Nut Allergy: Clinical and Allergological Features in Italian Children
Abstract
:1. Background
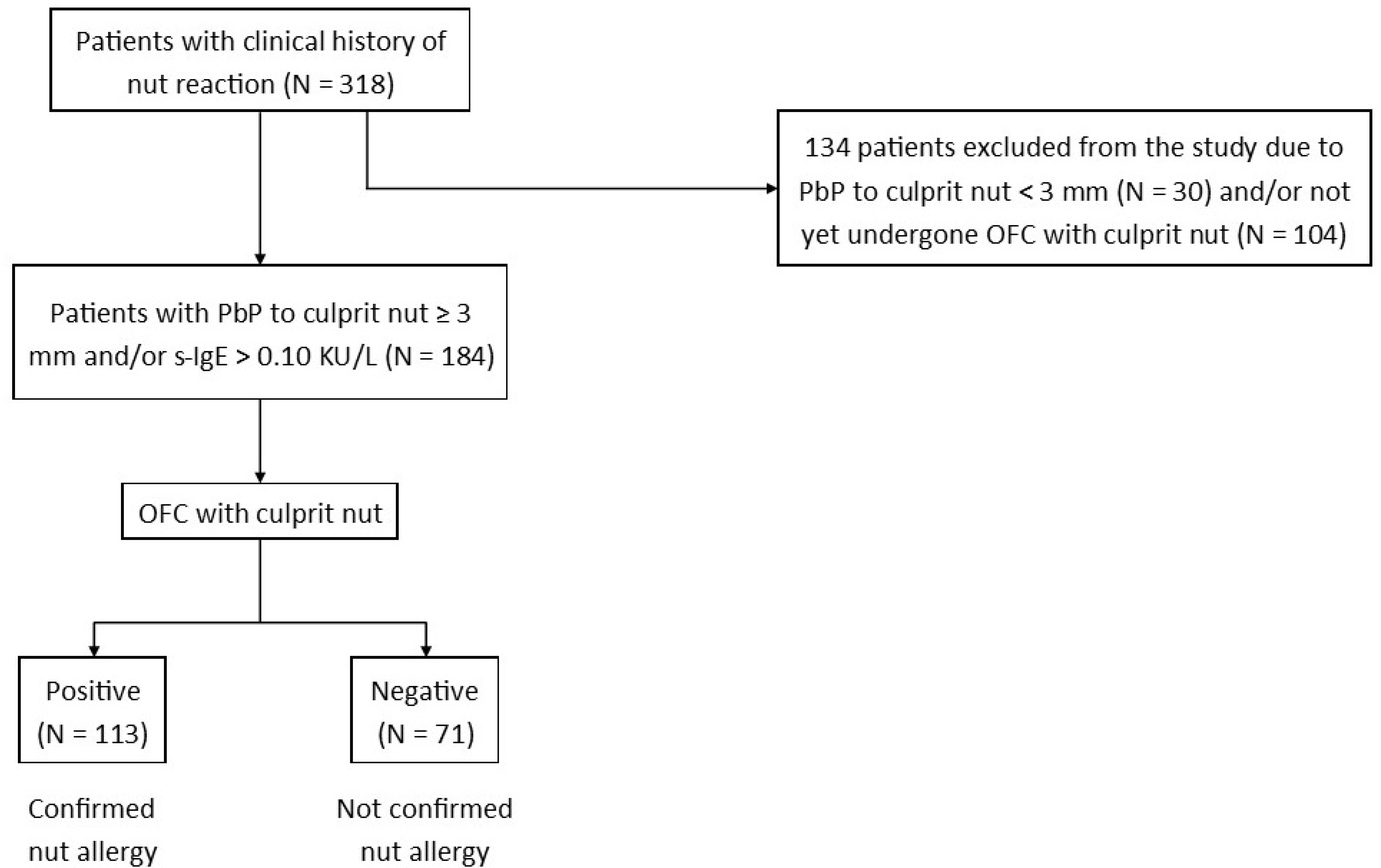
2. Materials and Methods
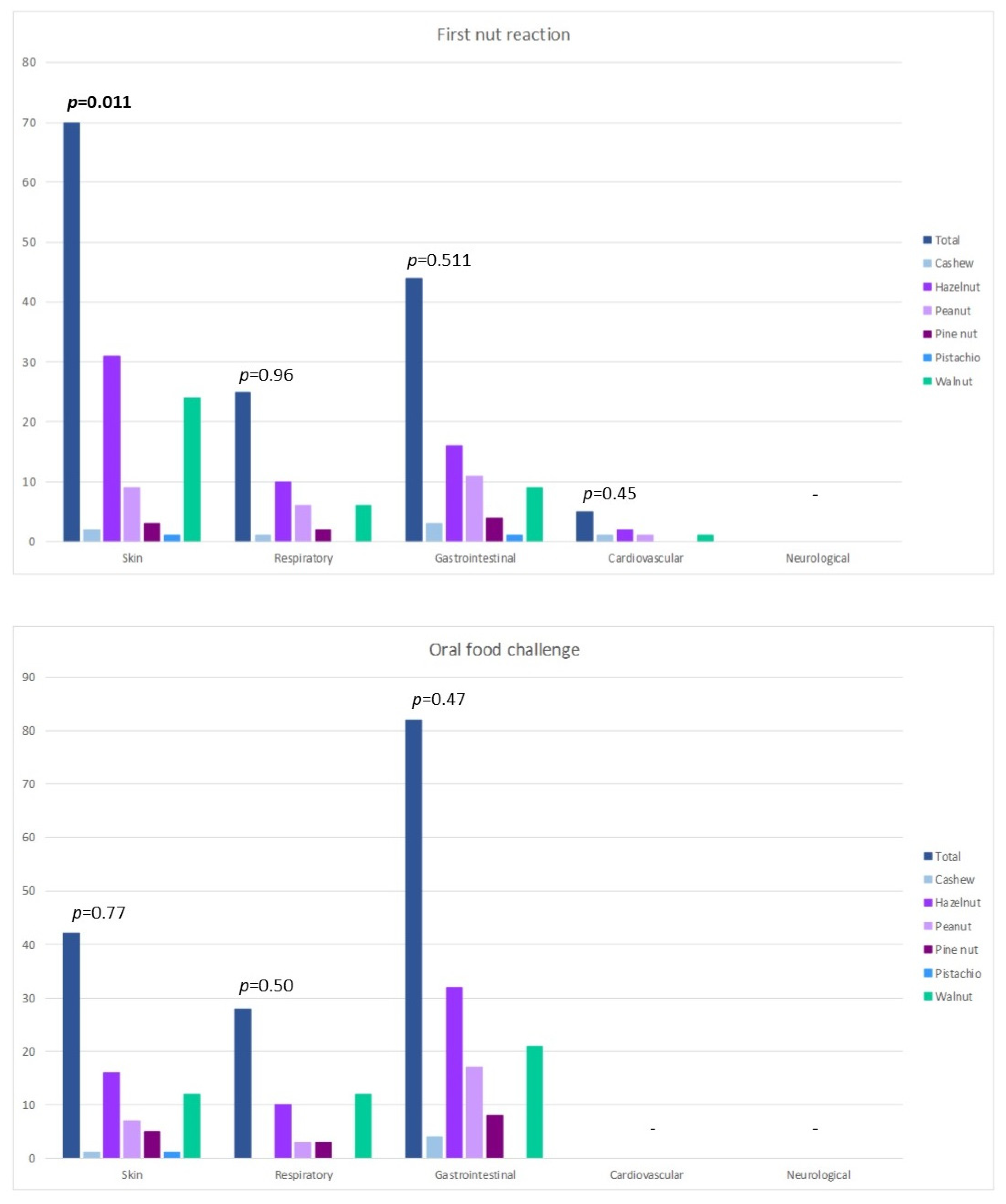
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFC | oral food challenge |
| PbP | prick by prick |
| s-IgE | serum-specific IgE |
| SPT | skin prick test |
| TN | tree nuts |
References
- Gupta, R.; Warren, C.; Smith, B.; Blumenstock, J.; Jiang, J.; Davis, M.; Nadeau, K. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Munoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar] [CrossRef]
- Dunngalvin, A.; Dubois, A.E.J.; Blok, B.M.J.F.; Hourihane, J.O.B. The Effects of Food Allergy on Quality of Life. Chem. Immunol. Allergy 2015, 101, 235–252. [Google Scholar]
- Primeau, M.; Kagan, R.; Joseph, L.; Lim, H.; Dufresne, C.; Duffy, C.; Prhcal, D.; Clarke, A. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin. Exp. Allergy 2000, 30, 1135–1143. [Google Scholar] [CrossRef]
- Logan, K.; Du Toit, G.; Giovannini, M.; Turcanu, V.; Lack, G. Pediatric Allergic Diseases, Food Allergy, and Oral Tolerance. Annu. Rev. Cell Dev. Biol. 2020, 36, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, P.G.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sekerel, B.E. Factors predicting anaphylaxis in children with tree nut allergies. Allergy Asthma Proc. 2019, 40, 180–186. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Lack, G.; Mazon, A.; Nieto, A.; Haddad, D.; Borugh, H.A.; Caubet, J.C. Managing Nut Allergy: A Remaining Clinical Challenge. J. Allergy Clin. Immunol. Pract. 2017, 5, 296–300. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Muñoz-furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef]
- de Leon, M.P.; Rolland, J.M.; O’Hehir, R.E. The peanut allergy epidemic: Allergen molecular characterisation and prospects for specific therapy. Expert Rev. Mol. Med. 2007, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Stiefel, G.; Anagnostou, K.; Boyle, R.J.; Brathwaite, N.; Ewan, P.; Fox, A.T.; Huber, P.; Luyt, D.; Till, S.J.; Venter, C.; et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin. Exp. Allergy 2017, 47, 719–739. