Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review
Abstract
:1. Introduction
1.1. Inflammatory Bowel Disease
1.2. IBD-Associated Anemia
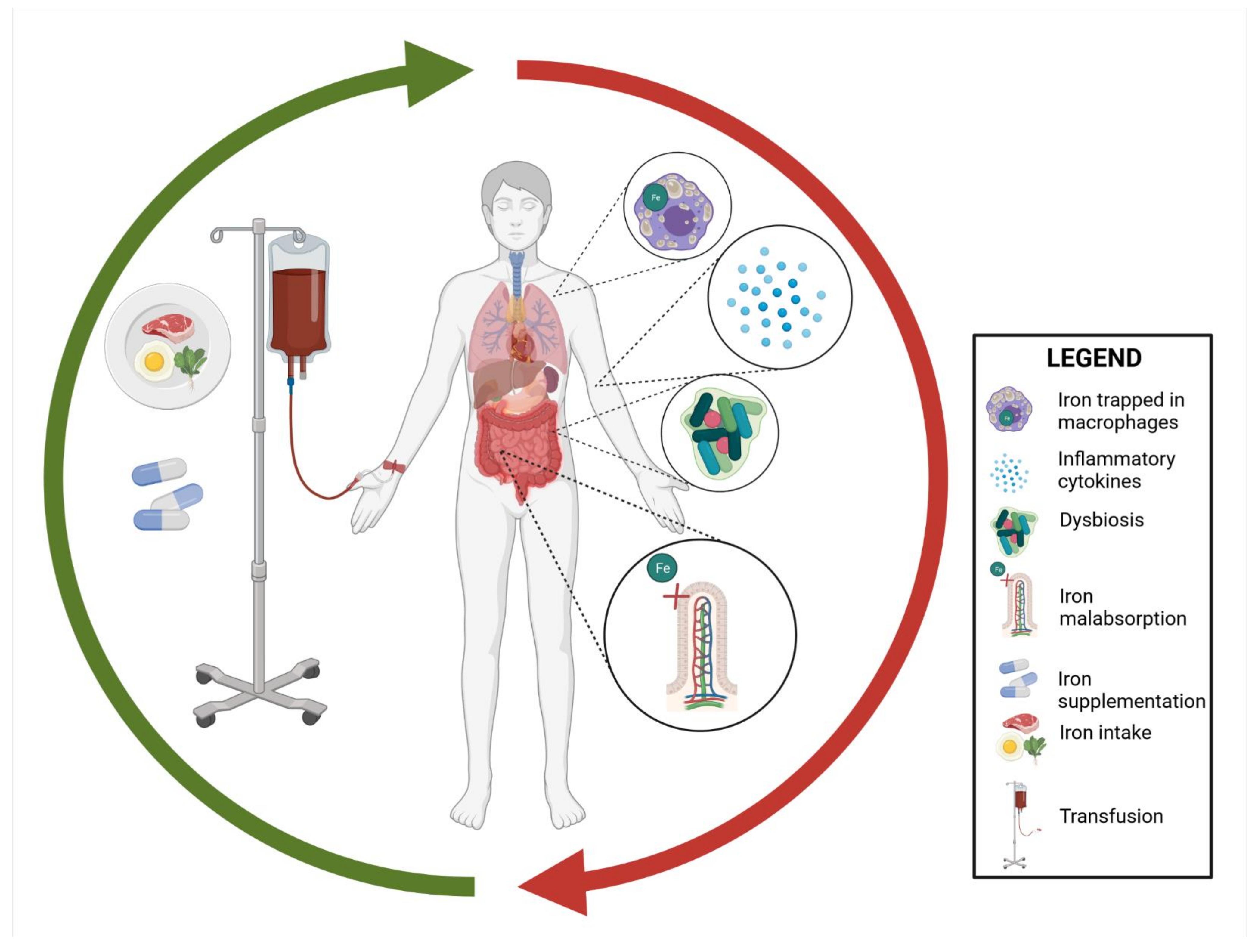
2. Iron Metabolism
- Impaired cognitive performance;
- Thyroid hormone dysfunction;
- Catecholamine dysfunction;
- Increased risk of infection;
- Increased exposure to stress and depression in postpartum anemia;
- Disturbances in the functioning of neurotransmitters;
- Poorer outcomes of cognitive and motor development in children;
- Loss of libido, deterioration of sex life.
