Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm
Abstract
1. Introduction
2. Materials and Methods
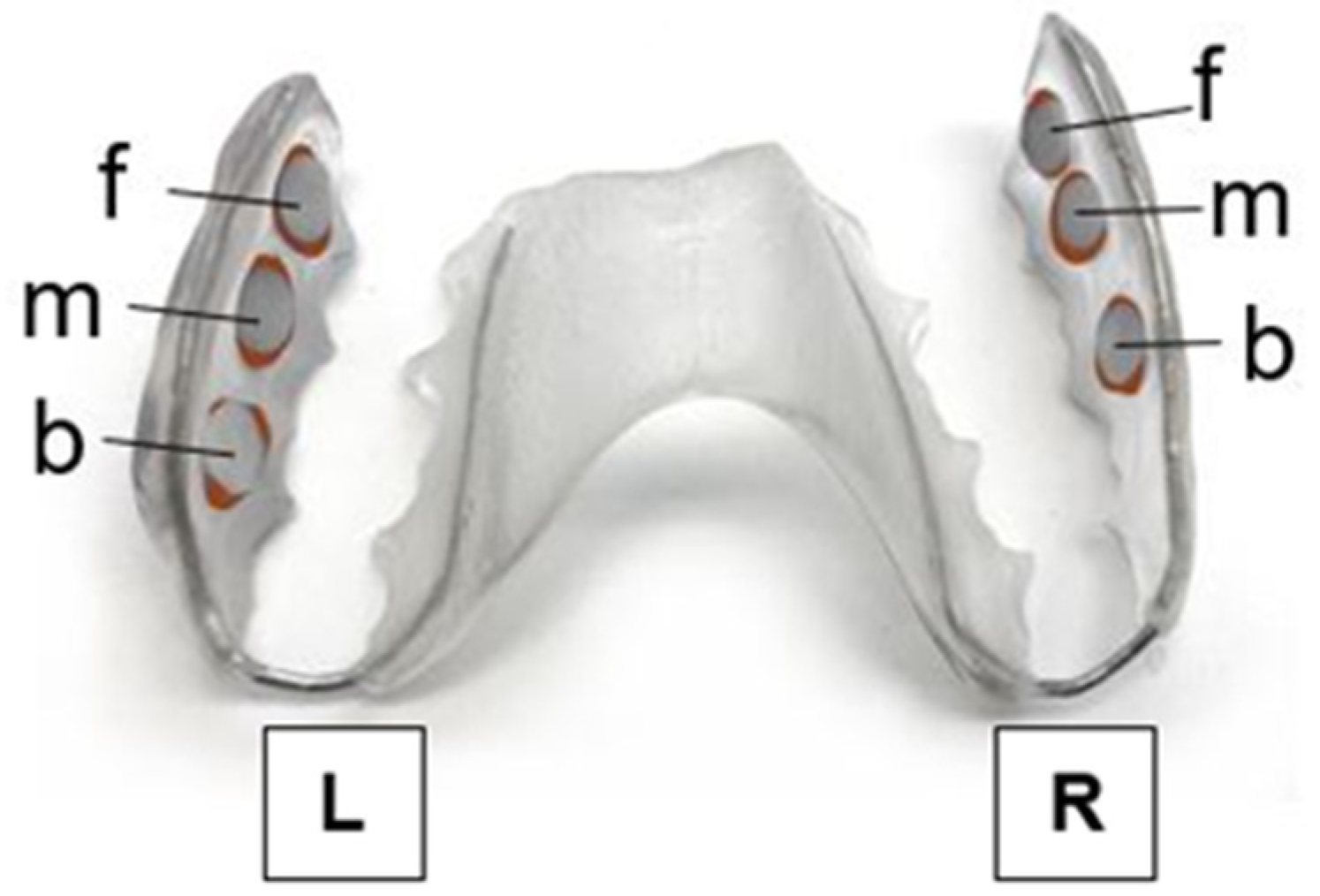
2.1. Selection of Study Participants and Test Specimens
2.2. Extract Preparation
2.3. Protocol for Treatment of the Initial Adhesion
2.4. Determination of Colony-Forming Units (CFU)
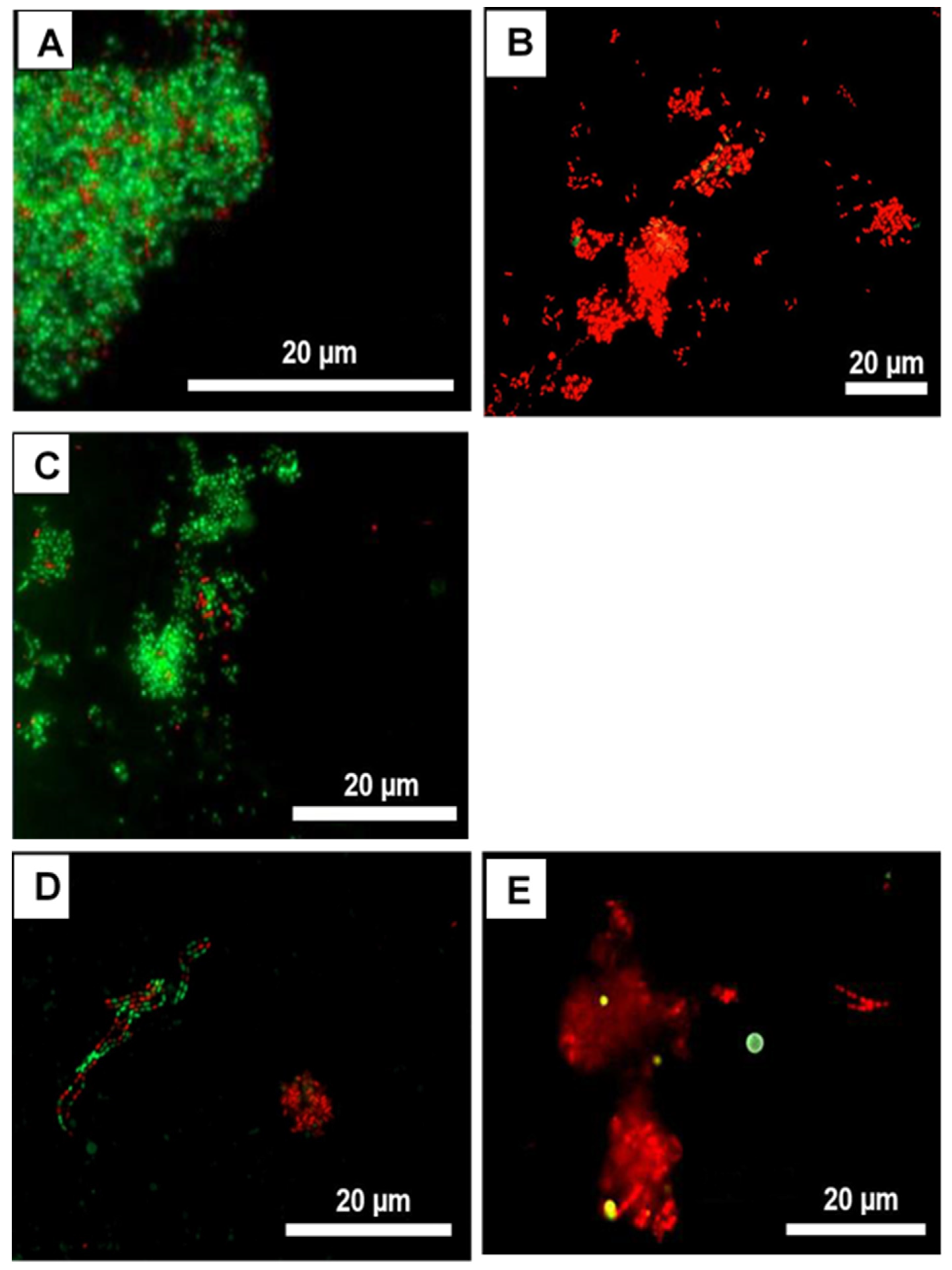
2.5. Live/Dead Staining and Fluorescence Microscopy
2.6. Statistical Analysis
3. Results
3.1. I. viscosa Extract Significantly Decreased the Viable Counts of Oral Microorganisms during Initial Adhesion
3.2. Live/Dead Assays Revealed High Bactericidal Activity for I. viscosa Extract against Oral Initial Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22. [Google Scholar] [CrossRef]
- Karygianni, L.; Al-Ahmad, A.; Argyropoulou, A.; Hellwig, E.; Anderson, A.C.; Skaltsounis, A.L. Natural Antimicrobials and Oral Microorganisms: A Systematic Review on Herbal Interventions for the Eradication of Multispecies Oral Biofilms. Front. Microbiol. 2015, 6, 1529. [Google Scholar] [CrossRef]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2015, 6, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. Biomed. Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Acquired resistance to chlorhexidine—Is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Gent, J.F.; Frank, M.E.; Hettinger, T.P. Taste confusions following chlorhexidine treatment. Chem. Senses 2002, 27, 73–80. [Google Scholar] [CrossRef][Green Version]
- Eley, B.M. Antibacterial agents in the control of supragingival plaque—A review. Br. Dent. J. 1999, 186, 286–296. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Karygianni, L.; Ruf, S.; Follo, M.; Hellwig, E.; Bucher, M.; Anderson, A.C.; Vach, K.; Al-Ahmad, A. Novel Broad-Spectrum Antimicrobial Photoinactivation of In Situ Oral Biofilms by Visible Light plus Water-Filtered Infrared A. Appl. Environ. Microbiol. 2014, 80, 7324–7336. [Google Scholar] [CrossRef]
