Vitamin D Status and Risk of Cystic Fibrosis-Related Diabetes: A Retrospective Single Center Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Database and Categorization
2.3. Statistical Analysis
3. Results
3.1. Subject Demographics
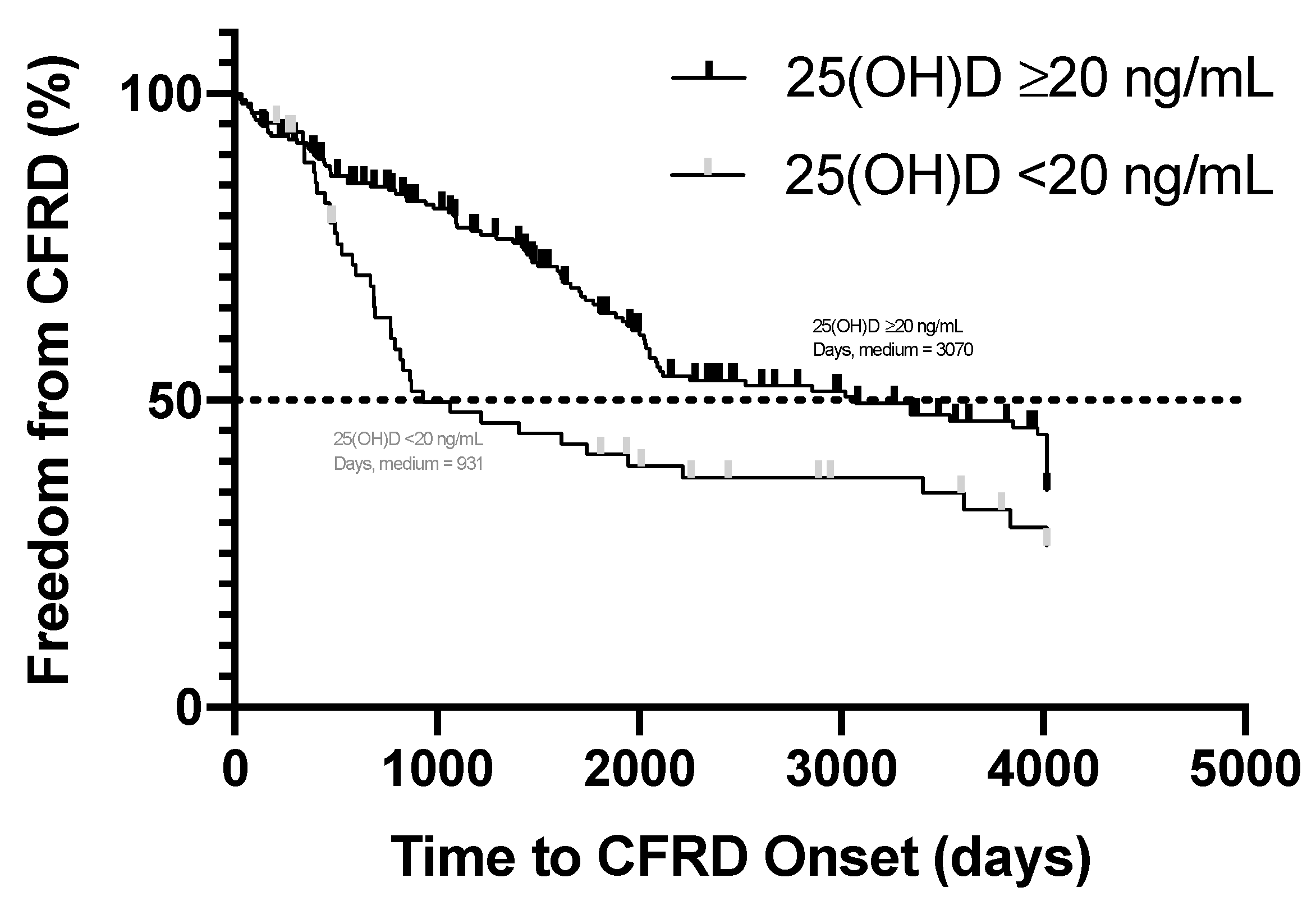
3.2. Time to Onset of Cystic Fibrosis-Related Diabetes
3.3. Influence of Other Risk Factors, Including Vitamin D Status, on the Development of CFRD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Strausbaugh, S.D.; Davis, P.B. Cystic fibrosis: A review of epidemiology and pathobiology. Clin. Chest Med. 2007, 28, 279–288. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolaso, C.; Groleau, V.; Schall, J.I.; Maqbool, A.; Mascarenhas, M.; Latham, N.E.; Dougherty, K.A.; Stallings, V.A. Fat-soluble vitamins in cystic fibrosis and pancreatic insufficiency: Efficacy of a nutrition intervention. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Donovan, D.S., Jr.; Papadopoulos, A.; Staron, R.B.; Addesso, V.; Schulman, L.; McGREGOR, C.; Cosman, F.; Lindsay, R.L.; Shane, E. Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Aris, R.M.; Renner, J.B.; Winders, A.D.; Buell, H.E.; Riggs, D.B.; Lester, G.E.; Ontjes, D.A. Increased rate of fractures and severe kyphosis: Sequelae of living into adulthood with cystic fibrosis. Ann. Intern. Med. 1998, 128, 186–193. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; González-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Kelly, A.; Stephenson, A.; Maguiness, K.; Enders, J.; Robinson, K.A.; Marshall, B.C.; Borowitz, D.; Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: Evidence-based recommendations from the Cystic Fibrosis Foundation. J. Clin. Endocrinol. Metab. 2012, 97, 1082–1093. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Pincikova, T.; Nilsson, K.; Moen, I.E.; Fluge, G.; Hollsing, A.; Knudsen, P.K.; Lindblad, A.; Mared, L.; Pressler, T.; Hjelte, L. Vitamin D deficiency as a risk factor for cystic fibrosis-related diabetes in the Scandinavian Cystic Fibrosis Nutritional Study. Diabetologia 2011, 54, 3007–3015. [Google Scholar] [CrossRef] [Green Version]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D deficiency and its treatment in cystic fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. 2), S66–S73. [Google Scholar] [CrossRef] [Green Version]
