The Influence of Supplementation with Zinc in Micro and Nano Forms on the Metabolism of Fatty Acids in Livers of Rats with Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Microsomes from Liver
2.3. Determination of Fatty Acids Methyl Esters in Hepatic Microsomes
2.4. Determination of Desaturases’ Activity
- D6D = (γ-linolenic acid (GLA), C18:3, n-6)/(LA, C18:2, n-6)
- D5D = (AA, C20:4, n-6)/(Dihomo-γ-linolenic acid (DGLA), C20:3, n-6)
- D9D-16 = (Palmitoleic acid, C16:1, n-7)/(Palmitic acid (PA), C16:0)
- D9D-18 = (Oleic acid (OL), C18:1, n-9)/(Stearic acid, C18:0)
- D9D total = ((Palmitoleic acid, C16:1, n-7) + (OL, C18:1, n-9))/((PA, C16:0) + (Stearic acid, C18:0))
2.5. Prostaglandin E2 Content in Liver
2.6. Determination of Fatty Acid Metabolites
2.7. Determination of Squalene, Cholesterol and Cholesterol-Oxidation Products
2.8. Statistical Analysis
3. Results
3.1. Fatty Acids Profile in Hepatic Microsomes
3.2. Analysis of Enzymes Activity
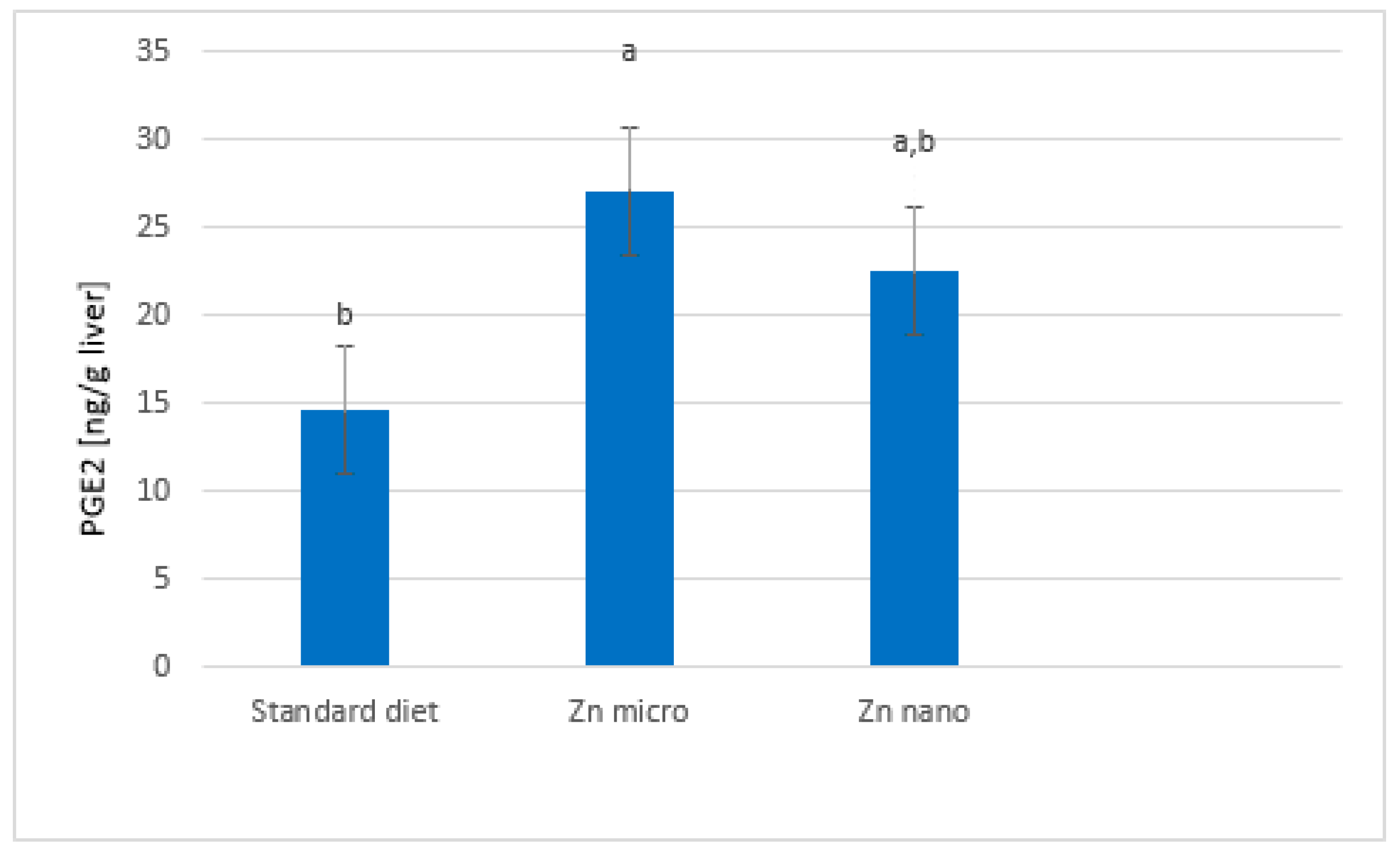
3.3. Liver PGE2 Levels
3.4. Determination of Fatty Acid Metabolites
3.5. Determination of Cholesterol and Oxysterols
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Breast Cancer Now Most Common Form of Cancer: WHO Taking Action. Available online: https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action (accessed on 9 August 2021).