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.; O’Flynn, N. Diagnosis and assessment of food allergy in children and young people in primary care and community settings: NICE clinical guideline. Br. J. Gen. Pract. 2011, 61, 473–475. [Google Scholar] [CrossRef]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elizur, A.; Appel, M.Y.; Nachshon, L.; Levy, M.B.; Epstein-Rigbi, N.; Golobov, K.; Goldberg, M. NUT Co Reactivity—ACquiring Knowledge for Elimination Recommendations (NUT CRACKER) study. Allergy 2018, 73, 593–601. [Google Scholar] [CrossRef]
- Giovannini, M.; Comberiati, P.; Piazza, M.; Chiesa, E.; Piacentini, G.L.; Boner, A.; Zanoni, G.; Peroni, D.G. Retrospective definition of reaction risk in Italian children with peanut, hazelnut and walnut allergy through component-resolved diagnosis. Allergol. Immunopathol. 2018, 47, 73–78. [Google Scholar] [CrossRef]
- Ortolani, C.; Ispano, M.; Pastorello, E.A.; Ansaloni, R.; Magri, G. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergic syndrome. J. Allergy Clin. Immunol. 1989, 83, 683–690. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niggemann, B.; Beyer, K. Time for a new grading system for allergic reactions? Allergy 2016, 71, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, P.G.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sackesen, C.; Sekerel, B.E. Phenotypical characterization of tree nuts and peanut allergies in east Mediterranean children. Allergol. Immunopathol. 2020, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Chan, E.S.; Sicherer, S. Peanut allergy: New advances and ongoing controversies. Pediatrics 2020, 145, e20192102. [Google Scholar] [CrossRef]
- Lyons, S.A.; Datema, M.R.; Le, T.M.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Clausen, M.; Dubakiene, R.; Fernández-Perez, C.; et al. Walnut Allergy Across Europe: Distribution of Allergen Sensitization Patterns and Prediction of Severity. J. Allergy Clin. Immunol. Pract. 2021, 9, 225–235.e10. [Google Scholar] [CrossRef]