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H.; Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J. Am. Diet. Assoc. 2008, 108, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Coriati, A.; Dubois, C.L.; Phaneuf, M.; Mailhot, M.; Lavoie, A.; Berthiaume, Y.; Rabasa-Lhoret, R. Relationship between vitamin D levels and glucose tolerance in an adult population with cystic fibrosis. Diabetes Metab. 2016, 42, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [Green Version]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Cristelo, C.; Machado, A.; Sarmento, B.; Gama, F.M. The roles of vitamin D and cathelicidin in type 1 diabetes susceptibility. Endocr. Connect. 2021, 10, R1–R12. [Google Scholar] [CrossRef]
- Zella, J.B.; McCary, L.C.; DeLuca, H.F. Oral administration of 1, 25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch. Biochem. Biophys. 2003, 417, 77–80. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Ostadmohammadi, V.; Lankarani, K.B.; Tabrizi, R.; Kolahdooz, F.; Heydari, S.T.; Kavari, S.H.; Mirhosseini, M.; Mafi, A.; Dastorani, M. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res. 2018, 50, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Z.; Guo, J.; Pan, A.; Chen, C.; Liu, L.; Liu, G. Association of Serum 25-Hydroxyvitamin D Concentrations with All-Cause and Cause-Specific Mortality among Individuals with Diabetes. Diabetes Care 2021, 44, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wan, Z.; Guo, J.; Liu, L.; Pan, A.; Liu, G. Association between Serum 25-hydroxyvitamin D Concentrations and Mortality among Adults with Prediabetes. J. Clin. Endocrinol. Metab. 2021, 106, e4039–e4048. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Tangpricha, V.; Zhang, X. Serum Vitamin D Concentration ≥75 nmol/L Is Related to Decreased Cardiometabolic and Inflammatory Biomarkers, Metabolic Syndrome, and Diabetes; and Increased Cardiorespiratory Fitness in US Adults. Nutrients 2020, 12, 730. [Google Scholar] [CrossRef] [Green Version]
- Chesdachai, S.; Tangpricha, V. Treatment of vitamin D deficiency in cystic fibrosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 36–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priemel, M.; von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef]
- West, N.E.; Lechtzin, N.; Merlo, C.A.; Turowski, J.B.; Davis, M.E.; Ramsay, M.Z.; Watts, S.L.; Stenner, S.P.; Boyle, M.P. Appropriate goal level for 25-hydroxyvitamin D in cystic fibrosis. Chest 2011, 140, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Lukemire, J.; Chen, Y.; Binongo, J.N.G.; Judd, S.E.; Michalski, E.S.; Lee, M.J.; Walker, S.; Ziegler, T.R.; Tirouvanziam, R.; et al. Vitamin D for the Immune System in Cystic Fibrosis (DISC): A double-blind, multicenter, randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2019, 109, 544–553. [Google Scholar] [CrossRef]