- Tang, X.; Loc, W.S.; Dong, C. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Nurgalieva, Z.; Liu, C.C.; Du, X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med. Oncol. 2011, 28, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2019, 51, 226–234. [Google Scholar] [CrossRef]
- Lappano, R.; Malaguarnera, R.; Belfiore, A.; Maggiolini, M. Recent advances on the stimulatory effects of metals in breast cancer. Mol. Cell. Endocrinol. 2017, 457, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jouybari, L.; Kiani, F.; Akbari, A.; Sanagoo, A.; Sayehmiri, F.; Aaseth, J.; Chartrand, M.S.; Sayehmiri, K.; Chirumbolo, S.; Bjørklundet, G. A meta-analysis of zinc levels in breast cancer. J. Trace Elem. Med. Biol. 2019, 56, 90–99. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef]
- Knez, M.; Pantovic, A.; Zekovic, M.; Pavlovic, Z.; Glibetic, M.; Zec, M. Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects? Nutrients 2020, 12, 93. [Google Scholar] [CrossRef]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef]
- Chimhashu, T.; Malan, L.; Baumgartner, J.; van Jaarsveld, P.J.; Galetti, V.; Moretti, D.; Smuts, C.M.; Zimmermann, M.B. Sensitivity of fatty acid desaturation and elongation to plasma zinc concentration: A randomised controlled trial in beninese children. Br. J. Nutr. 2018, 119, 610–619. [Google Scholar] [CrossRef]
- Yary, T.; Voutilainen, S.; Tuomainen, T.P.; Ruusunen, A.; Nurmi, T.; Virtanen, J.K. Serum n-6 polyunsaturated fatty acids, delta5- and delta6-desaturase activities, and risk of incident type 2 diabetes in men: The Kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 2016, 103, 1337–1343. [Google Scholar] [CrossRef]
- Stawarska, A.; Czerwonka, M.; Wyrębiak, R.; Wrzesień, R.; Bobrowska-Korczak, B. Zinc Affects Cholesterol Oxidation Products and Fatty Acids Composition in Rats’ Serum. Nutrients 2021, 13, 1563. [Google Scholar] [CrossRef]
- Gonzalez-Nunez, D.; Claria, J.; Rivera, F.; Poch, E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension 2001, 37, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Zukowski, S.; Kowal-Bielecka, O.; DuBuske, L.M.; Bodzenta-Lukaszyk, A. Concentrations of 15-HETE and PGE2 in Induced Sputum of Allergic Asthma Patients. J. Allergy Clin. Immunol. 2006, 117, S200. [Google Scholar] [CrossRef]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Douglas-Jones, A.; Mansel, R.E. Levels of expression of lipoxygenases and cyclooxygenase-2 in human breast cancer. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 275–281. [Google Scholar] [CrossRef]
- O’Flaherty, J.T.; Wooten, R.E.; Samuel, M.P.; Thomas, M.J.; Levine, E.A.; Case, L.D.; Akman, S.A.; Edwards, I.J. Fatty Acid Metabolites in Rapidly Proliferating Breast Cancer. PLoS ONE 2013, 8, e63076. [Google Scholar] [CrossRef] [PubMed]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef]