- Cai, H.; Chen, J.; Panagodage Perera, N.K.; Liang, X. Effects of Herbal Mouthwashes on Plaque and Inflammation Control for Patients with Gingivitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Evid. Based Complement. Alternat. Med. 2020, 2020, 2829854. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Groppo, F.C.; Bergamaschi, C.d.C.; Cogo, K.; Franz-Montan, M.; Motta, R.H.L.; de Andrade, E.D. Use of phytotherapy in dentistry. Phytother. Res. 2008, 22, 993–998. [Google Scholar] [CrossRef]
- Maoz, M.; Neeman, I. Effect of Inula viscosa extract on chitin synthesis in dermatophytes and Candida albicans. J. Ethnopharmacol. 2000, 71, 479–482. [Google Scholar] [CrossRef]
- Gökbulut, A.; Ozhan, O.; Satilmiş, B.; Batçioğlu, K.; Günal, S.; Sarer, E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat. Prod. Commun. 2013, 8, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Zarga, M.H.A.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Omezzine, F. In vitro assessment of Inula spp. organic extracts for their antifungal activity against some pathogenic and antagonistic fungi. Afr. J. Microbiol. Res. 2011, 5, 3527–3531. [Google Scholar] [CrossRef]
- Hertel, S.; Graffy, L.; Pötschke, S.; Basche, S.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Inula viscosa on the pellicle’s protective properties and initial bioadhesion in-situ. Arch. Oral Biol. 2016, 71, 87–96. [Google Scholar] [CrossRef]
- Jahn, R.; Schönfelder, P. Exkursionsflora für Kreta; Ulmer: Stuttgart, Germany, 1995; ISBN 3800134780. [Google Scholar]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Hernández, V.; Recio, M.C.; Máñez, S.; Giner, R.M.; Ríos, J.-L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar] [CrossRef]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A-D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [CrossRef]
- Máñez, S.; Hernández, V.; Giner, R.-M.; Ríos, J.-L.; Recio, M.D.C. Inhibition of pro-inflammatory enzymes by inuviscolide, a sesquiterpene lactone from Inula viscosa. Fitoterapia 2007, 78, 329–331. [Google Scholar] [CrossRef]