- Calamelli, E.; Trozzo, A.; Di Blasi, E.; Serra, L.; Bottau, P. Hazelnut allergy. Medicina 2021, 57, 67. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, J.M.; Bagley, K.; Kulis, M. Tree nut allergies: Allergen homology, cross-reactivity, and implications for therapy. Clin. Exp. Allergy 2018, 48, 762–772. [Google Scholar] [CrossRef]
- Haroun-Díaz, E.; Azofra, J.; González-Mancebo, E.; de las Heras, M.; Pastor-Vargas, C.; Esteban, V.; Villalba, M.; Díaz-Perales, A.; Cuesta-Herranz, J. Nut allergy in two different areas of Spain: Differences in clinical and molecular pattern. Nutrients 2017, 9, 909. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.A.; Assa’ad, A.; Burks, W.A.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, H.S.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, 51–58. [Google Scholar] [CrossRef]
- Brough, H.A.; Caubet, J.-C.; Mazon, A.; Haddad, D.; Bergmann, M.M.; Wassenberg, J.; Panetta, V.; Gourgey, R.; Radulovic, S.; Nieto, M.; et al. Defining challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter European study. J. Allergy Clin. Immunol. 2020, 145, 1231–1239. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Burks, A.W.; Sampson, H.A. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics 1998, 102, e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midun, E.; Radulovic, S.; Brough, H.; Caubet, J.C. Recent advances in the management of nut allergy. World Allergy Organ. J. 2021, 14, 100491. [Google Scholar] [CrossRef]
- Maloney, J.M.; Rudengren, M.; Ahlstedt, S.; Bock, S.A.; Sampson, H.A. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J. Allergy Clin. Immunol. 2008, 122, 145–151. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Peters, R.; Tang, M.L.K.; Dharmage, S.; Ponsonby, A.L.; Gurrin, L.; Perrett, K.; Koplin, J.; Allen, K.J.; Dwyer, T.; et al. Patterns of tree nut sensitization and allergy in the first 6 years of life in a population-based cohort. J. Allergy Clin. Immunol. 2019, 143, 644–650.e5. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Conover-Walker, M.K.; Matsui, E.C.; Wood, R.A. The natural history of tree nut allergy. J. Allergy Clin. Immunol. 2005, 116, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Couch, C.; Franxman, T.; Greenhawt, M. Characteristics of tree nut challenges in tree nut allergic and tree nut sensitized individuals. Ann. Allergy, Asthma Immunol. 2017, 118, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Anagnostou, K. Safety of Oral Food Challenges in Early Life. Children 2018, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, E.M.; Becker, A.B. Oral food challenge outcomes in a pediatric tertiary care center. Allergy Asthma Clin. Immunol. 2017, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, K.M.; Amalanayagam, S.; Shreffler, W.G.; Noone, S.; Sicherere, S.H.; Sampson, H.A.; Nowak-Wegrzyn, A. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J. Allergy Clin. Immunol. 2009, 124, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.A.; Cox, A.L.; Vitale, M.; Sampson, H.A. Outcomes of office-based, open food challenges in the management of food allergy. J. Allergy Clin. Immunol. 2011, 128, 1120–1122. [Google Scholar] [CrossRef] [Green Version]
- Ballini, G.; Gavagni, C.; Guidotti, C.; Ciolini, G.; Liccioli, G.; Giovannini, M.; Sarti, L.; Ciofi, D.; Novembre, E.; Mori, F.; et al. Frequency of positive oral food challenges and their outcomes in the allergy unit of a tertiary-care pediatric hospital. Allergol. Immunopathol. 2021, 49, 120–130. [Google Scholar] [CrossRef]
| Dose (mg) | Almond (mg of Protein) | Cashew (mg of Protein) | Hazelnut (mg of Protein) | Peanut (mg of Protein) | Pine Nut (mg of Protein) | Pistachio (mg of Protein) | Walnut (mg of Protein) | |
|---|---|---|---|---|---|---|---|---|
| 5 | 1.05 | 0.9 | 0.7 | 1.3 | 0.7 | 1 | 0.75 | |
| 10 | 2.1 | 1.8 | 1.4 | 2.6 | 1.4 | 2 | 1.5 | |
| 25 | 5.25 | 4.5 | 3.5 | 6.5 | 3.5 | 5 | 3.75 | |
| 50 | 10.5 | 9 | 7 | 13 | 7 | 10 | 7.5 | |
| 100 | 21 | 18 | 14 | 26 | 14 | 20 | 15 | |
| 150 | 31.5 | 27 | 21 | 39 | 21 | 30 | 22.5 | |
