Gut Microbiota and Mycobiota Evolution Is Linked to Memory Improvement after Bariatric Surgery in Obese Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Cognitive Functions
2.3. Fecal Sample Collection and Sequencing
2.4. Bacterial and Fungal Sequence Analyses
2.5. Statistical Analyses
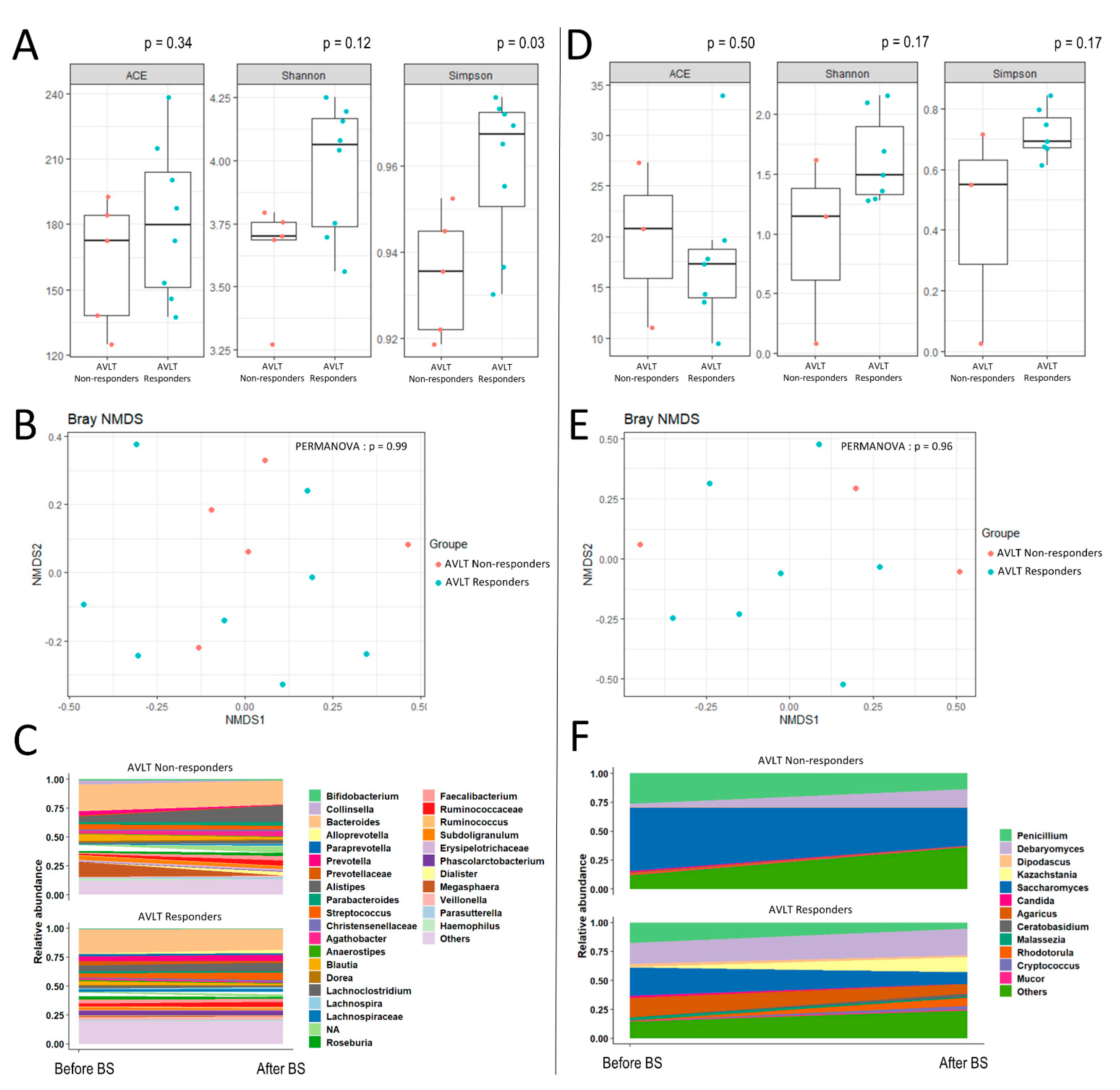
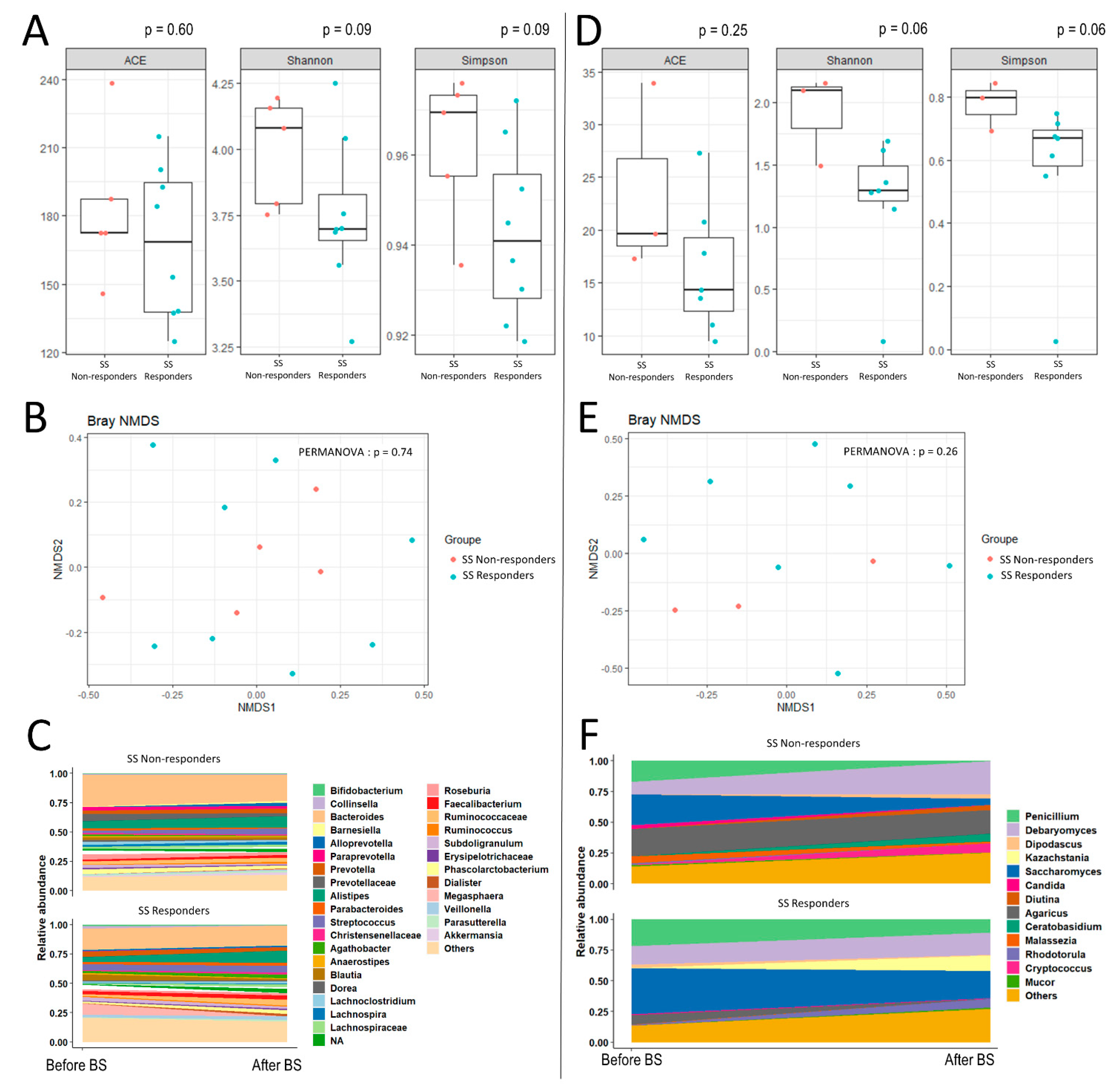
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Contreras-Rodríguez, O.; Blasco, G.; Coll, C.; Biarnés, C.; Miranda-Olivos, R.; Latorre, J.; Moreno-Navarrete, J.-M.; et al. Obesity Impairs Short-Term and Working Memory through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab. 2020, 32, 548–560.e7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Gourley, D.D.; Dekhtyar, M.; Haley, A.P. Cognition, Brain Structure, and Brain Function in Individuals with Obesity and Related Disorders. Curr. Obes. Rep. 2020, 9, 544–549. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Steinert, R.E.; Rehman, A.; Souto Lima, E.J.; Agamennone, V.; Schuren, F.H.J.; Gero, D.; Schreiner, P.; Vonlanthen, R.; Ismaeil, A.; Tzafos, S.; et al. Roux-en-Y gastric bypass surgery changes fungal and bacterial microbiota in morbidly obese patients-A pilot study. PLoS ONE 2020, 15, e0236936. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Farin, W.; Oñate, F.P.; Plassais, J.; Bonny, C.; Beglinger, C.; Woelnerhanssen, B.; Nocca, D.; Magoules, F.; Le Chatelier, E.; Pons, N.; et al. Impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: A metagenomic comparative analysis. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2020, 16, 852–862. [Google Scholar] [CrossRef] [PubMed]
- McGlennon, T.W.; Buchwald, J.N.; Pories, W.J.; Yu, F.; Roberts, A.; Ahnfeldt, E.P.; Menon, R.; Buchwald, H. Bypassing TBI: Metabolic Surgery and the Link between Obesity and Traumatic Brain Injury-a Review. Obes. Surg. 2020, 30, 4704–4714. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Profitlich, T.; Rangus, I.; Heßler, S.; Witte, A.V.; Grittner, U.; Ordemann, J.; Flöel, A. Bariatric Surgery and Brain Health—A Longitudinal Observational Study Investigating the Effect of Surgery on Cognitive Function and Gray Matter Volume. Nutrients 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Bo, T.; Xi, L.; Xu, X.; He, N.; Zhan, Y.; Li, W.; Liang, P.; Chen, Y.; Shi, J.; et al. Reversal of Functional Brain Activity Related to Gut Microbiome and Hormones After VSG Surgery in Patients with Obesity. J. Clin. Endocrinol. Metab. 