3. Iron in the Diet of IBD Patients
3.1. Iron and Its Requirements
3.2. Dietary Sources of IBD
- Patients should eat meat and fish. The best way to prepare meals is by boiling and roasting (due to the possible gastrointestinal symptoms);
- Good sources of iron are also green vegetables; however, it should be noted that vegetables contain many compounds inhibiting iron absorption;
- Legumes contain relatively high amounts of iron. Nevertheless, legumes are hard to digest and may be poorly tolerated by patients suffering from IBD;
- Patients should reduce the intake of tea and coffee because some of the compounds present in coffee and tea inhibit iron absorption;
- Patients should eat iron-rich plant products with food containing vitamin C, for example, spinach (also a source of iron) with lemon juice (vitamin C source), which will increase iron absorption.
4. Iron and Microbiota
5. Iron Supplementation in IBD
- 1st generation—high molecular weight iron dextran;
- 2nd generation—low molecular weight iron dextran;
- a.
- Ferrous gluconate (Ferrlecit);
- b.
- Iron sucrose (venofer);
- 3rd generation;
- c.
- Ferumoxytol;
- d.
- Iron carboxymaltose (Ferinject);
- e.
- Iron isomaltoside (Monover).
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aksan, A.; Beales, I.L.; Baxter, G.; Ramirez de Arellano, A.; Gavata, S.; Valentine, W.J.; Hunt, B. Evaluation of the cost-effectiveness of iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease in the UK. CEOR 2021, 13, 541–552. [Google Scholar] [CrossRef]
- Gargallo-Puyuelo, C.J.; Alfambra, E.; García-Erce, J.A.; Gomollon, F. Iron treatment may be difficult in inflammatory diseases: Inflammatory bowel disease as a paradigm. Nutrients 2018, 10, 1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L.; ECCO—EpiCom. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, M.; Barańska, M.; Małecka-Panas, E.; Talar-Wojnarowska, R. The prevalence, characteristics, and determinants of anaemia in newly diagnosed patients with inflammatory bowel disease. Prz. Gastroenterol. 2019, 14, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Halfvarson, J.; Rodríguez-Lago, I.; Hedin, C.R.H.; Jess, T.; Dubinsky, M.; Croitoru, K.; Colombel, J.-F. Results of the seventh scientific workshop of ECCO: Precision medicine in ibd-prediction and prevention of inflammatory bowel disease. J. Crohn’s Colitis 2021, 15, 1443–1454. [Google Scholar] [CrossRef]
- Akhuemonkhan, E.; Parian, A.; Miller, K.; Hanauer, S.; Hutfless, S. Prevalence and screening for anaemia in mild to moderate Crohn’s disease and ulcerative colitis in the United States, 2010–2014. BMJ Open Gastroenterol. 2017, 4, e000155. [Google Scholar] [CrossRef] [Green Version]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Ramos-Rivers, C.; Regueiro, M.; Koutroumpakis, E.; Click, B.; Schwartz, M.; Swoger, J.; Baidoo, L.; Hashash, J.G.; Barrie, A.; et al. Five-year period prevalence and characteristics of anemia in a large us inflammatory bowel disease cohort. J. Clin. Gastroenterol. 2016, 50, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Foteinogiannopoulou, K.; Karmiris, K.; Axiaris, G.; Velegraki, M.; Gklavas, A.; Kapizioni, C.; Karageorgos, C.; Kateri, C.; Katsoula, A.; Kokkotis, G.; et al. The burden and management of anemia in Greek patients with inflammatory bowel disease: A retrospective, multicenter, observational study. BMC Gastroenterol. 2021, 21, 269. [Google Scholar] [CrossRef]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; de la Morena, F.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in European countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef]