| 300 | 63 | 54 | 42 | 78 | 42 | 60 | 45 | |
| 600 | 126 | 108 | 84 | 156 | 84 | 120 | 90 | |
| 1200 | 252 | 216 | 168 | 312 | 168 | 240 | 180 | |
| 2000 | 420 | 360 | 280 | 520 | 280 | 400 | 300 | |
| 4000 | 840 | 720 | 560 | 1040 | 560 | 800 | 600 | |
| Cumulative dose | 8440 | 1172.4 | 1519.2 | 1181.6 | 2194.4 | 1181.6 | 1688 | 1266 |
| Total (N = 113) | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Pistachio (N = 1) | Walnut (N = 32) | p | |
|---|---|---|---|---|---|---|---|---|
| Male (N = %) | 74; 65 | 3; 75 | 33; 77 | 12; 55 | 5; 45 | 1; 100 | 20; 62 | 0.27 |
| Age (months) (median; min; max) | 42; 8; 175 | |||||||
| AD (N = %) | 46; 41 | 0; 0 | 20; 47 | 6; 27 | 4; 36 | 1; 100 | 15; 47 | 0.22 |
| Asthma (N = %) | 33; 29 | 0; 0 | 14; 33 | 5; 23 | 5; 45 | 1; 100 | 8; 25 | 0.27 |
| Rhinitis (N = %) | 49; 43 | 1; 25 | 21; 49 | 7; 32 | 3; 27 | 1; 100 | 16; 50 | 0.38 |
| Other FA (N = %) | 34; 30 | 2; 50 | 17; 40 | 2; 9 | 2; 18 | 1; 100 | 10; 31 | 0.07 |
| Family history of allergy (N = %) | 85; 75 | 4; 100 | 33; 77 | 13; 59 | 9; 81 | 1; 100 | 25; 78 | 0.39 |
| Age at first reaction (months) (mean ± SD; min; max) | 57 ± 43; 8; 175 | 102 ± 71; 24; 172 | 45 ± 39; 8; 175 | 60 ± 41; 18; 154 | 103 ± 44; 48; 174 | - | 50 ± 33; 12; 125 | 0.00017 |
| PbP (mm) (mean ± SD; min; max) | 7 ± 3; 3; 15 | 8 ± 2; 6; 10 | 7 ± 3; 3; 15 | 6 ± 3; 3; 10 | 7 ± 2; 3; 10 | - | 7 ± 3; 3; 15 | 0.47 |
| s-IgE (KU/L) (mean ± SD; min; max) | 21 ± 32; 0.11; 100 | 3 ± 2; 1.7; 4.93 | 26 ± 35; 0.16; 100 | 31 ± 41; 0.12; 100 | 9 ± 19; 0.11; 66.3 | - | 14 ± 25; 0.3; 96.2 | 0.94 |
| Nut | Other Nuts Allergies | |||||
|---|---|---|---|---|---|---|
| Cashew (N = %) | Hazelnut (N = %) | Peanut (N = %) | Pine Nut (N = %) | Pistachio (N = %) | Walnut (N = %) | |
| Cashew (N = 4) | - | 0; 0 | 0; 0 | 0; 0 | 1; 25 | 0; 0 |
| Hazelnut (N = 43) | 2; 5 | 3; 7 | 2; 5 | 1; 2 | 3; 7 | |
| Peanut (N = 22) | 0; 0 | 2; 9 | 0; 0 | 0; 0 | 1; 5 | |
| Pine nut (N = 11) | 1; 9 | 1; 9 | 0; 0 | 0; 0 | 2; 18 | |
| Pistachio (N = 1) | 0; 0 | 1; 100 | 0; 0 | 0; 0 | 0; 0 | |