2021, 106, e3619–e3633. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; Tinahones, F.J. Shifts in gut microbiota and their metabolites induced by bariatric surgery. Impact of factors shaping gut microbiota on bariatric surgery outcomes. Rev. Endocr. Metab. Disord. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.C.E.; Seeley, R.J. How does “metabolic surgery” work its magic? New evidence for gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Malloy-Diniz, L.F.; Lasmar, V.A.P.; Gazinelli, L.D.S.R.; Fuentes, D.; Salgado, J.V. The Rey Auditory-Verbal Learning Test: Applicability for the Brazilian elderly population. Braz. J. Psychiatry 2007, 29, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loring, D.W.; Strauss, E.; Hermann, B.P.; Barr, W.B.; Perrine, K.; Trenerry, M.R.; Chelune, G.; Westerveld, M.; Lee, G.P.; Meador, K.J.; et al. Differential neuropsychological test sensitivity to left temporal lobe epilepsy. J. Int. Neuropsychol. Soc. JINS 2008, 14, 394–400. [Google Scholar] [CrossRef]
- Drozdick, L.W.; Raiford, S.E.; Wahlstrom, D.; Weiss, L.G. The Wechsler Adult Intelligence Scale—Fourth Edition and the Wechsler Memory Scale—Fourth Edition. In Contemporary Intellectual Assessment: Theories, Tests, and Issues, 4th ed.; The Guilford Press: New York, NY, USA, 2018; pp. 486–511. ISBN 978-1-4625-3578-1. [Google Scholar]
- Vandenborght, L.-E.; Enaud, R.; Urien, C.; Coron, N.; Girodet, P.-O.; Ferreira, S.; Berger, P.; Delhaes, L. Type 2–high asthma is associated with a specific indoor mycobiome and microbiome. J. Allergy Clin. Immunol. 2021, 147, 1296–1305.e6. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- Pauvert, C.; Buée, M.; Laval, V.; Edel-Hermann, V.; Fauchery, L.; Gautier, A.; Lesur, I.; Vallance, J.; Vacher, C. Bioinformatics matters: The accuracy of plant and soil fungal community data is highly dependent on the metabarcoding pipeline. Fungal Ecol. 2019, 41, 23–33. [Google Scholar] [CrossRef]
- McKnight, D.T.; Huerlimann, R.; Bower, D.S.; Schwarzkopf, L.; Alford, R.A.; Zenger, K.R. microDecon: A highly accurate read-subtraction tool for the post-sequencing removal of contamination in metabarcoding studies. Environ. DNA 2019, 1, 14–25. [Google Scholar] [CrossRef]
