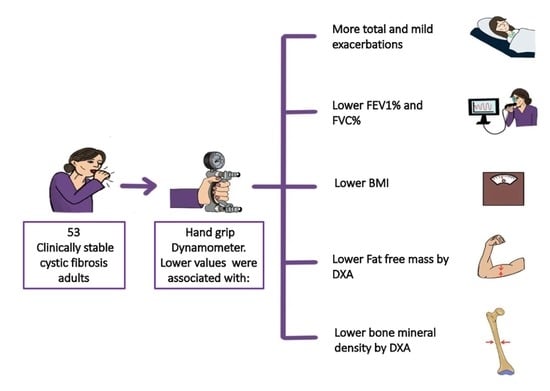
Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Anthropometric and Body Composition Parameters
2.2. Dietary Questionnarie
2.3. Assessment of Respiratory Status
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Culhane, S.; George, C.; Pearo, B.; Spoede, E. Malnutrition in Cystic Fibrosis. Nutr. Clin. Pr. 2013, 28, 676–683. [Google Scholar] [CrossRef]
- Ziegler, B.; Rovedder, P.M.E.; Lukrafka, J.L.; Oliveira, C.L.; Menna-Barreto, S.S.; Dalcin, P.D.T.R. Capacidade submáxima de exercício em pacientes adolescentes e adultos com fibrose cística. J. Bras. Pneumol. 2007, 33, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Blau, H.; Mussaffi-Georgy, H.; Fink, G.; Kaye, C.; Szeinberg, A.; Spitzer, S.A.; Yahav, J. Effects of an Intensive 4-Week Summer Camp on Cystic Fibrosis. Chest 2002, 121, 1117–1122. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jochem, C.; Leitzmann, M.; Volaklis, K.; Aune, D.; Strasser, B. Association Between Muscular Strength and Mortality in Clinical Populations: A Systematic Review and Meta-Analysis. J. Am. Med Dir. Assoc. 2019, 20, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionescu, A.A.; Chatham, K.; Davies, C.A.; Nixon, L.S.; Enright, S.; Shale, D.J. Inspiratory Muscle Function and Body Composition in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 1998, 158, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Legroux-Gérot, I.; Leroy, S.; Prudhomme, C.; Perez, T.; Flipo, R.M.; Wallaert, B.; Cortet, B. Bone loss in adults with cystic fibrosis: Prevalence, associated factors, and usefulness of biological markers. Jt. Bone Spine 2012, 79, 73–77. [Google Scholar] [CrossRef]
- Rovedder, P.M.E.; Borba, G.C.; Anderle, M.; Flores, J.; Ziegler, B.; Barreto, S.S.M.; Dalcin, P.D.T.R. Peripheral muscle strength is associated with lung function and functional capacity in patients with cystic fibrosis. Physiother. Res. Int. 2019, 24, e1771. [Google Scholar] [CrossRef]
- DeLion, M.; Braux, J.; Jourdain, M.-L.; Guillaume, C.; Bour, C.; Gangloff, S.; Le Pimpec-Barthes, F.; Sermet-Gaudelus, I.; Jacquot, J.; Velard, F. Overexpression of RANKL in osteoblasts: A possible mechanism of susceptibility to bone disease in cystic fibrosis. J. Pathol. 2016, 240, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.H.M.; Iwanicki, C.; McCaffery, H.; Nasr, S.Z. The Association of Grip Strength, Body Mass Index, and Lung Function in Youth with Cystic Fibrosis. Nutr. Clin. Pr. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Wells, G.D.; Wilkes, D.L.; Schneiderman, J.E.; Thompson, S.; Coates, A.L.; Ratjen, F. Physiological correlates of pulmonary function in children with cystic fibrosis. Pediatr. Pulmonol. 2013, 49, 878–884. [Google Scholar] [CrossRef]
- Sahlberg, M.E.; Svantesson, U.; Thomas, E.M.L.M.; Strandvik, B. Muscular Strength and Function in Patients With Cystic Fibrosis. Chest 2005, 127, 1587–1592. [Google Scholar] [CrossRef] [Green Version]
- Curiel, M.D.; De La Peña, J.L.C.; Perez, J.H.; Cano, R.P.; Rapado, A.; Martínez, I.R. Study of bone mineral density in lumbar spine and femoral neck in a Spanish population. Osteoporos. Int. 1997, 7, 59–64. [Google Scholar] [CrossRef]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group; World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 1994; Volume 843, pp. 1–129. [Google Scholar]
- Kanis JA on behalf of the WSG. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health; WHO Collaborating Centre, University of Sheefield: Sheffield, UK, 2008; pp. 5–7. [Google Scholar]
- 2019 ISCD Official Positions—Adults; The International Society For Clinical Densitometry (ISCD): Middletown, CT, USA, 2019.
