Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials & Methods
2.1. Study Population and Data Collection
2.2. Sampling, DNA Extraction, Sequencing, and Data Analysis
2.3. Strategy for Clustering Gut Microbiota
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda) 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Microbe-Associated Metabolites as Targets for Incident Type 2 Diabetes. J. Diabetes Investig. 2021, 12, 476–478. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The Gut Microbiome of Healthy Japanese and Its Microbial and Functional Uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, A.S.; Ninomiya, K.; Uneyama, H. The Role of the Japanese Traditional Diet in Healthy and Sustainable Dietary Patterns around the World. Nutrients 2018, 10, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Livingstone, M.B.E.; Sasaki, S. Thirteen-Year Trends in Dietary Patterns among Japanese Adults in the National Health and Nutrition Survey 2003–2015: Continuous Westernization of the Japanese Diet. Nutrients 2018, 10, 994. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Sugiyama, T.; Yamaguchi, Y.; Tamura, Y.; Izawa, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; Ogasawara, N.; Goto, C.; et al. Gut Microbiota Disorders Cause Type 2 Diabetes Mellitus and Homeostatic Disturbances in Gut-Related Metabolism in Japanese Subjects. J. Clin. Biochem. Nutr. 2019, 64, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, A.R.; Giger, M.L.; Drukker, K.; Li, H.; Yuan, Y.; Bhooshan, N. Exploring Nonlinear Feature Space Dimension Reduction and Data Representation in Breast Cadx with Laplacian Eigenmaps and T-SNE. Med. Phys. 2010, 37, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [Green Version]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic Fat Obesity Presents the Greatest Risk for Incident Type 2 Diabetes: A Population-Based Longitudinal Study. Int. J. Obes. (Lond) 2019, 43, 139–148. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR from Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Ohkura, T.; Shiochi, H.; Fujioka, Y.; Sumi, K.; Yamamoto, N.; Matsuzawa, K.; Izawa, S.; Kinoshita, H.; Ohkura, H.; Kato, M.; et al. 20/(Fasting C-Peptide × Fasting Plasma Glucose) is a Simple and Effective Index of Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A Preliminary Report. Cardiovasc. Diabetol. 