- Eetemadi, A.; Rai, N.; Pereira, B.M.P.; Kim, M.; Schmitz, H.; Tagkopoulos, I. The Computational Diet: A Review of Computational Methods Across Diet, Microbiome, and Health. Front. Microbiol. 2020, 11, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, N.T.; Li, F.; Wang, S.; Kuhn, L. metamicrobiomeR: An R package for analysis of microbiome relative abundance data using zero-inflated beta GAMLSS and meta-analysis across studies using random effects models. BMC Bioinform. 2019, 20, 188. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Sze, M.A.; Schloss, P.D. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. mBio 2016, 7, e01018-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.-H.; Wu, C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; von Schwartzenberg, R.J.; Mai, K.; Zeitz, U.; Witte, A.V.; Hampel, D.; Szela, A.-M.; Fabian, S.; Grittner, U.; Spranger, J.; et al. Caloric Restriction in Older Adults-Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb. Cortex 2017, 27, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Verdi, S.; Jackson, M.A.; Beaumont, M.; Bowyer, R.C.E.; Bell, J.T.; Spector, T.D.; Steves, C.J. An Investigation Into Physical Frailty as a Link Between the Gut Microbiome and Cognitive Health. Front. Aging Neurosci. 2018, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Canipe, L.G.; Sioda, M.; Cheatham, C.L. Diversity of the gut-microbiome related to cognitive behavioral outcomes in healthy older adults. Arch. Gerontol. Geriatr. 2021, 96, 104464. [Google Scholar] [CrossRef] [PubMed]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet Lond. Engl. 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Guo, R.; Yu, W.; Zhang, F.; Wu, F.; Shang, J. The Alteration in Composition and Function of Gut Microbiome in Patients with Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 8842651. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solas, M.; Milagro, F.I.; Ramírez, M.J.; Martínez, J.A. Inflammation and gut-brain axis link obesity to cognitive dysfunction: Plausible pharmacological interventions. Curr. Opin. Pharmacol. 2017, 37, 87–92. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zheng, Z.; Wan, X.; Cao, J.; Wei, R.; Duan, J. The role of gut microbiota and amino metabolism in the effects of improvement of islet β-cell function after modified jejunoileal bypass. Sci. Rep. 2021, 11, 4809. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Moran, T.H.; Papantoni, A.; Speck, C.; Bakker, A.; Kamath, V.; Carnell, S.; Steele, K.E. Short-term improvements in cognitive function following vertical sleeve gastrectomy and Roux-en Y gastric bypass: A direct comparison study. Surg. Endosc. 2020, 34, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