- Bhimavarapu, A.; Deng, Q.; Bean, M.; Lee, N.; Ziegler, T.R.; Alvarez, J.; Tangpricha, V. Factors Contributing to Vitamin D Status at Hospital Admission for Pulmonary Exacerbation in Adults with Cystic Fibrosis. Am. J. Med. Sci. 2021, 361, 75–82. [Google Scholar] [CrossRef] [PubMed]
| All Subjects | Vitamin D Deficient (25(OH)D < 20 ng/mL) | Vitamin D Not Deficient (25(OH)D ≥ 20 ng/mL) | |
|---|---|---|---|
| Subjects n (%) | 253 | 64 (25.3) | 189 (74.7) |
| Age at Entry, y | 27.1 (±9.0) | 26.9 (±8.3) | 27.1 (±9.2) |
| Gender, male | 132 (52.2) | 39 (60.1) | 93 (49.2) |
| Gender, female | 121 (47.8) | 25 (39.1) | 96 (50.8) |
| Race, Caucasian or White a | 231 (91.3) | 47 (73.4) | 186 (97.4) |
| Race, African American or Black a | 18 (7.1) | 16 (25) | 2 (1.1) |
| Days without Diabetes Mellitus b | 1917.1 (±1394.5) | 2161.2 (±1627.6) | 2410.7 (±1667.6) |
| BMI, kg/m2 | 21.8 (±3.4) | 21.8 (±3.8) | 21.7 (±3.3) |
| BMI at goal c | 165 (65.2) | 45 (70.3) | 120 (63.5) |
| BMI d, <25 kg/m2 | 211 (83.4) | 53 (82.8) | 158 (83.6) |
| Developed CFRD e | 133 (52.6) | 41 (64.1) | 92 (48.7) |
| 25(OH)D, ng/mL | 31.8 (±14.0) | 12.5 (±4.4) | 36.9 (±15.5) |
| Compared Value | X2. Df | p-Value | |
|---|---|---|---|
| Vitamin D Deficiency a | CF Mutation Type b | 0.27, 2 | 0.88 |
| Vitamin D Deficiency | Develop CFRD | 4.54, 1 | 0.03 * |
| Vitamin D Deficiency | Gender, Male or Female | 2.63, 1 | 0.10 |
| Vitamin D Deficiency | Pancreatic Enzyme Usage c | 0.66, 1 | 0.42 |
| Develop CFRD d | Gender, Male or Female | 0.43, 1 | 0.51 |
| Vitamin D Insufficiency e | Develop CFRD | 0.03, 1 | 0.87 |
| Compared Value | t-Score, df | p-Value | |
|---|---|---|---|
| Develop CFRD a | First 25(OH)D, ng/mL b | 0.22, 251 | 0.83 |
| Develop CFRD | BMI, kg/m2 | 1.74, 251 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wu, M.; Alvarez, J.A.; Tangpricha, V. Vitamin D Status and Risk of Cystic Fibrosis-Related Diabetes: A Retrospective Single Center Cohort Study. Nutrients 2021, 13, 4048. https://doi.org/10.3390/nu13114048
Peng Y, Wu M, Alvarez JA, Tangpricha V. Vitamin D Status and Risk of Cystic Fibrosis-Related Diabetes: A Retrospective Single Center Cohort Study. Nutrients. 2021; 13(11):4048. https://doi.org/10.3390/nu13114048
Chicago/Turabian StylePeng, Yiqing, Malinda Wu, Jessica A. Alvarez, and Vin Tangpricha. 2021. "Vitamin D Status and Risk of Cystic Fibrosis-Related Diabetes: A Retrospective Single Center Cohort Study" Nutrients 13, no. 11: 4048. https://doi.org/10.3390/nu13114048
APA StylePeng, Y., Wu, M., Alvarez, J. A., & Tangpricha, V. (2021). Vitamin D Status and Risk of Cystic Fibrosis-Related Diabetes: A Retrospective Single Center Cohort Study. Nutrients, 13(11), 4048. https://doi.org/10.3390/nu13114048