2013, 12, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, M.; Matsushita, Y.; Fukuda, K.; Wakura, T.; Okabe, K.; Koshimizu, Y.; Fukushima, Y.; Kobashi, C.; Yamazaki, Y.; Honoki, H.; et al. Secretory Units of Islets in Transplantation Index Is a Useful Predictor of Insulin Requirement in Japanese Type 2 Diabetic Patients. J. Diabetes Investig. 2014, 5, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, H.; Sanada, M.; Kitada, K.; Terashima, T.; Kim, H.; Sakaue, Y.; Fujitani, M.; Kawai, H.; Maeda, K.; Kashiwagi, A. Rationale and Usefulness of Newly Devised Abbreviated Diagnostic Criteria and Staging for Diabetic Polyneuropathy. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. 1), S178–S183. [Google Scholar] [CrossRef]
- Mineoka, Y.; Ishii, M.; Hashimoto, Y.; Tanaka, M.; Nakamura, N.; Katsumi, Y.; Isono, M.; Fukui, M. Relationship between Limited Joint Mobility of Hand and Carotid Atherosclerosis in Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2017, 132, 79–84. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Skipping Breakfast Is Associated with Glycemic Variability in Patients with Type 2 Diabetes. Nutrition 2020, 71, 110639. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Inoue, R.; Ohue-Kitano, R.; Tsukahara, T.; Tanaka, M.; Masuda, S.; Inoue, T.; Yamakage, H.; Kusakabe, T.; Hasegawa, K.; Shimatsu, A.; et al. Prediction of Functional Profiles of Gut Microbiota from 16S RRNA Metagenomic Data Provides a More Robust Evaluation of Gut Dysbiosis Occurring in Japanese Type 2 Diabetic Patients. J. Clin. Biochem. Nutr. 2017, 61, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Takagi, T.; Naito, Y.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Kamada, K.; Ishikawa, T.; Inoue, R.; Okuda, K.; Tsujimoto, Y.; et al. Changes in the Gut Microbiota Are Associated with Hypertension, Hyperlipidemia, and Type 2 Diabetes Mellitus in Japanese Subjects. Nutrients 2020, 12, 2996. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Osaka, T.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Intake of Sucrose Affects Gut Dysbiosis in Patients with Type 2 Diabetes. J. Diabetes Investig. 2020, 11, 1623–1634. [Google Scholar] [CrossRef]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. Msystems 2018, 3, e00021-18. [Google Scholar] [CrossRef] [Green Version]
