- Ivarsson, A.; Myléus, A.; Norström, F.; van der Pals, M.; Rosén, A.; Högberg, L.; Danielsson, L.; Halvarsson, B.; Hammarroth, S.; Hernell, O.; et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013, 131, e687–e694. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, A.; Mustalahti, K.; Kaukinen, K.; Viskari, H.; Volodicheva, V.; Haapala, A.; Ilonen, J.; Knip, M.; Mäki, M.; Hyöty, H.; et al. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann. Med. 2008, 40, 223–231. [Google Scholar] [CrossRef]
- Ravikumara, M.; Tuthill, D.P.; Jenkins, H.R. The changing clinical presentation of coeliac disease. Arch. Dis. Child. 2006, 91, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Garampazzi, A.; Rapa, A.; Mura, S.; Capelli, A.; Valori, A.; Boldorini, R.; Oderda, G. Clinical pattern of celiac disease is still changing. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.A.; Jenkins, H.R. The epidemiology of coeliac disease in South Wales: A 28-year perspective. Arch. Dis. Child. 2013, 98, 405–407. [Google Scholar] [CrossRef]
- Walker-Smith, J.A.G.S. Revised criteria for diagnosis of coeliac disease. Report of working group of European society of paediatric gastroenterology and nutrition. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar] [CrossRef]
- Van Kalleveen, M.W.; de Meij, T.; Plötz, F.B. Clinical spectrum of paediatric coeliac disease: A 10-year single-centre experience. Eur. J. Pediatr. 2018, 177, 593–602. [Google Scholar] [CrossRef]
- Roma, E.; Panayiotou, J.; Karantana, H.; Constantinidou, C.; Siakavellas, S.I.; Krini, M.; Syriopoulou, V.P.; Bamias, G. Changing pattern in the clinical presentation of pediatric celiac disease: A 30-year study. Digestion 2009, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Vanderhoof, J.A. Chronic diarrhea. Pediatr. Rev. 1998, 19, 418–422. [Google Scholar] [CrossRef]
- Pedicelli, S.; Peschiaroli, E.; Violi, E.; Cianfarani, S. Controversies in the definition and treatment of idiopathic short stature (ISS). J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 105–115. [Google Scholar] [CrossRef]
- Baker, R.D.; Greer, F.R.; Bhatia, J.J.S.; Abrams, S.A.; Daniels, S.R.; Schneider, M.B.; Silverstein, J.; Stettler, N.; Thomas, D.W.; Grummer-Strawn, L.; et al. Clinical report—Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Plunkett, A.; Beattie, R.M. Recurrent abdominal pain in childhood. J. R. Soc. Med. 2005, 98, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.; Dale, A. Chronic constipation in children. Br. Med. J. 2006, 333, 1051–1055. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Ivarsson, A.; Persson, L.Å.; Nyström, L.; Hernell, O. The Swedish coeliac disease epidemic with a prevailing twofold higher risk in girls compared to boys may reflect gender specific risk factors. Eur. J. Epidemiol. 2003, 18, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C.; Reingold, S.C.; O’Looney, P.A.; Blankenhorn, E.; Brinley, F.; Collier, E.; Duquette, P.; Fox, H.; Giesser, B.; Gilmore, W.; et al. Biomedicine: A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef]
- Tapsas, D.; Hollén, E.; Stenhammar, L.; Fälth-Magnusson, K. The clinical presentation of coeliac disease in 1030 Swedish children: Changing features over the past four decades. Dig. Liver Dis. 2016, 48, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cilleruelo, M.L.; Roman-Riechmann, E.; Sanchez-Valverde, F.; Donat, E.; Manuel-Ramos, J.; Martín-Orte, E.; López, M.J.; García-Novo, D.; García, S.; Pavón, P.; et al. Spanish national registry of celiac disease: Incidence and clinical presentation. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 522–526. [Google Scholar] [CrossRef]