- Resál, T.; Farkas, K.; Molnár, T. Iron deficiency anemia in inflammatory bowel disease: What do we know? Front. Med. 2021, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Aksan, A.; Schoepfer, A.; Juillerat, P.; Vavricka, S.; Bettencourt, M.; Ramirez de Arellano, A.; Gavata, S.; Morin, N.; Valentine, W.J.; Hunt, B. Iron formulations for the treatment of iron deficiency anemia in patients with inflammatory bowel disease: A cost-effectiveness analysis in Switzerland. Adv. Ther. 2021, 38, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Henriksson, I.; Brus, O.; Zhulina, Y.; Nyhlin, N.; Tysk, C.; Montgomery, S.; Halfvarson, J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: A population-based cohort study. Aliment. Pharmacol. Ther. 2018, 48, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; di Pietro, M.L.; Gasbarrini, A.; Boccia, S.; Poscia, A.; Stojanovic, J.; Kheiraoui, F.; Proli, E.M.; Fabrizio, L.; Favaretti, C.; et al. Sustainability of endovenous iron deficiency anaemia treatment: Hospital-based health technology assessment in ibd patients. BioMed Res. Int. 2017, 2017, e3470893. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.A.; Chun, J.; Im, J.P.; Lee, H.J.; Han, K.; Soh, H.; Park, S.; Kim, J.S. Anemia is associated with the risk of Crohn’s disease, not ulcerative colitis: A nationwide population-based cohort study. PLoS ONE 2020, 15, e0238244. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Reyes, E.; Ofman, J. Prevalence and outcomes of anemia in inflammatory bowel disease: A systematic review of the literature. Am. J. Med. 2004, 116, 44S–49S. [Google Scholar] [CrossRef] [PubMed]
- De Antunes, C.V.A.; Neto, A.E.H.; de Nascimento, C.R.A.; Chebli, L.A.; Moutinho, I.L.D.; do Pinheiro, B.V.; Reboredo, M.M.; Malaguti, C.; Castro, A.C.S.; Chebli, J.M.F. Anemia in inflammatory bowel disease outpatients: Prevalence, risk factors, and etiology. BioMed Res. Int. 2015, 2015, 728925. [Google Scholar] [CrossRef] [Green Version]
- Gomollón, F.; Gisbert, J.P. Anemia and inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 4659–4665. [Google Scholar] [CrossRef]
- Bengi, G.; Keyvan, H.; Durmaz, S.B.; Akpınar, H. Frequency, types, and treatment of anemia in Turkish patients with inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 4186–4196. [Google Scholar] [CrossRef]
- Niepel, D.; Klag, T.; Malek, N.P.; Wehkamp, J. Practical guidance for the management of iron deficiency in patients with inflammatory bowel disease. Therap. Adv. Gastroenterol. 2018, 11, 1756284818769074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.; Dignass, A.U. Management of iron deficiency anemia in inflammatory bowel disease—A practical approach. Ann. Gastroenterol. 2013, 26, 104–113. [Google Scholar] [PubMed]
- Shu, W.; Pang, Z.; Xu, C.; Lin, J.; Li, G.; Wu, W.; Sun, S.; Li, J.; Li, X.; Liu, Z. Anti-tnf-α monoclonal antibody therapy improves anemia through downregulating hepatocyte hepcidin expression in inflammatory bowel disease. Mediat. Inflamm. 2019, 2019, 4038619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybyszewska, J.; Zekanowska, E. The role of hepcidin, ferroportin, hcp1, and dmt1 protein in iron absorption in the human digestive tract. Prz. Gastroenterol. 2014, 9, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitha, S.; Bashir, M.; Ali, T. Iron deficiency anemia in inflammatory bowel disease. World J. Gastrointest. Pathophysiol. 2015, 6, 62–72. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- De Benoist, B.; World Health Organization; Centers for Disease Control and Prevention (U.S.). Worldwide Prevalence of Anaemia 1993–2005 of: WHO Global Database of Anaemia; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159665-7. [Google Scholar]
- Yokoi, K. Investigating the essentiality and requirements of iron from the ancient to the present. Biol. Trace Elem. Res. 2019, 188, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wiafe, M.A.; Apprey, C.; Annan, R.A. Patterns of dietary iron intake, iron status, and predictors of haemoglobin levels among early adolescents in a rural ghanaian district. J. Nutr. Metab. 2020, 2020, 3183281. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization: Rome, Italy, 2004; ISBN 978-92-4-154612-6. [Google Scholar]
- Śliwińska, A.; Luty, J.; Aleksandrowicz-Wrona, E.; Małgorzewicz, S. Iron status and dietary iron intake in vegetarians. Adv. Clin. Exp. Med. 2018, 27, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Skolmowska, D.; Głąbska, D. Analysis of heme and non-heme iron intake and iron dietary sources in adolescent menstruating females in a national polish sample. Nutrients 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Office of Dietary Supplements—Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-Consumer/ (accessed on 26 June 2021).