- van der Maaten, L.; Hinton, G. Visualizing Data Using T-SNE. J. Mach. Learn. Res. 2008, 9, 2431–2456. [Google Scholar]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese Traditional Dietary Fungus Koji Aspergillus Oryzae Functions as a Prebiotic for Blautia Coccoides through Glycosylceramide: Japanese Dietary Fungus Koji Is a New Prebiotic. SpringerPlus 2016, 5, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
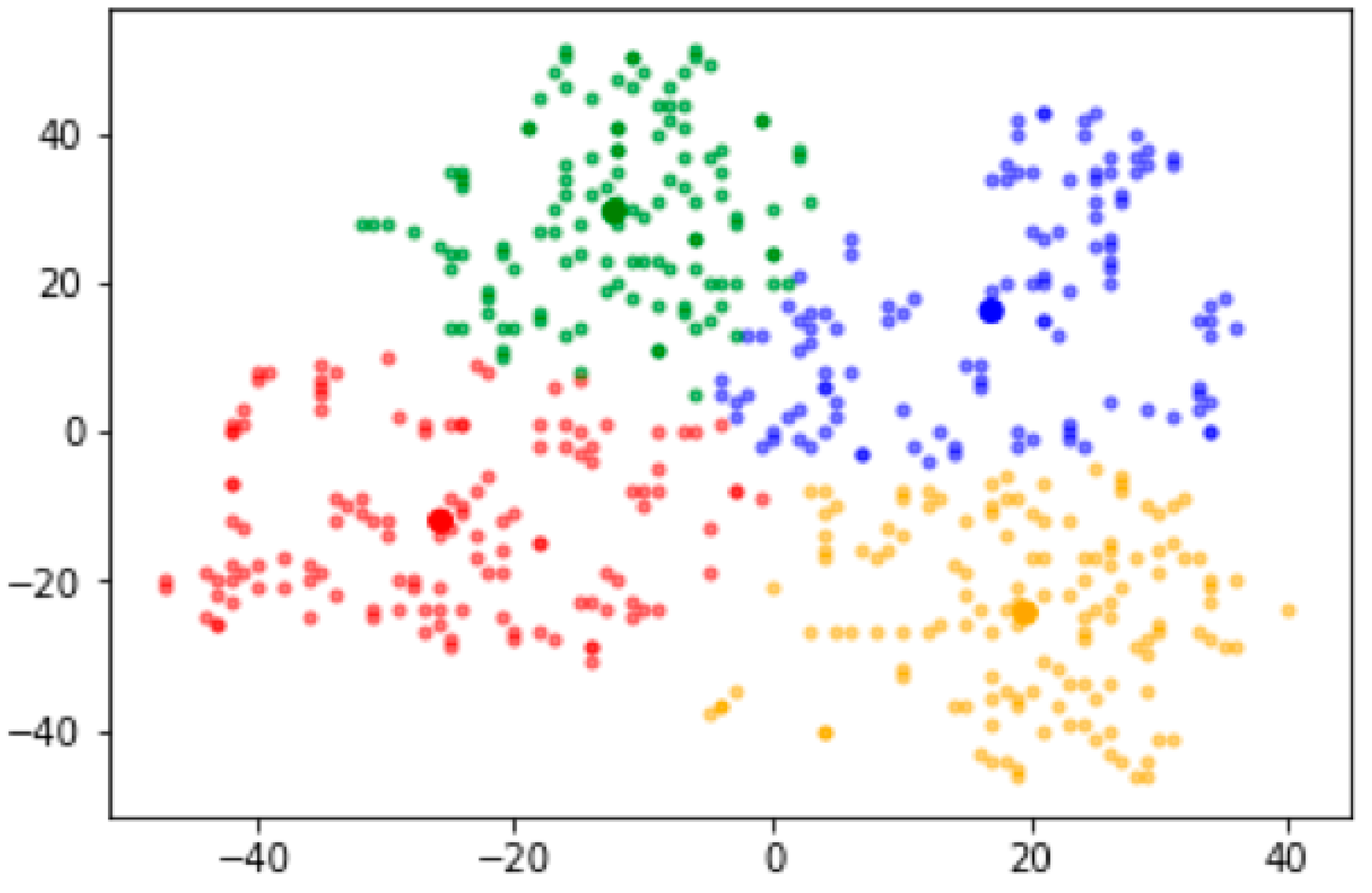

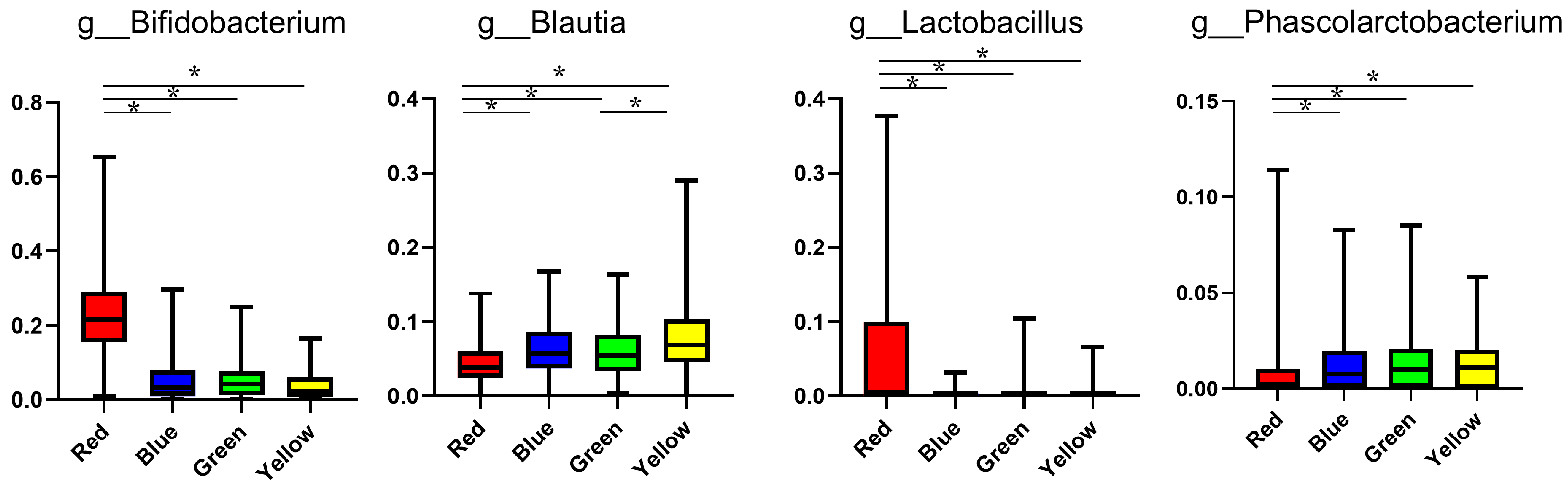
| Group | Red | Blue | Green | Yellow | p Value * |
|---|---|---|---|---|---|
| Number | 129 | 116 | 118 | 134 | - |
| Type 2 diabetes, % (n) | 86.8 (112) | 69.8 (81) | 76.3 (90) | 74.6 (100) | 0.012 |
| Male sex, % (n) | 48.1 (62) | 57.8 (67) | 57.6 (68) | 48.5 (65) | 0.237 |
| Age, years, mean (SD) | 67.8 (10.9) | 64.5 (12.5) | 67.5 (10.6) | 65.3 (11.2) | 0.057 |
| BMI, kg/m2, mean (SD) | 23.8 (4.0) | 24.2 (4.8) | 23.3 (3.6) | 23.6 (3.9) | 0.424 |
| Odds Ratio (95% CI) | p Value | |
|---|---|---|
| Red group | Ref. | - |
| Blue group | 0.34 (0.18–0.66) | 0.001 |
| Green group | 0.46 (0.24–0.90) | 0.024 |
| Yellow group | 0.47 (0.24–0.89) | 0.021 |
| Men | 1.66 (1.08–2.55) | 0.022 |
| Age, years | 1.01 (0.99–1.03) | 0.257 |
| Group | Red | Blue | Green | Yellow | p Value * |
|---|---|---|---|---|---|
| Number | 112 | 81 | 90 | 100 | - |
| Male sex, % (n) | 47.3 (53) | 60.5 (49) | 61.1 (55) | 55.0 (55) | 0.172 |
| Age, years, mean (SD) | 67.5 (10.9) | 66.2 (10.9) | 68.2 (9.5) | 64.8 (10.9) | 0.122 |
| BMI, kg/m2, mean (SD) | 24.1 (4.0) | 25.0 (4.9) | 23.8 (3.6) | 24.2 (3.8) | 0.295 |
| Duration of diabetes, years, mean (SD) | 14.8 (10.1) | 13.6 (9.3) | 15.1 (11.7) | 12.2 (8.8) | 0.164 |
| Family history of diabetes, % (n) | 46.4 (52) | 40.7 (33) | 37.8 (34) | 53 (53) | 0.16 |
| Habitual alcohol intake, % (n) | 5.4 (6) | 13.6 (11) | 5.6 (5) | 18 (18) | 0.006 |
| Smoking status | 0.403 | ||||
| Nonsmoker, % (n) | 66.1 (74) | 51.9 (42) | 60 (54) | 57 (57) | |
| Past smoker, % (n) | 25 (28) | 28.4 (23) | 25.6 (23) | 29 (29) | |
| Current smoker, % (n) | 8.9 (10) | 19.8 (16) | 14.4 (13) | 14 (14) | |