- Marasco, G.; Di Biase, A.R.; Schiumerini, R.; Eusebi, L.H.; Iughetti, L.; Ravaioli, F.; Scaioli, E.; Colecchia, A.; Festi, D. Gut microbiota and celiac disease. Dig. Dis. Sci. 2016, 61, 1461–1472. [Google Scholar] [CrossRef]
- Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; De Giorgio, R.; Festi, D.; Caio, G. Probiotics, prebiotics and other dietary supplements for gut microbiota modulation in celiac disease patients. Nutrients 2020, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, A.R.; Marasco, G.; Ravaioli, F.; Dajti, E.; Colecchia, L.; Righi, B.; D’Amico, V.; Festi, D.; Iughetti, L.; Colecchia, A. Gut microbiota signatures and clinical manifestations in celiac disease children at onset: A pilot study. J. Gastroenterol. Hepatol. 2021, 36, 446–454. [Google Scholar] [CrossRef]
- Marasco, G.; Colecchia, A.; Festi, D. Dysbiosis in Celiac disease patients with persistent symptoms on gluten-free diet: A condition similar to that present in irritable bowel syndrome patients? Am. J. Gastroenterol. 2015, 110, 598. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Baker, R.D.; Ly, E.K.; Kozielski, R.; Baker, S.S. Presenting pattern of pediatric celiac disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Almallouhi, E.; King, K.S.; Patel, B.; Wi, C.; Juhn, Y.J.; Murray, J.A.; Absah, I. Increasing incidence and altered presentation in a population-based study of pediatric celiac disease in North America. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lurz, E.; Scheidegger, U.; Spalinger, J.; Schöni, M.; Schibli, S. Clinical presentation of celiac disease and the diagnostic accuracy of serologic markers in children. Eur. J. Pediatr. 2009, 168, 839–845. [Google Scholar] [CrossRef]
- Kivelä, L.; Kaukinen, K.; Lähdeaho, M.L.; Huhtala, H.; Ashorn, M.; Ruuska, T.; Hiltunen, P.; Visakorpi, J.; Mäki, M.; Kurppa, K. Presentation of celiac disease in Finnish children is no longer changing: A 50-year perspective portions of this study were presented orally at the 47th annual meeting of the European society for pediatric gastroenterology, hepatology and nutrition (ESPGHAN), June 9–12, 2014, Jerusalem, Israel. J. Pediatr. 2015, 167, 1109–1115. [Google Scholar] [CrossRef]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R. Accuracy of diagnostic antibody tests for coeliac disease in children: Summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef]
- Smarrazzo, A.; Misak, Z.; Costa, S.; Mičetić-Turk, D.; Abu-Zekry, M.; Kansu, A.; Abkari, A.; Bouziane-Nedjadi, K.; Ben Hariz, M.; Roma, E.; et al. Diagnosis of celiac disease and applicability of ESPGHAN guidelines in Mediterranean countries: A real life prospective study. BMC Gastroenterol. 2017, 17, 17. [Google Scholar] [CrossRef][Green Version]
- Bishop, J.; Reed, P.; Austin, P.; Hurst, M.; Ameratunga, R.; Craigie, A.; McFarlane, J.; Chin, S.E.; Mouat, S.M.; Evans, H.M. Prospective evaluation of the ESPGHAN guidelines for diagnosis of celiac disease in New Zealand children. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 749–754. [Google Scholar] [CrossRef]
- Riznik, P.; Balogh, M.; Bódi, P.; De Leo, L.; Dolinsek, J.; Guthy, I.; Gyimesi, J.; Horváth, Á.; Kis, I.; Klemenak, M.; et al. The use of biopsy and “no-biopsy” approach for diagnosing paediatric coeliac disease in the central European region. Gastroenterol. Res. Pract. 2019, 2019, 9370397. [Google Scholar] [CrossRef]