- Olveira, G. Manual de Nutricion Clinica y Dietetica, 3rd ed.; Ediciones Díaz de Santos, S.A.: Madrid, Spain, 2016. [Google Scholar]
- Torralvo, F.J.S.; Porras, N.; Fernandez, J.A.; Torres, F.G.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Martínez, G.R.; Olveira, G.; et al. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Casado-Miranda, E.; Padilla, A.; Dorado, A.; Rojo-Martinez, G.; Porras, N.; Garcia-Escobar, E.; Soriguer, F. Markers for the Validation of Reported Dietary Intake in Adults with Cystic Fibrosis. J. Am. Diet. Assoc. 2009, 109, 1704–1711. [Google Scholar] [CrossRef]
- Jimenez, A. Tablas de Composición de Alimentos; Barcelona Novartis Consum Heal SA: Barcelona, Spain, 2002. [Google Scholar]
- Mataix, J. Tablas de Composición de Alimentos Españoles; Granada Univ Granada: Granada, Spain, 2003. [Google Scholar]
- Spanish Food Composition Database. Available online: https://www.bedca.net/bdpub/ (accessed on 22 November 2020).
- FoodData Central; US Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2019.
- Available online: https://fdc.nal.usda.gov/download-datasets.html (accessed on 22 November 2020).
- Máiz, L.; Baranda, F.; Coll, R.; Prados, C.; Vendrell, M.; Escribano, A.; Gartner, S.; de Gracia, S.; Martínez, M.; Salcedo, A.; et al. Normativa del diagnóstico y el tratamiento de la afección respiratoria en la fibrosis quística. Arch. Bronconeumol. 2001, 37, 316–324. [Google Scholar] [CrossRef]
- Gur, M.; Bar-Yoseph, R.; Diab, G.; Hanna, M.; Rozen, G.; Daud, F.; Keidar, Z.; Toukan, Y.; Masarweh, K.; Nir, V.; et al. Understanding the interplay between factors that influence bone mineral density in CF. Pediatr. Pulmonol. 2020, 55, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Rikkonen, T.; Sirola, J.; Salovaara, K.; Tuppurainen, M.; Jurvelin, J.S.; Honkanen, R.; Kröger, H. Muscle Strength and Body Composition Are Clinical Indicators of Osteoporosis. Calcif. Tissue Int. 2012, 91, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Sanchis, J.; Agusti-Vidal, A.; Segarra, F.; Navajas, D.; Rodriguez-Roisin, R.; Casan, P.; Sans, S. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986, 22, 217–224. [Google Scholar]
- Gruet, M.; Troosters, T.; Verges, S. Peripheral muscle abnormalities in cystic fibrosis: Etiology, clinical implications and response to therapeutic interventions. J. Cyst. Fibros. 2017, 16, 538–552. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, S.; Zemel, B.S.; Stallings, V.A.; Rubenstein, R.C.; Kelly, A. Body Composition and Pulmonary Function in Cystic Fibrosis. Front. Pediatr. 2014, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Hussey, J.; Gormley, J.; Leen, G.; Greally, P. Peripheral muscle strength in young males with cystic fibrosis. J. Cyst. Fibros. 2002, 1, 116–121. [Google Scholar] [CrossRef] [Green Version]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; the A.S.P.E.N. Board of Directors. Consensus Statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V.; Academy of Nutrition and Dietetics; American Society for Parenteral and Enteral Nutrition; et al. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators Recommended for the Identification and Documentation of Pediatric Malnutrition (Undernutrition). Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Ms, H.T.G.; McDonald, C.M.; Derrick, J.W.; Eggett, D.L.; Bellini, S.G. Evaluating Changes in Handgrip Strength in Children With Cystic Fibrosis: A Pilot Study. Nutr. Clin. Pr. 2018, 33, 261–267. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Gaspar, I.; Porras, N.; Núñez, G.M.M.; Rubio-Martín, E.; Colomo, N.; Rojo-Martínez, G.; Soriguer, F. Fat-Free Mass Depletion and Inflammation in Patients with Bronchiectasis. J. Acad. Nutr. Diet. 2012, 112, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Puhan, M.; Hebestreit, H.; Kriemler, S. The 1-min sit-to-stand test—A simple functional capacity test in cystic fibrosis? J. Cyst. Fibros. 2015, 15, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Bolívar, V.; Olveira, G.; Porras, N.; Acosta, E.; Rubio-Martín, E.; Tapia-Guerrero, M.J.; Abuin-Fernández, J. Osteopenia and Osteoporosis in Patients with Bronchiectasis: Association with Respiratory Parameters, Body Composition, Muscle Strength and Bone Remodeling Biomarkers. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Olveira, G.; Olveira, C.; Doña, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martínez, E.; Rojo-Martínez, G.; Martín-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2015, 35, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
| CF | CF | ||||
|---|---|---|---|---|---|
| Total (n = 53) | Dynamometry ≥ p10 (n = 42) | Dynamometry < p10 (n = 11) | p | ||
| Age | (m ± SD) | 28.3 ± 8.1 | 30.4 ± 7.6 | 20.5 ± 4.6 | <0.01 |
| Sex Male Female | n (%) | 22 (41.5) 31 (58.5) | 17 (40.5) 25 (59.5) | 5 (45.5) 6 (54.5) | NS |
| RESPIRATORY STATUS | |||||
| Bronchorrhea (mL) | (m ± SD) | 20.5 ± 20.2 | 18.6 ± 20.4 | 26.5 ± 19.2 | NS |
| Total exacerbations | (m ± SD) | 2.3 ± 1.8 | 2.0 ± 1.5 | 3.6 ± 2.2 | <0.01 |
| Severe exacerbations | (m ± SD) | 0.41 ± 0.80 | 0.4 ± 0.8 | 0.5 ± 0.8 | NS |
| FEV1 (%) | (m ± SD) | 63.3 ± 25.6 | 67.5 ± 25.2 | 56.6 ± 21.4 | <0.001 |
| FVC (%) | (m ± SD) | 75.3 ± 22.0 | 79.6 ± 18.2 | 73.9 ± 23.0 | <0.001 |
| FEV1/FVC (%) | (m ± SD) | 66.7 ± 12.0 | 68.0 ± 15.7 | 61.4 ± 17.8 | NS |
| Colonisations | |||||
| S. Aureus colonisation | n (%) | 42 (79.2) | 32 (76.2) | 10 (90.9) | NS |
| H. influenzae colonisation | n (%) | 23 (43.4) | 20 (47.6) | 3 (27.3) | NS |
| P. aeruginosa colonisation | n (%) | 41 (77.4) | 31 (73.8) | 10 (90.6) | NS |
| DIETARY QUESTIONNAIRE | |||||
| Mean calorie intake | (m ± SD) | 3444.0 ± 750.6 | 3324.4 ± 638.0 | 3683.2 ± 1000.6 | NS |
| Calories/kg of body weight | (m ± SD) | 47.5 ± 11.6 | 45.6 ± 10.6 | 54.0 ± 5.0 | NS |
| Proteins (%) | (m ± SD) | 16.2 ± 2.4 | 16.1 ± 2.5 | 16.3 ± 2.6 | NS |
| Proteins/kg of body weight | (m ± SD) | 1.9 ± 0.6 | 1.9 ± 0.6 | 2.2 ± 0.6 | NS |
| Vitamin A (UI) | (m ± SD) | 6202.2 ± 4445.9 | 5730.0 ± 4616.9 | 7820.9 ± 3629.5 | NS |
| Vitamin D (UI) | (m ± SD) | 3400.4 ± 2855.5 | 2961.1 ± 2515.1 | 4906.3 ± 3620.1 | NS |
| Vitamin E (mg) | (m ± SD) | 269.3 ± 175.0 | 261.2 ± 187.5 | 297.0 ± 131.3 | NS |
| Vitamin K (mcg) | (m ± SD) | 1078.5 ± 1596.7 | 1160.8 ± 1755.7 | 776.7 ± 778.0 | NS |
| BONE STATUS | |||||