| Exercise, % (n) | 42 (47) | 50.6 (41) | 44.4 (40) | 54 (54) | 0.293 |
| Neuropathy, % (n) | 24.1 (27) | 28.4 (23) | 24.4 (22) | 21 (21) | 0.721 |
| Retinopathy, % (n) | 23.2 (26) | 22.2 (18) | 27.8 (25) | 12 (12) | 0.052 |
| Nephropathy, % (n) | 43.8 (49) | 49.4 (40) | 44.4 (40) | 35 (35) | 0.252 |
| History of cardiovascular disease, % (n) | 18.8 (21) | 12.4 (10) | 18.9 (17) | 6 (6) | 0.025 |
| Medication use | |||||
| Sulfonylurea, % (n) | 25 (28) | 16.1 (13) | 24.4 (22) | 24 (24) | 0.452 |
| Glinide, % (n) | 16.1 (18) | 7.4 (86) | 3.3 (3) | 4 (4) | 0.002 |
| Dipeptidyl peptidase-4 inhibitor, % (n) | 59.8 (67) | 43.2 (35) | 54.4 (49) | 42 (42) | 0.029 |
| Biguanide, % (n) | 40.2 (45) | 45.7 (37) | 27.8 (25) | 49 (49) | 0.019 |
| Thiazolidinedione, % (n) | 6.3 (7) | 4.9 (4) | 3.3 (3) | 0 (0) | 0.096 |
| α-Glucosidase inhibitor, % (n) | 30.4 (34) | 6.2 (5) | 4.4 (4) | 4 (4) | <0.001 |
| Sodium glucose co-transporter 2 inhibitor, % (n) | 14.3 (16) | 23.5 (19) | 15.6 (14) | 13 (13) | 0.238 |
| Glucagon-like peptide-1 analog, % (n) | 12.5 (14) | 14.8 (12) | 16.7 (15) | 21 (21) | 0.398 |
| Insulin, % (n) | 26.8 (30) | 23.5 (19) | 25.6 (23) | 17 (17) | 0.355 |
| Renin angiotensin system inhibitor, % (n) | 48.2 (54) | 48.2 (39) | 45.6 (41) | 36 (36) | 0.258 |
| Calcium channel blocker, % (n) | 32.1 (36) | 25.9 (21) | 31.1 (28) | 23 (23) | 0.427 |
| Diuretic, % (n) | 13.4 (15) | 11.1 (9) | 7.8 (7) | 8 (8) | 0.488 |
| α blocker, % (n) | 1.8 (2) | 7.4 (6) | 6.7 (6) | 1 (1) | 0.045 |
| β blocker, % (n) | 9.8 (11) | 6.2 (5) | 5.6 (5) | 2 (2) | 0.124 |
| Statin, % (n) | 42.0 (47) | 40.7 (3) | 36.7 (33) | 35 (35) | 0.71 |
| Fibrate, % (n) | 2.7 (3) | 9.9 (8) | 2.2 (2) | 3 (3) | 0.038 |
| Eicosapentaenoic acid, % (n) | 7.1 (8) | 2.5 (2) | 1.1 (1) | 6 (6) | 0.133 |
| Ezetimibe, % (n) | 2.7 (3) | 7.4 (6) | 2.2 (2) | 0 (0) | 0.282 |
| Group | Red | Blue | Green | Yellow | p Value * |
|---|---|---|---|---|---|
| Number | 112 | 81 | 90 | 100 | - |
| Systolic blood pressure, mmHg, mean (SD) | 134.0 (17.7) | 134.7 (19.3) | 134.8 (20.0) | 133.5 (18.3) | 0.963 |
| Diastolic blood pressure, mmHg, mean (SD) | 77.9 (11.6) | 80.3 (11.6) | 78.9 (11.5) | 78.7 (10.3) | 0.524 |
| Glucose, mg/dL (SD) | 147.5 (50.9) | 149.7 (46.5) | 150.5 (50.0) | 148.9 (49.0) | 0.977 |