- Tucci, F.; Astarita, L.; Abkari, A.; Abu-Zekry, M.; Attard, T.; Ben Hariz, M.; Bilbao, J.R.; Boudraa, G.; Boukthir, S.; Costa, S.; et al. Celiac disease in the Mediterranean area. BMC Gastroenterol. 2014, 14, 24. [Google Scholar] [CrossRef]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef]
- Losurdo, G.; Di Leo, M.; Santamato, E.; Giangaspero, A.; Rendina, M.; Luigiano, C.; Ierardi, E.; Di Leo, A. May antitransglutaminase levels predict severity of duodenal lesions in adults with celiac disease? Medicina 2021, 57, 1212. [Google Scholar] [CrossRef]
- Penny, H.A.; Raju, S.A.; Lau, M.S.; Marks, L.J.S.; Baggus, E.M.R.; Bai, J.C.; Bassotti, G.; Bontkes, H.J.; Carroccio, A.; Danciu, M.; et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021, 70, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Gidrewicz, D.; Potter, K.; Trevenen, C.L.; Lyon, M.; Butzner, J.D. Evaluation of the ESPGHAN celiac guidelines in a North American pediatric population. Am. J. Gastroenterol. 2015, 110, 760–767. [Google Scholar] [CrossRef]
- Reilly, N.R.; Husby, S.; Sanders, D.S.; Green, P.H.R. Coeliac disease: To biopsy or not? Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 60–66. [Google Scholar] [CrossRef]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The diagnostic accuracy of serologic tests for celiac disease: A systematic review. Gastroenterology 2005, 128, S38–S46. [Google Scholar] [CrossRef] [PubMed]
| Patients n = 340, n (%) or Mean (SD) | |
|---|---|
| Sex (Female) (%) | 223 (65.6) |
| Age at diagnosis (Months) | 76.6 (44.3) |
| Body mass index (BMI) percentile at diagnosis | 38.1 (30.4) |
| Human Leukocyte Antigen (HLA) | |
| DQ2 | 291 (85.6) |
| DQ8 | 20 (5.9) |
| DQ2/DQ8 | 29 (8.5) |
| IgA Anti-tTG antibodies (U/mL) | 110.0 (118.8) |
| IgA deficit | 6 (1.7) |
| EMA+ | 304 (86.1) |
| Esophagogastroduodenoscopy performed | 295 (86.8) |
| Marsh Classification at biopsy | |
| 2 | 51 (15) |
| 3 | 244 (71.8) |
| Biopsy not performed | 45 (13.2) |
| Family history of celiac disease | 93 (27.4) |
| Total n (%) n. 295 | 2004–2008 n (%) n. 98 | 2009–2014 n (%) n. 197 | p | |
|---|---|---|---|---|
| Gastrointestinal symptoms | ||||
| Diarrhea | 24 (8.1) | 12 (12.2) | 12 (6.1) | 0.071 |
| Constipation | 18 (6.1) | 3 (3.1) | 15 (7.7) | 0.122 |
| Abdominal pain | 105 (35.6) | 29 (29.6) | 76 (38.8) | 0.121 |
| Changes in bowel habits | 10 (3.4) | 3 (3.1) | 7 (3.6) | 0.820 |
| Bloating | 83 (28.1) | 32 (32.7) | 51 (26) | 0.234 |
| Nausea/Vomit | 30 (10.2) | 10 (10.2) | 20 (10.2) | 1 |
| Lack of appetite | 32 (10.8) | 7 (7.1) | 25 (12.8) | 0.145 |
| Gastro-esophageal reflux | 4 (1.4) | 2 (2) | 2 (1) | 0.476 |
| Extra-intestinal symptoms | ||||
| Hyposomia | 48 (16.3) | 21 (21.4) | 27 (13.8) | 0.094 |
| Anemia | 55 (18.6) | 18 (18.4) | 37 (18.9) | 0.916 |
| Slow growth | 54 (18.3) | 22 (22.5) | 32 (16.3) | 0.201 |
| Headache | 20 (6.8) | 4 (4.1) | 16 (8.2) | 0.190 |
| Epilepsy | 4 (1.4) | 1 (1) | 3 (1.5) | 0.722 |
| Asthenia | 25 (8.5) | 3 (3.1) | 22 (11.2) | 0.018 |
| Atopic dermatitis | 29 (9.8) | 14 (14.3) | 15 (7.7) | 0.072 |
| Dermatitis herpetiformis | 18 (6.1) | 7 (7.1) | 11 (5.6) | 0.606 |
| High transaminase levels | 16 (5.4) | 4 (4.1) | 12 (6.1) | 0.467 |
| Muscle hypotrofia | 4 (1.4) | 3 (3.1) | 1 (0.5) | 0.075 |
| Arthritis | 3 (1) | 0 | 3 (1.5) | 0.218 |
| Aphtosis | 8 (2.7) | 1 (1) | 7 (3.6) | 0.205 |