| Overall | AVLT Responders | Symbol Span Responders | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | ||
| N | 13 | 9 (69%) | 4 (31%) | 8 (62%) | 5 (38%) | ||
| Female | 11 (85%) | 9 (100%) | 2(50%) | 0.53 | 7 (75%) | 5 (100%) | 0.99 |
| Age (years) | 48 ± 12 | 47 ± 13 | 44 ± 12 | 0.70 | 45 ± 15 | 45 ± 10 | 0.92 |
| Obesity duration (years) | 25 ± 8 | 29.3 ± 6.0 | 22.7 ± 8.2 | 0.20 | 30 ± 6 | 22 ± 7 | 0.10 |
| BMI (kg/m2) | 43 ± 5 | 43 ± 6 | 43 ± 4 | 0.96 | 44 ± 5 | 43 ± 5 | 0.75 |
| History of diabetes | 4 (31%) | 3 (33%) | 1 (25%) | 0.05 | 2 (25%) | 2 (40%) | 0.99 |
| HOMA | 4 [2; 7] | 12 [3; 66] | 4 [2; 5] | 0.14 | 5 [4; 66] | 3 [2; 5] | 0.09 |
| CRP (mg/L) | 6 [4; 13] | 4 [3; 11] | 9 [5; 15] | 0.22 | 9 [5; 13] | 5 [3; 15] | 0.33 |
| Weight loss (%) | 18 [14; 25] | 21 [11; 34] | 18 [14; 22] | 0.76 | 18 [13; 28] | 17 [14; 22] | 0.88 |
| Vit B1 postop | 149 [128; 178] | 156 [121; 191] | 149 [133; 175] | 0.99 | 156 [127; 173] | 149 [128; 186] | 0.62 |
| Vit B12 postop | 361 [314; 520] | 381 [320; 442] | 361 [306; 663] | 0.99 | 343 [295; 390] | 379 [320; 754] | 0.57 |
| Type of surgery | |||||||
| SG | 5 (56%) | 2 (50%) | 0.99 | 5 (62%) | 2 (40%) | 0.59 | |
| Gastric bypass | 4 (44%) | 2 (50%) | 3 (37%) | 3 (60%) | |||
| Kingdom | Phylum | Class | Order | Family | Genus | Odds Ratio (Log) | 95% CI | FDR Adjusted p-Value |
|---|---|---|---|---|---|---|---|---|
| Bacteria | Firmicutes | Negativicutes | Selenomonadales | Veillonellaceae | Dialister | −1.7 | (−1.9, −1.5) | <0.0001 |
| Bacteria | Firmicutes | Negativicutes | Selenomonadales | Veillonellaceae | Megasphaera | −1.6 | (−1.6, −1.6) | <0.0001 |
| Bacteria | Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | −1 | (−1.7, −0.3) | 0.03 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Peptostreptococcaceae | Romboutsia | −0.6 | (−0.6, −0.6) | <0.0001 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Christensenellaceae | Christensenellaceae | −0.6 | (−0.8, −0.3) | 0.005 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | −0.6 | (−0.9, −0.3) | 0.01 |