- Iuliano, L. Pathways of Cholesterol Oxidation via Non-Enzymatic Mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.J.V.; Sampaio, G.R.; Ferrari, C.K.B.; Torres, E.A.F.S. Oxidation of Cholesterol in Foods and Its Importance for Human Health. Food Rev. Int. 2012, 28, 47–70. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent Aspects of the Effects of Zinc on Human Health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Wathurapatha, W.; Ishara, M.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G. Effects of Zinc Supplementation on Serum Lipids: A Systematic Review and Meta-Analysis. Nutr. Metab. 2015, 12, 26. [Google Scholar] [CrossRef]
- Bobrowska-Korczak, B.; Gątarek, P.; Skrajnowska, D.; Bielecki, W.; Wyrębiak, R.; Kovalczuk, T.; Wrzesień, R.; Kałużna-Czaplińska, J. Effect of zinc supplementation on the serum metabolites profile at the early stage of breast cancer in rats. Nutrients 2020, 12, 3457. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animals. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lowry, D.H.; Rosenbrough, J.J.; Farr, A.A.; Randal, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Frohberg, P.; Drutkowski, G.; Wobst, I. Monitoring eicosanoid biosynthesis via lipoxygenase and cyclooxygenase pathways in human whole blood by single HPLC run. J. Pharm. Biomed. Anal. 2006, 41, 1317–1324. [Google Scholar] [CrossRef]
- Jelińska, M.; Skrajnowska, D.; Wrzosek, M.; Domańska, K.; Bielecki, W.; Zawistowska, M.; Bobrowska-Korczak, B. Inflammation factors and element supplementation in cancer. J. Trace Elem. Med. Biol. 2020, 59, 126450. [Google Scholar] [CrossRef]
- Białek, A.; Jelińska, M.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. The Effect of Diet Supplementation with Pomegranate and Bitter Melon on Lipidomic Profile of Serum and Cancerous Tissues of Rats with Mammary Tumours. Antioxidants 2020, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pekiner, B.; Pennock, J.F. Fatty acids in plasma and red blood cell membranes in humans, rats, rabbits and dogs. Biochem. Mol. Biol. Int. 1995, 37, 221–229. [Google Scholar] [PubMed]
- Nutrient Requirements of Laboratory Animals, Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, Fourth Revised Edition, National Research Council, 1995. Available online: http://www.nap.edu/catalog/4758.html (accessed on 11 November 2019).
- Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. cis-9, trans-11 CLA derived endogenously from trans-11 18:1 reduces cancer risk in rats. J. Nutr. 2003, 133, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.L.; Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by Δ9-desaturase in rats. J. Nutr. 2004, 134, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.C.; Rojas, P.; Carrasco, F.; Basfi-fer, K.; Valenzuela, R.; Codoceo, J.; Inostroza, J.; Ruz, M. Fatty acid desaturation in red blood cell membranes of patients with type 2 diabetes is improved by zinc supplementation. J. Trace Elem. Med. Biol. 2020, 62, 126571. [Google Scholar] [CrossRef]
- He, C.; Qu, X.; Wan, J.; Rong, R.; Huang, L.; Cai, C.; Zhou, K.; Gu, Y.; Qian, S.Y.; Kang, J.X. Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS ONE 2012, 7, e47567. [Google Scholar] [CrossRef]
- Larre, S.; Tran, N.; Fan, C.; Hamadeh, H.; Champigneulles, J.; Azzouzi, R.; Cussenot, O.; Mangin, P.; Olivier, J.L. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008, 87, 14–19. [Google Scholar] [CrossRef]