- Karygianni, L.; Follo, M.; Hellwig, E.; Burghardt, D.; Wolkewitz, M.; Anderson, A.; Al-Ahmad, A. Microscope-based imaging platform for large-scale analysis of oral biofilms. Appl. Environ. Microbiol. 2012, 78, 8703–8711. [Google Scholar] [CrossRef]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmüller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2013, 17, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Müller, N.; Wiedmann-Al-Ahmad, M.; Sava, I.; Hübner, J.; Follo, M.; Schirrmeister, J.; Hellwig, E. Endodontic and salivary isolates of Enterococcus faecalis integrate into biofilm from human salivary bacteria cultivated in vitro. J. Endod. 2009, 35, 986–991. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Follo, M.; Selzer, A.-C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.L.; Boom, G.; Arends, J. Calcium distribution in human and bovine surface enamel. Caries Res. 1973, 7, 349–359. [Google Scholar] [CrossRef]
- Nakamichi, I.; Iwaku, M.; Fusayama, T. Bovine teeth as possible substitutes in the adhesion test. J. Dent. Res. 1983, 62, 1076–1081. [Google Scholar] [CrossRef]
- Teruel, J.d.D.; Alcolea, A.; Hernández, A.; Ruiz, A.J.O. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral Sci. 2006, 19, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.-S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Spitzmüller, B.; Lux, H.C.; Altenburger, M.; Al-Ahmad, A.; Hannig, M. Efficacy of enzymatic toothpastes for immobilisation of protective enzymes in the in situ pellicle. Arch. Oral Biol. 2010, 55, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Attin, T.; Hannig, M.; Henze, E.; Brinkmann, K.; Zech, R. Immobilisation and activity of human alpha-amylase in the acquired enamel pellicle. Arch. Oral Biol. 2004, 49, 469–475. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. Effects of commonly used food preservatives on biofilm formation of Streptococcus mutans in vitro. Arch. Oral Biol. 2008, 53, 765–772. [Google Scholar] [CrossRef]
- Grande, M.; Piera, F.; Cuenca, A.; Torres, P.; Bellido, I.S. Flavonoids from Inula viscosa. Planta Med. 1985, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Mayer, K.; Roitman, J.N. Exudate flavonoids of Inula viscosa. Phytochemistry 1991, 30, 2445–2446. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H. Isoflavanones from the heartwood of Swartzia polyphylla and their antibacterial activity against cariogenic bacteria. Chem. Pharm. Bull. 1992, 40, 2970–2974. [Google Scholar] [CrossRef]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmüller, B.; Al-Ahmad, A.; Hannig, M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008, 36, 540–545. [Google Scholar] [CrossRef]