| Fungi | Mucoromycota | Mucoromycetes | Mucorales | Mucoraceae | Mucor | 0.2 | (0.2, 0.2) | <0.0001 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Anaerostipes | 0.3 | (0.2, 0.4) | 0.005 |
| Fungi | Ascomycota | Saccharomycetes | Saccharomycetales | Saccharomycetal | Candida | 0.3 | (0.3, 0.3) | <0.0001 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae | 0.7 | (0.3, 1.1) | 0.01 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Barnesiellaceae | Barnesiella | 0.7 | (0.5, 1) | <0.0001 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospira | 0.8 | (0.3, 1.4) | 0.04 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | 0.9 | (0.4, 1.3) | 0.006 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Alloprevotella | 1 | (1, 1) | <0.0001 |
| Fungi | Basidiomycota | Microbotryomycetes | Sporidiobolales | Sporidiobolaceae | Rhodotorula | 1 | (1, 1) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | 1.2 | (1, 1.4) | <0.0001 |
| Fungi | Basidiomycota | Malasseziomycetes | Malasseziales | Malasseziaceae | Malassezia | 1.5 | (1.4, 1.6) | <0.0001 |
| Fungi | Ascomycota | Saccharomycetes | Saccharomycetales | Dipodascaceae | Dipodascus | 2.6 | (2.6, 2.6) | <0.0001 |
| Fungi | Basidiomycota | Agaricomycetes | Agaricales | Agaricaceae | Agaricus | 4.2 | (4.2, 4.2) | <0.0001 |
| Kingdom | Phylum | Class | Order | Family | Genus | Odds Ratio (Log) | 95% CI | FDR Adjusted p-Value |
|---|---|---|---|---|---|---|---|---|
| Fungi | Basidiomycota | Malasseziomycetes | Malasseziales | Malasseziaceae | Malassezia | −3.8 | (−3.8, −3.7) | <0.0001 |
| Bacteria | Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | −2.6 | (−2.6, −2.6) | <0.0001 |
| Fungi | Ascomycota | Saccharomycetes | Saccharomycetales | Dipodascaceae | Dipodascus | −2.5 | (−2.5, −2.5) | <0.0001 |
| Bacteria | Firmicutes | Negativicutes | Selenomonadales | Veillonellaceae | Megasphaera | −2.4 | (−2.4, −2.3) | <0.0001 |
| Fungi | Ascomycota | Saccharomycetes | Saccharomycetales | Saccharomycetal | Candida | −1.7 | (−1.7, −1.7) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotellaceae | −1.6 | (−2.6, −0.7) | 0.0084 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Barnesiellaceae | Barnesiella | −1.4 | (−1.6, −1.2) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Alloprevotella | −1.2 | (−1.2, −1.2) | <0.0001 |