- Canzoniero, L.M.; Turetsky, D.M.; Choi, D.W. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J. Neurosci. 1999, 19, Rc31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Aizenman, E.; DeFranco, D.B.; Rosenberg, P.A. Intracellular Zinc Release, 12-Lipoxygenase Activation and MAPK Dependent Neuronal and Oligodendroglial Death. Mol. Med. 2007, 13, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kulig, W.; Cwiklik, L.; Jurkiewicz, P.; Rog, T.; Vattulainen, I. Cholesterol Oxidation Products and Their Biological Importance. Chem. Phys. Lipids 2016, 199, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Samman, S.; Roberts, D.C.K. Zinc and Cholesterol Metabolism. Nutr. Res. 1988, 8, 559–570. [Google Scholar] [CrossRef]
- Costarelli, L.; Muti, E.; Malavolta, M.; Giacconi, R.; Cipriano, C.; Sartini, D.; Emanuelli, M.; Silvestrini, M.; Provinciali, L.; Gobbi, B.; et al. Modulation of Genes Involved in Zinc Homeostasis in Old Low-Grade Atherosclerotic Patients under Effects of HMG-CoA Reductase Inhibitors. Rejuvenation Res. 2008, 11, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, A.; Le Blanc, S.; Olivares, M.; Pizarro, F.; Ruz, M.; Arredondo, M. Iron, Copper, and Zinc Transport: Inhibition of Divalent Metal Transporter 1 (DMT1) and Human Copper Transporter 1 (HCTR1) by ShRNA. Biol. Trace Elem. Res. 2012, 146, 281–286. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, S.; Athira, S.; Suresh Babu, S.; Varma, H.; Mohanan, P. Determination of the Bioavailability of Zinc Oxide Nanoparticles Using ICP-AES and Associated Toxicity. Colloids Surf. B 2020, 188, 110767. [Google Scholar] [CrossRef]


| Standard Diet | Micro-Zn | Nano-Zn | p-Value * | |
|---|---|---|---|---|
| SFA | ||||
| Lauric acid (C12:0) | 1.61 ± 0.33 | 1.94 ± 0.15 | 2.28 ± 1.33 | n.s. |
| Myristic acid (C14:0) | 7.31 ± 1.97 | 7.04 ± 3.01 | 10.3 ± 5.7 | n.s. |
| Pentadecanoic acid (C15:0) | 5.94 ± 1.00 | 6.23 ± 1.94 | 7.25 ± 3.19 | n.s. |
| Palmitic acid (C16:0) | 838 ± 135 | 982 ± 251 | 1159 ± 543 | n.s. |
| Heptadecanoic acid (C17:0) | 17.1 ± 2.9 | 19.3 ± 2.5 | 18.5 ± 3.9 | n.s. |
| Stearic acid (C18:0) | 790 ± 187 b | 1044 ± 242 a,b | 1110 ± 136 a | 0.020 |
| Eicosanoic acid (C20:0) | n.d. ± 0.43 | 0.50 ± 0.17 a | 0.33 ± 0.06 a | <0.001 |
| ∑SFA | 1660 ± 305 | 2062 ± 176 | 2309 ± 691 | n.s. |
| MUFA | ||||
| 9-Hexadecenoic acid (C16:1 n-9) | 4.31 ± 1.35 | 4.54 ± 1.98 | 6.78 ± 3.70 | n.s. |
| Palmitoleic acid (C16:1 n-7) | 14.2 ± 6.2 | 15.1 ± 6.0 | 25.4 ± 16.2 | n.s. |
| Oleic acid (C18:1 n-9 OL) | 229 ± 86 | 282 ± 90 | 416 ± 210 | n.s. |
| Vaccenic acid (C18:1 n-7) | 46.6± 9.7 b | 60.4 ± 7.3 a,b | 77.6 ± 31.6 a | 0.032 |
| Eicosenoic acid (C20:1 n-9) | 0.27 ± 0.46 | 1.37 ± 0.28 a | 1.67 ± 0.58 a | <0.001 |
| ∑MUFA | 295 ± 102 | 364 ± 104 | 528 ± 262 | n.s. |
| PUFA | ||||
| Linoleic acid (C18:2 n-6 LA) | 699 ± 230 | 716 ± 236 | 1126 ± 584 | n.s. |
| γ-Linolenic acid (C18:3 n-6 GLA) | 7.02 ± 3.93 | 6.43 ± 4.24 | 15.6 ± 10.9 | n.s. |
| α-Linolenic acid (C18:3 n-3 ALA) | 19.9 ± 12.3 | 19.1 ± 14.0 | 39.1 ± 20.6 | n.s. |
| Eicosadienoic acid (C20:2 n-6) | 2.38 ± 0.86 | 2.12 ± 0.53 | 2.22 ± 0.68 | n.s. |
| Dihomo-γ-linolenoic (C20:3 n-6 DGLA) | 5.92 ± 1.21 | 5.66 ± 0.81 | 5.71 ± 2.03 | n.s. |