| Walnut (N = 32) | 0; 0 | 6; 19 | 1; 3 | 0; 0 | 0; 0 | |
| Severity | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Walnut (N = 32) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP | s-IgE | ||
| First reaction | Mild | - | - | 7 ± 3 | 26 ± 35 | 7 ± 3 | 34 ± 45 | 7 ± 3 | 1 ± 1 | 6 ± 3 | 15 ± 28 | 1.31 | 0.56 |
| Moderate | 8 ± 3 | 2 ± 1 | 9 ± 2 | 45 ± 47 | 5 ± 2 | 58 ± 60 | 8 ± 1 | 6 ± 5 | - | - | 0.20 | 0.27 | |
| Severe | - | - | 6 ± 3 | 13 ± 21 | 4 ± 2 | 26 ± 42 | - | - | 7 ± 4 | 2 ± 2 | 0.31 | 0.80 | |
| Oral food challenge | Mild | 8 ± 2 | 3 ± 2 | 7 ± 3 | 24 ± 35 | 6 ± 2 | 29 ± 41 | 7 ± 2 | 11 ± 23 | 6 ± 3 | 8 ± 11 | 0.52 | 0.78 |
| Moderate | - | - | 6 ± 1 | 45 ± 34 | 6 ± 4 | 56 ± 43 | - | - | 9 ± 5 | 54 ± 49 | 0.40 | 0.84 | |
| Severe | - | - | 6 ± 3 | - | - | - | 6 ± 1 | 3 ± 3 | 6 ± 3 | 3 ± 3 | 0.97 | 0.76 | |
| First Reaction | Oral Food Challenge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Allergens | Available Data (N=%) | Value (KU/L) (Mean ± SD; Min; Max) | Mild (KU/L) (Mean ± SD) | Moderate (KU/L) (Mean ± SD) | Severe (KU/L) (Mean ± SD) | p | Mild (KU/L) (Mean ± SD) | Moderate (KU/L) (Mean ± SD) | Severe (KU/L) (Mean ± SD) | p |
| Ara h 1 | 22; 100 | 33 ± 41; 0.15; 100 | 46 ± 51 | 28 ± 32 | 39 ± 40 | 0.80 | 34 ± 42 | 27 ± 45 | - | 0.98 |
| Ara h 2 | 22; 100 | 38 ± 39; 0.6; 100 | 41 ± 47 | 32 ± 33 | 48 ± 42 | 0.92 | 34 ± 39 | 59 ± 45 | - | 0.64 |
| Ara h 3 | 21; 95 | 19 ± 32; 0.11; 100 | 39 ± 43 | 11 ± 14 | 3 ± 3 | 0.88 | 20 ± 33 | - | - | - |
| Ara h 8 | 21; 95 | 1 ± 1; 0.15; 2.17 | 1 ±1 | - | 1 ± 1 | 0.53 | 0 ± 0 | - | - | - |
| Ara h 9 | 21; 95 | 16 ± 22; 0.88; 40.8 | 16 ± 22 | - | - | - | 3 ± 4 | - | - | - |
| Jug r 1 | 16; 50 | 11 ± 22; 0.27; 88.3 | 4 ± 4 | - | - | - | 13 ± 25 | 4 ± 5 | 4 ± 4 | 0.93 |
| Jur r 3 | 16; 50 | 3 ± 6; 0.12; 16 | 4 ± 7 | - | - | - | 3 ± 7 | - | - | - |
| Cor a 1 | 32; 74 | 8 ± 11; 0.16; 32.3 | 8 ± 13 | 12 ± 14 | 5 ± 7 | 0.42 | 7 ± 10 | - | - | - |
| Cor a 8 | 32; 74 | 5 ± 11; 0.11; 36.6 | 3 ± 4 | 0 ± 0 | 20 ± 23 | 0.51 | 6 ± 11 | - | - | - |