| Fungi | Basidiomycota | Agaricomycetes | Agaricales | Agaricaceae | Agaricus | −1.1 | (−1.1, −1.1) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Paraprevotella | −1 | (−1.6, −0.3) | 0.0315 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospira | −1 | (−1.4, −0.5) | 0.0062 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Clostridiacea | Clostridium | −0.8 | (−1.1, −0.5) | 0.0016 |
| Fungi | Mucoromycota | Mucoromycetes | Mucorales | Mucoraceae | Mucor | −0.5 | (−0.5, −0.4) | <0.0001 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Anaerostipes | 0.2 | (0.1, 0.4) | 0.0148 |
| Bacteria | Firmicutes | Clostridia | Clostridiales | Christensenellaceae | Christensenellaceae | 0.4 | (0.1, 0.7) | 0.0315 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Tannerellaceae | Parabacteroides | 0.7 | (0.2, 1.2) | 0.0315 |
| Bacteria | Firmicutes | Negativicutes | Selenomonadales | Veillonellaceae | Dialister | 0.8 | (0.6, 1) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | 1.4 | (1.3, 1.6) | <0.0001 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | NA | NA | 1.6 | (1, 2.2) | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enaud, R.; Cambos, S.; Viaud, E.; Guichoux, E.; Chancerel, E.; Marighetto, A.; Etchamendy, N.; Clark, S.; Mohammedi, K.; Cota, D.; et al. Gut Microbiota and Mycobiota Evolution Is Linked to Memory Improvement after Bariatric Surgery in Obese Patients: A Pilot Study. Nutrients 2021, 13, 4061. https://doi.org/10.3390/nu13114061
Enaud R, Cambos S, Viaud E, Guichoux E, Chancerel E, Marighetto A, Etchamendy N, Clark S, Mohammedi K, Cota D, et al. Gut Microbiota and Mycobiota Evolution Is Linked to Memory Improvement after Bariatric Surgery in Obese Patients: A Pilot Study. Nutrients. 2021; 13(11):4061. https://doi.org/10.3390/nu13114061
Chicago/Turabian StyleEnaud, Raphaël, Sophie Cambos, Esther Viaud, Erwan Guichoux, Emilie Chancerel, Aline Marighetto, Nicole Etchamendy, Samantha Clark, Kamel Mohammedi, Daniela Cota, and et al. 2021. "Gut Microbiota and Mycobiota Evolution Is Linked to Memory Improvement after Bariatric Surgery in Obese Patients: A Pilot Study" Nutrients 13, no. 11: 4061. https://doi.org/10.3390/nu13114061
APA StyleEnaud, R., Cambos, S., Viaud, E., Guichoux, E., Chancerel, E., Marighetto, A., Etchamendy, N., Clark, S., Mohammedi, K., Cota, D., Delhaes, L., & Gatta-Cherifi, B. (2021). Gut Microbiota and Mycobiota Evolution Is Linked to Memory Improvement after Bariatric Surgery in Obese Patients: A Pilot Study. Nutrients, 13(11), 4061. https://doi.org/10.3390/nu13114061