| Hemoglobin A1c, % (SD) | 7.30 (1.41) | 7.54 (1.38) | 7.38 (1.16) | 7.27 (1.14) | 0.508 |
| C-peptide index, mean (SD) | 1.23 (0.74) | 1.33 (0.72) | 1.18 (0.69) | 1.26 (0.70) | 0.628 |
| Aspartate aminotransferase, IU/L, mean (SD) | 23.8 (12.1) | 26.1 (11.5) | 21.2 (8.8) | 23.7 (8.8) | 0.026 |
| Alanine aminotransferase, IU/L, mean (SD) | 24.8 (19.6) | 26.0 (16.4) | 21.5 (15.1) | 23.7 (14.1) | 0.303 |
| Gamma-glutamyltransferase, IU/L, mean (SD) | 31.9 (24.3) | 42.3 (48.4) | 29.4 (20.8) | 38.4 (29.0) | 0.025 |
| Creatinine, μmol/L (SD) | 69.9 (30.8) | 74.9 (25.7) | 80.0 (44.1) | 71.8 (23.2) | 0.134 |
| eGFR, mL/min/1.73 m2, mean (SD) | 72.0 (21.4) | 67.6 (16.4) | 66.7 (20.8) | 69.9 (17.7) | 0.208 |
| Uric acid, μmol/L, mean (SD) | 299.7 (69.7) | 306.1 (75.0) | 306.1 (78.2) | 317.7 (76.5) | 0.373 |
| Triglycerides, mmol/L, mean (SD) | 1.44 (1.06) | 1.63 (0.91) | 1.37 (0.71) | 1.60 (0.93) | 0.16 |
| HDL cholesterol, mmol/L, mean (SD) | 1.62 (0.47) | 1.53 (0.46) | 1.50 (0.39) | 1.56 (0.42) | 0.278 |
| Group | Red | Blue | Green | Yellow | p Value * |
|---|---|---|---|---|---|
| Number | 112 | 81 | 90 | 100 | - |
| Total energy intake, kcal/day, mean (SD) | 1687 (621) | 1804 (534) | 1769 (733) | 1714 (626) | 0.294 |
| Total energy intake, kcal/day/IBW, mean (SD) | 30.5 (11.4) | 31.6 (8.6) | 29.6 (9.9) | 29.4 (9.8) | 0.29 |
| Protein intake, g/day/IBW, mean (SD) | 1.27 (0.58) | 1.28 (0.41) | 1.25 (0.47) | 1.26 (0.52) | 0.78 |
| Protein intake/energy intake, % (SD) | 16.6 (3.6) | 16.3 (3.3) | 17.1 (3.4) | 17.1 (3.5) | 0.341 |
| Animal protein intake, g/day/IBW, mean (SD) | 0.78 (0.47) | 0.78 (0.32) | 0.76 (0.36) | 0.79 (0.41) | 0.794 |
| Vegetable protein intake, g/day/IBW, mean (SD) | 0.50 (0.17) | 0.50 (0.15) | 0.49 (0.18) | 0.47 (0.15) | 0.402 |
| Fat intake, g/day/IBW, mean (SD) | 0.97 (0.49) | 0.98 (0.32) | 0.94 (0.37) | 0.95 (0.36) | 0.696 |
| Fat intake/energy intake, % (SD) | 28.6 (6.3) | 28.0 (5.6) | 28.8 (7.0) | 29.0 (6.3) | 0.811 |
| Animal fat intake, g/day/IBW, mean (SD) | 0.48 (0.31) | 0.48 (0.19) | 0.46 (0.21) | 0.46 (0.23) | 0.623 |
| Vegetable fat intake, g/day/IBW, mean (SD) | 0.49 (0.22) | 0.50 (0.18) | 0.48 (0.20) | 0.49 (0.18) | 0.925 |
| Carbohydrate intake, g/day/IBW, mean (SD) | 3.94 (1.41) | 4.00 (1.27) | 3.80 (1.49) | 3.58 (1.37) | 0.071 |