| Recurrent infections | 32 (10.8) | 11 (11.2) | 21 (10.7) | 0.895 |
| Tooth enamel alterations | 1 (0.3) | 0 | 1 (0.5) | 0.479 |
| Associated diseases | ||||
| Thiroiditis | 2 (0.7) | 2 (2) | 0 | 0.045 |
| Down’s syndrome | 1 (0.3) | 1 (1) | 0 | 0.157 |
| Allergies | 40 (13.5) | 18 (18.4) | 22 (11.2) | 0.092 |
| Asthma | 10 (3.4) | 6 (6.1) | 4 (2) | 0.069 |
| Toddler Age (0–3 Years) (n = 70) n (%) | Primary School Age (4–12 Years) (n = 200) n (%) | High School Age (>12 Years) (n = 25) n (%) | p | |
|---|---|---|---|---|
| Gastrointestinal symptoms | ||||
| Diarrhea | 10 (14.3) | 13 (6.5) | 1 (0.4) | 0.091 |
| Constipation | 6 (8.6) | 12 (6) | 0 | 0.307 |
| Abdominal pain | 13 (18.6) | 79 (39.5) | 13 (52) | 0.001 |
| Changes in bowel habits | 3 (4.3) | 7 (3.5) | 0 | 0.590 |
| Bloating | 40 (57.1) | 38 (19) | 5 (20) | <0.001 |
| Nausea/Vomit | 10 (14.3) | 19 (9.5) | 1 (4) | 0.299 |
| Lack of appetite | 9 (12.9) | 21 (10.5) | 2 (8) | 0.772 |
| Gastro-esophageal reflux | 1 (1.4) | 3 (1.5) | 0 | 0.827 |
| Extra-intestinal symptoms | ||||
| Hyposomia | 16 (22.9) | 29 (14.5) | 3 (12) | 0.226 |
| Anemia | 12 (17.1) | 39 (19.5) | 4 (16) | 0.845 |
| Slow growth | 21 (30) | 30 (15) | 3 (12) | 0.015 |
| Weight loss | 5 (7.1) | 2 (1) | 0 | 0.011 |
| Headache | 0 | 14 (7) | 6 (24) | <0.001 |
| Epilepsy | 1 (1.4) | 3 (1.5) | 0 | 0.827 |
| Asthenia | 0 | 19 (9.5) | 6 (24) | 0.001 |
| Atopic dermatitis | 3 (4.3) | 21 (10.5) | 5 (20) | 0.066 |
| Dermatitis herpetiformis | 1 (1.4) | 3 (1.5) | 13 (52) | 0.733 |
| High transaminase levels | 12 (17.1) | 4 (2) | 0 | <0.001 |
| Muscle hypotropia | 1 (1.4) | 3 (1.5) | 0 | 0.827 |
| Arthritis | 1 (1.4) | 2 (1) | 0 | 0.830 |
| Aphtosis | 0 | 8 (4) | 0 | 0.140 |
| Recurrent infections | 4 (5.7) | 27 (13.5) | 1 (4) | 0.099 |
| Tooth enamel alterations | 0 | 1 (0.5) | 0 | 0.787 |
| Associated diseases | ||||
| Thyroiditis | 0 | 2 (1) | 0 | 0.618 |
| Down’s syndrome | 1 (1.4) | 0 | 0 | 0.201 |
| Diabetes type 1 | 1 (1.4) | 2 (1) | 1 (4) | 0.457 |
| Allergies | 9 (12.9) | 26 (13) | 5 (20) | 0.621 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Biase, A.R.; Marasco, G.; Ravaioli, F.; Colecchia, L.; Dajti, E.; Lecis, M.; Passini, E.; Alemanni, L.V.; Festi, D.; Iughetti, L.; et al. Clinical Presentation of Celiac Disease and Diagnosis Accuracy in a Single-Center European Pediatric Cohort over 10 Years. Nutrients 2021, 13, 4131. https://doi.org/10.3390/nu13114131
Di Biase AR, Marasco G, Ravaioli F, Colecchia L, Dajti E, Lecis M, Passini E, Alemanni LV, Festi D, Iughetti L, et al. Clinical Presentation of Celiac Disease and Diagnosis Accuracy in a Single-Center European Pediatric Cohort over 10 Years. Nutrients. 2021; 13(11):4131. https://doi.org/10.3390/nu13114131
Chicago/Turabian StyleDi Biase, Anna Rita, Giovanni Marasco, Federico Ravaioli, Luigi Colecchia, Elton Dajti, Marco Lecis, Erica Passini, Luigina Vanessa Alemanni, Davide Festi, Lorenzo Iughetti, and et al. 2021. "Clinical Presentation of Celiac Disease and Diagnosis Accuracy in a Single-Center European Pediatric Cohort over 10 Years" Nutrients 13, no. 11: 4131. https://doi.org/10.3390/nu13114131
APA StyleDi Biase, A. R., Marasco, G., Ravaioli, F., Colecchia, L., Dajti, E., Lecis, M., Passini, E., Alemanni, L. V., Festi, D., Iughetti, L., & Colecchia, A. (2021). Clinical Presentation of Celiac Disease and Diagnosis Accuracy in a Single-Center European Pediatric Cohort over 10 Years. Nutrients, 13(11), 4131. https://doi.org/10.3390/nu13114131