| Arachidonic acid (C20:4 n-6 AA) | 630 ± 206 | 720 ± 101 | 861 ± 372 | n.s. |
| Eicosapentaenoic acid (C20:5 n-3 EPA) | 9.11 ± 2.91 | 8.03 ± 3.15 | 12.9 ± 5.5 | n.s. |
| Docosapentaenoic acid (C22:5 n-6 DPA) | 15.3 ± 3.1 | 15.9 ± 5.3 | 18.5 ± 8.8 | n.s. |
| Docosahexaenoic acid (C22:6 n-3 DHA) | 187 ± 51 | 215 ± 39 | 224 ± 99 | n.s. |
| ∑PUFA | 1476 ± 411 | 1710 ± 277 | 2306 ± 1081 | n.s. |
| n-3 | 231 ± 55 | 258 ± 45 | 295 ± 129 | n.s. |
| n-6 | 1245 ± 373 | 1451 ± 250 | 2011 ± 960 | n.s. |
| Standard Diet | Micro-Zn | Nano-Zn | p-Value * | |
|---|---|---|---|---|
| SFA (%) | 48.8 ± 4.1 | 50.1 ± 5.1 | 46.3 ± 4.7 | n.s. |
| MUFA (%) | 8.56 ± 1.97 | 8.74 ± 1.92 | 10.0 ± 1.4 | n.s. |
| PUFA (%) | 42.6 ± 3.3 | 41.2 ± 3.8 | 43.7 ± 4.0 | n.s. |
| n-6/n-3 PUFA | 5.42 ± 1.13 | 5.67 ± 0.89 | 6.80 ± 1.03 | n.s. |
| (MUFA+PUFA)/SFA | 1.06 ± 0.17 | 1.02 ± 0.22 | 1.18 ± 0.21 | n.s. |
| PUFA/SFA | 0.88 ± 0.14 | 0.84 ± 0.18 | 0.96 ± 0.18 | n.s. |
| PI | 140 ± 17 | 134 ± 9 | 128 ± 12 | n.s. |
| Standard Diet | Zn Micro | Zn Nano | p-Value * | |
|---|---|---|---|---|
| HODE | 19.6 ± 5.4 a | 7.8 ± 1.1 | 17.6 ± 6.6 a | 0.0017 |
| 15-HETE | 6.2 ± 1.6 | 5.5 ± 1.8 | 6.3 ± 1.7 | n.s. |
| 12-HETE | 4.6 ± 0.9 a | 2.5 ± 1.2 | 4.4 ± 0.9 a | <0.0001 |
| 5-HETE | 6.5 ± 1.8 a | 3.9 ± 1.4 | 6.1 ±1.3 a | 0.0045 |
| [μg/ mL] | Standard Diet | Zn Micro | Zn Nano | p-Value * |
|---|---|---|---|---|
| Squalene | 7.10 ± 4.79 | 10.4 ± 4.2 | 8.47 ± 3.44 | n.s. |
| Cholesterol | 152 ± 37 b | 258 ± 85 a | 204 ± 41 a,b | 0.006 |
| 7K-Ch | 1.10 ± 0.58 a,b | 2.26 ± 1.58 a | 0.89 ± 0.37 b | 0.026 |
| 7α-OH-Ch | 0.39 ± 0.17 a,b | 0.66 ± 0.34 a | 0.33 ± 0.09 b | 0.022 |
| 7β-OH-Ch | 0.17 ± 0.09 a | 0.38 ± 0.24 | 0.17 ± 0.05 a | 0.018 |
| 5.6βE-Ch | 0.44 ± 0.21 a,b | 0.81 ± 0.45 a | 0.38 ± 0.13 b | 0.020 |
| ∑COPs | 2.11 ± 1.02 a,b | 4.10 ± 2.51 a | 1.77 ± 0.61 b | 0.019 |
| COPs/Ch [%] | 1.40 ± 0.66 | 1.55 ± 0.83 | 0.86 ± 0.22 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarska, A.; Czerwonka, M.; Jelińska, M.; Piasecka, I.; Bobrowska-Korczak, B. The Influence of Supplementation with Zinc in Micro and Nano Forms on the Metabolism of Fatty Acids in Livers of Rats with Breast Cancer. Nutrients 2021, 13, 3821. https://doi.org/10.3390/nu13113821
Stawarska A, Czerwonka M, Jelińska M, Piasecka I, Bobrowska-Korczak B. The Influence of Supplementation with Zinc in Micro and Nano Forms on the Metabolism of Fatty Acids in Livers of Rats with Breast Cancer. Nutrients. 2021; 13(11):3821. https://doi.org/10.3390/nu13113821
Chicago/Turabian StyleStawarska, Agnieszka, Małgorzata Czerwonka, Małgorzata Jelińska, Iga Piasecka, and Barbara Bobrowska-Korczak. 2021. "The Influence of Supplementation with Zinc in Micro and Nano Forms on the Metabolism of Fatty Acids in Livers of Rats with Breast Cancer" Nutrients 13, no. 11: 3821. https://doi.org/10.3390/nu13113821
APA StyleStawarska, A., Czerwonka, M., Jelińska, M., Piasecka, I., & Bobrowska-Korczak, B. (2021). The Influence of Supplementation with Zinc in Micro and Nano Forms on the Metabolism of Fatty Acids in Livers of Rats with Breast Cancer. Nutrients, 13(11), 3821. https://doi.org/10.3390/nu13113821