- Lipiński, P.; Starzyński, R.R.; Styś, A.; Staroń, R.; Gajowiak, A. Niedokrwistość na tle niedoboru żelaza w diecie. Kosm. Probl. Nauk. Biol. 2014, 63, 373–379. [Google Scholar]
- Banjari, I.; Hjartåker, A. Dietary sources of iron and vitamin b12: Is this the missing link in colorectal carcinogenesis? Med. Hypotheses 2018, 116, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zijp, I.M.; Korver, O.; Tijburg, L.B.M. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements—Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 26 June 2021).
- Hunt, J.R. Dietary and physiological factors that affect the absorption and bioavailability of iron. Int. J. Vitam. Nutr. Res. 2005, 75, 375–384. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Carrier, J.; Aghdassi, E.; Cullen, J.; Allard, J.P. Iron supplementation increases disease activity and vitamin e ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2002, 132, 3146–3150. [Google Scholar] [CrossRef] [Green Version]
- Ettreiki, C.; Gadonna-Widehem, P.; Mangin, I.; Coëffier, M.; Delayre-Orthez, C.; Anton, P.M. Juvenile ferric iron prevents microbiota dysbiosis and colitis in adult rodents. World J. Gastroenterol. 2012, 18, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with ibd. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef]
- Tompkins, G.R.; O’Dell, N.L.; Bryson, I.T.; Pennington, C.B. The effects of dietary ferric iron and iron deprivation on the bacterial composition of the mouse intestine. Curr. Microbiol. 2001, 43, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calvé, A.; Santos, M.M. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, D.; Vaghefi-Vaezzadeh, N.; Roland, N.; Bouvard, G.; Arhan, P.; Bureau, F.; Neuville, D.; Maubois, J.L. Influence of short-chain fatty acids on iron absorption by proximal colon. Scand. J. Gastroenterol. 2002, 37, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Wandersman, C.; Stojiljkovic, I. Bacterial heme sources: The role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 2000, 3, 215–220. [Google Scholar] [CrossRef]
- Otto, B.R.; Sparrius, M.; Verweij-van Vught, A.M.; MacLaren, D.M. Iron-regulated outer membrane protein of bacteroides fragilis involved in heme uptake. Infect. Immun. 1990, 58, 3954–3958. [Google Scholar] [CrossRef] [Green Version]
- Archibald, F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol. Lett. 1983, 19, 29–32. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Clark, H.M.; McIlvin, M.; Vazquez, C.; Palmere, S.L.; Grab, D.J.; Seshu, J.; Hart, P.J.; Saito, M.; Culotta, V.C. A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 2013, 288, 8468–8478. [Google Scholar] [CrossRef] [Green Version]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.S.; Pleasants, J.R.; Wostmann, B.S. Effect of intestinal microflora on iron and zinc metabolism, and on activities of metalloenzymes in rats. J. Nutr. 1972, 102, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, R.H.; Conrad, M.E.; Crosby, W.H. Measurement of total body iron in animals using whole-body liquid scintillation detectors. Proc. Soc. Exp. Biol. Med. 1962, 111, 115–119. [Google Scholar] [CrossRef]
- Mücke, V.; Mücke, M.M.; Raine, T.; Bettenworth, D. Diagnosis and treatment of anemia in patients with inflammatory bowel disease. Ann. Gastroenterol. 2017, 30, 15–22. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Soendergaard, C.; Vikner, M.E.; Weiss, G. Rational management of iron-deficiency anaemia in inflammatory bowel disease. Nutrients 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, O.H.; Ainsworth, M.; Coskun, M.; Weiss, G. Management of iron-deficiency anemia in inflammatory bowel disease: A systematic review. Medicine 2015, 94, e963. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Peyrin-Biroulet, L.; Danese, S. Oral iron for ibd patients: Lessons learned at time of Covid-19 pandemic. J. Clin. Med. 2020, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Tsantes, A.; Peyrin-Biroulet, L.; Danese, S. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease. Medicine 2016, 95, e2308. [Google Scholar] [CrossRef]