- Little, J.W. Complementary and alternative medicine: Impact on dentistry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 137–145. [Google Scholar] [CrossRef]
- West, I.; Maibach, H.I. Contact urticaria syndrome from multiple cosmetic components. Contact Dermat. 1995, 32, 121. [Google Scholar] [CrossRef]
- Hadley, S.; Petry, J.J. Valerian. Am. Fam. Phys. 2003, 67, 1755–1758. [Google Scholar] [PubMed]
- Huntley, A.L.; Thompson Coon, J.; Ernst, E. The safety of herbal medicinal products derived from Echinacea species: A systematic review. Drug Saf. 2005, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shalom, R.; Bergman, M.; Grossman, S.; Azzam, N.; Sharvit, L.; Fares, F. Inula Viscosa Extract Inhibits Growth of Colorectal Cancer Cells in vitro and in vivo Through Induction of Apoptosis. Front. Oncol. 2019, 9, 227. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of Inula viscosa Aiton Leaves. J. Food Saf. 2016, 36, 77–88. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients 2021, 13, 4029. https://doi.org/10.3390/nu13114029
Kurz H, Karygianni L, Argyropoulou A, Hellwig E, Skaltsounis AL, Wittmer A, Vach K, Al-Ahmad A. Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients. 2021; 13(11):4029. https://doi.org/10.3390/nu13114029
Chicago/Turabian StyleKurz, Hannah, Lamprini Karygianni, Aikaterini Argyropoulou, Elmar Hellwig, Alexios Leandros Skaltsounis, Annette Wittmer, Kirstin Vach, and Ali Al-Ahmad. 2021. "Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm" Nutrients 13, no. 11: 4029. https://doi.org/10.3390/nu13114029
APA StyleKurz, H., Karygianni, L., Argyropoulou, A., Hellwig, E., Skaltsounis, A. L., Wittmer, A., Vach, K., & Al-Ahmad, A. (2021). Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients, 13(11), 4029. https://doi.org/10.3390/nu13114029