| Cor a 9 | 33; 77 | 22 ± 36; 0.11; 100 | 21 ± 33 | 50 ± 57 | 9 ± 18 | 0.14 | 20 ± 34 | 49 ± 20 | - | 0.92 |
| Cor a 14 | 31; 71 | 16 ± 23; 0.11; 90 | 19 ± 27 | 16 ± 17 | 7 ± 10 | 0.08 | 17 ± 24 | 12 ± 12 | - | 0.95 |
| Total (N = 113) | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Pistachio (N = 1) | Walnut (N = 32) | p | |
|---|---|---|---|---|---|---|---|---|
| Mild (N = %) | 91; 81 | 4; 100 | 36; 84 | 18; 82 | 8; 73 | 1; 100 | 24; 75 | 0.77 |
| Protein ingested (mean (mg) ± SD) | 283 ± 630 | 79 ± 35 | 279 ± 518 | 472 ± 1054 | 199 ± 350 | - | 221 ± 478 | 0.65 |
| Moderate (N = %) | 13; 11 | 0; 0 | 5; 12 | 3; 14 | 1; 9 | 0; 0 | 4; 12 | 0.97 |
| Protein ingested (mean (mg) ± SD) | 376 ± 686 | - | 764 ± 1074 | 123 ± 75 | - | - | 168 ± 47 | 0.36 |
| Severe (N = %) | 9; 8 | 0; 0 | 2; 5 | 1; 5 | 2; 18 | 0; 0 | 4; 12 | 0.58 |
| Protein ingested (mean (mg) ± SD) | 69 ± 72 | - | 43 ± 32 | - | 104 ± 136 | - | 44 ± 56 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliati, S.; Barni, S.; Giovannini, M.; Liccioli, G.; Sarti, L.; Alicandro, T.; Paladini, E.; Perferi, G.; Azzari, C.; Novembre, E.; et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients 2021, 13, 4076. https://doi.org/10.3390/nu13114076
Tagliati S, Barni S, Giovannini M, Liccioli G, Sarti L, Alicandro T, Paladini E, Perferi G, Azzari C, Novembre E, et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients. 2021; 13(11):4076. https://doi.org/10.3390/nu13114076
Chicago/Turabian StyleTagliati, Sylvie, Simona Barni, Mattia Giovannini, Giulia Liccioli, Lucrezia Sarti, Tatiana Alicandro, Erika Paladini, Giancarlo Perferi, Chiara Azzari, Elio Novembre, and et al. 2021. "Nut Allergy: Clinical and Allergological Features in Italian Children" Nutrients 13, no. 11: 4076. https://doi.org/10.3390/nu13114076
APA StyleTagliati, S., Barni, S., Giovannini, M., Liccioli, G., Sarti, L., Alicandro, T., Paladini, E., Perferi, G., Azzari, C., Novembre, E., & Mori, F. (2021). Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients, 13(11), 4076. https://doi.org/10.3390/nu13114076