- Stein, J.; Bager, P.; Befrits, R.; Gasche, C.; Gudehus, M.; Lerebours, E.; Magro, F.; Mearin, F.; Mitchell, D.; Oldenburg, B.; et al. Anaemia management in patients with inflammatory bowel disease: Routine practice across nine European countries. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Hoffman, C.; Vel, S.; Greco, M.; Szabo, H.; Wilson, B.; Avedano, L. Anaemia from a patient perspective in inflammatory bowel disease: Results from the european federation of Crohn’s and ulcerative colitis association’s online survey. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1385–1391. [Google Scholar] [CrossRef]

| Type of Anemia | Cause |
|---|---|
| IDA | • Iron loss from bleeding |
| • Decreased iron intake from enterocytes | |
| • Impaired iron absorption | |
| ACD | • Inhibition of erythropoiesis due to inflammatory cytokines |
| • Iron trapped in macrophages | |
| • Dysfunction of iron transport | |
| Vitamin B12 and foliate deficiency-associated anemia | • Malabsorption |
| • Extensive small bowel resection | |
| Drug-induced anemia | • Thiopurines, Sulfasalazine |
| • Methotrexate |
| Group | Age (Years) | Total Requirements (95th Percentile, mg/Day) | Recommended Iron Intake for Different Diet Bioavailability (95th Percentile, mg/Day) | |
|---|---|---|---|---|
| 15% (High Bioavailability) | 10% (Low Bioavailability) | |||
| Infants and children | 0.5–1.0 | 0.93 | 6.20 | 9.30 |
| 1–3 | 0.58 | 3.90 | 5.80 | |
| 7–10 | 0.63 | 4.20 | 6.30 | |
| 11–14 | 0.89 | 5.90 | 8.90 | |
| Females | 11–14 PM | 1.40 | 9.30 | 14.00 |
| 11–14 | 3.27 | 21.80 | 32.70 | |
| 15–17 | 3.10 | 20.70 | 31.00 | |
| 18+ | 2.94 | 19.60 | 29.40 | |
| Postmenopausal females | - | 1.13 | 7.50 | 11.30 |
| Lactating females | - | 1.50 | - | - |
| Males | 11–14 | 1.46 | 9.70 | 14.60 |
| 15–17 | 1.88 | 12.50 | 18.80 | |
| 18+ | 1.37 | 9.1 | 13.70 | |
| Product | Iron Content (mg/100 g) |
|---|---|
| Pork liver | 19 |
| Cow’s milk | 0.03 |
| Herring | 1.1 |
| Lentils | 8.6 |
| Chocolate | 0.3–0.5 |
| Beef | 3.1 |
| Egg | 1.3 |
| Broccoli | 1.1 |
| Pasta | 2.1 |
| Type of Iron | Factors Determining Iron Status | |
|---|---|---|
| Heme iron | Amount of dietary heme iron Contents of calcium in meal Food preparation | |
| Non-heme | Balance between enhancing and inhibiting dietary factors Amount of available non-heme iron | |
| Enhancing factors | Inhibiting factors | |
| Non-heme iron | Ascorbic acid | Phytate and phosphates |
| Meat, fish, seafood | Iron-binding phenolic compounds | |
| Fermented vegetables or sauces (e.g., soy sauce) | Calcium | |
| Soya | ||
| Fe 2+ | Fe3+ |
|---|---|
| Ferrous fumarate | Iron protein sucinylate |
| Ferrous sulphate | Iron polymaltose complex |
| Ferrous gluconate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahadea, D.; Adamczewska, E.; Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review. Nutrients 2021, 13, 4008. https://doi.org/10.3390/nu13114008
Mahadea D, Adamczewska E, Ratajczak AE, Rychter AM, Zawada A, Eder P, Dobrowolska A, Krela-Kaźmierczak I. Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review. Nutrients. 2021; 13(11):4008. https://doi.org/10.3390/nu13114008
Chicago/Turabian StyleMahadea, Dagmara, Ewelina Adamczewska, Alicja Ewa Ratajczak, Anna Maria Rychter, Agnieszka Zawada, Piotr Eder, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review" Nutrients 13, no. 11: 4008. https://doi.org/10.3390/nu13114008
APA StyleMahadea, D., Adamczewska, E., Ratajczak, A. E., Rychter, A. M., Zawada, A., Eder, P., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review. Nutrients, 13(11), 4008. https://doi.org/10.3390/nu13114008