| BMD | (m ± SD) | 1.092 ± 0.237 | 1.169 ± 0.255 | 0.991 ± 0.097 | <0.05 |
| T-score | (m ± SD) | −0.430 ± 1.184 | −0.323 ± 1.123 | −1.900 ± 1.228 | <0.05 |
| Z-score | (m ± SD) | −0.449± 1.145 | −0.172 ± 1.051 | −1.530 ± 0.830 | <0.001 |
| Normal | n (%) | 36 (67.9) | 32 (76.2) | 4 (36.4) | <0.05 |
| Osteopenia | n (%) | 11 (20.8) | 8 (19.0) | 3 (27.2) | |
| Osteoporosis | n (%) | 6 (11.3) | 2 (4.8) | 4 (36.4) | |
| NUTRITIONAL STATUS | |||||
| BMI | (m ± SD) | 21.7 ± 3.4 | 22.1 ± 3.7 | 20.2 ± 1.8 | <0.05 |
| Fat mass (kg) | (m ± SD) | 15.4 ± 8.1 | 15.9 ± 8.6 | 13.4 ± 4.6 | NS |
| Fat-free mass (kg) | (m ± SD) | 42.9 ± 9.7 | 43.1 ± 9.2 | 42.1 ± 12.2 | NS |
| Fat mass (%) | (m ± SD) | 25.9 ± 9.8 | 26.0 ± 9.7 | 25.4 ± 11.2 | NS |
| Fat-free mass (%) | (m ± SD) | 74.1 ± 9.9 | 74.0 ± 9.8 | 74.6 ±11.2 | NS |
| FFMI (kg/talla2) | (m ± SD) | 16.3 ± 2.5 | 16.2 ± 2.4 | 15.8 ± 2.9 | NS |
| Malnourished (FFMI < 17/15) | n (%) | 26 (49.1) | 20 (47.6) | 6 (54.5) | |
| Mean dynamometry | (m ± SD) | 30.2 ± 11.3 | 30.4 ± 11.9 | 19.5 ± 9.5 | <0.001 |
| Maximum dynamometry | (m ± SD) | 31.7 ± 11.6 | 33.9 ± 10.9 | 20.8 ± 9.2 | <0.01 |
| Age | Bronchorrhea | Total Exacerbations | Mild Exacerbations | Severe Exacerbations | FEV1 (%) | FVC (%) | FEV1/FVC (%) | |
|---|---|---|---|---|---|---|---|---|
| Mean dynamometry (r) | 0.334 | 0.051 | −0.067 | −0.024 | −0.110 | 0.222 | 0.302 | 0.042 |
| p | <0.01 | NS | NS | NS | NS | NS | <0.05 | NS |
| Maximum dynamometry (r) | 0.312 | 0.072 | −0.117 | −0.074 | −0.135 | 0.232 | 0.331 | 0.057 |
| p | <0.05 | NS | NS | NS | NS | NS | <0.05 | NS |
| BMD | T-Score | Z-Score | BMI | FFMI | Fat Mass (kg) | Fat-Free Mass (kg) | |
|---|---|---|---|---|---|---|---|
| Mean dynamometry (r) | 0.291 | 0.156 | 0.272 | 0.400 | 0.548 | −0.035 | 0.327 |
| p | <0.05 | NS | NS | <0.01 | <0.001 | NS | <0.05 |
| Maximum dynamometry (r) | 0.289 | 0.120 | 0.246 | 0.388 | 0.676 | −0.039 | 0.331 |
| p | <0.05 | NS | NS | <0.01 | <0.001 | NS | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Bolívar, V.; Olveira, C.; Ruiz-García, I.; Porras, N.; García-Olivares, M.; Sánchez-Torralvo, F.J.; Girón, M.V.; Alonso-Gallardo, S.P.; Olveira, G. Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis. Nutrients 2021, 13, 4107. https://doi.org/10.3390/nu13114107
Contreras-Bolívar V, Olveira C, Ruiz-García I, Porras N, García-Olivares M, Sánchez-Torralvo FJ, Girón MV, Alonso-Gallardo SP, Olveira G. Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis. Nutrients. 2021; 13(11):4107. https://doi.org/10.3390/nu13114107
Chicago/Turabian StyleContreras-Bolívar, Victoria, Casilda Olveira, Ignacio Ruiz-García, Nuria Porras, Maria García-Olivares, Francisco José Sánchez-Torralvo, Maria Victoria Girón, Silvia P. Alonso-Gallardo, and Gabriel Olveira. 2021. "Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis" Nutrients 13, no. 11: 4107. https://doi.org/10.3390/nu13114107
APA StyleContreras-Bolívar, V., Olveira, C., Ruiz-García, I., Porras, N., García-Olivares, M., Sánchez-Torralvo, F. J., Girón, M. V., Alonso-Gallardo, S. P., & Olveira, G. (2021). Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis. Nutrients, 13(11), 4107. https://doi.org/10.3390/nu13114107