| Carbohydrate intake/energy intake, % (SD) | 52.2 (8.2) | 50.7 (8.8) | 51.2 (9.5) | 48.8 (8.6) | 0.06 |
| Fiber intake, g/day, mean (SD) | 11.8 (5.0) | 11.9 (4.9) | 13.0 (5.4) | 12.1 (5.0) | 0.337 |
| Sucrose intake, g/day, mean (SD) | 12.8 (8.6) | 13.5 (9.7) | 10.3 (8.6) | 10.5 (6.7) | 0.032 |
| Salt intake, g/day, mean (SD) | 10.5 (3.7) | 10.9 (3.3) | 10.9 (4.8) | 10.7 (3.8) | 0.778 |
| Seaweed intake, g/day, mean (SD) | 14.5 (17.0) | 12.8 (12.4) | 12.8 (13.0) | 13.6 (13.7) | 0.990 |
| Soybean food intake, g/day, mean (SD) | 160.5 (110.1) | 174.0 (115.5) | 202.7 (126.7) | 186.5 (132.4) | 0.048 |
| Tofu and fried tofu intake, g/day, mean (SD) | 51.2 (36.2) | 48.4 (40.3) | 52.1 (41.4) | 47.7 (35.3) | 0.689 |
| Fermented soybean food intake, g/day, mean (SD) | 109.3 (96.1) | 125.6 (103.0) | 150.7 (113.2) | 138.9 (118.7) | 0.011 |
| Natto intake, g/day, mean (SD) | 11.1 (15.7) | 11.0 (14.3) | 14.0 (16.7) | 15.8 (20.3) | 0.062 |
| Miso soup intake, g/day, mean (SD) | 98.2 (10.3) | 114.6 (12.0) | 136.7 (11.4) | 123.0 (10.7) | 0.03 |
| Alcohol consumption, g/day, mean (SD) | 4.75 (14.9) | 11.1 (25.6) | 5.23 (15.2) | 10.71 (21.1) | 0.011 |
| Japanese sake intake, g/day, mean (SD) | 4.08 (2.52) | 9.38 (2.93) | 4.79 (2.79) | 3.21 (2.62) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, Y.; Hashimoto, Y.; Hamaguchi, M.; Ando, S.; Kaji, A.; Sakai, R.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; et al. Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 3816. https://doi.org/10.3390/nu13113816
Kondo Y, Hashimoto Y, Hamaguchi M, Ando S, Kaji A, Sakai R, Inoue R, Kashiwagi S, Mizushima K, Uchiyama K, et al. Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus. Nutrients. 2021; 13(11):3816. https://doi.org/10.3390/nu13113816
Chicago/Turabian StyleKondo, Yuriko, Yoshitaka Hashimoto, Masahide Hamaguchi, Shinto Ando, Ayumi Kaji, Ryosuke Sakai, Ryo Inoue, Saori Kashiwagi, Katsura Mizushima, Kazuhiko Uchiyama, and et al. 2021. "Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus" Nutrients 13, no. 11: 3816. https://doi.org/10.3390/nu13113816
APA StyleKondo, Y., Hashimoto, Y., Hamaguchi, M., Ando, S., Kaji, A., Sakai, R., Inoue, R., Kashiwagi, S., Mizushima, K., Uchiyama, K., Takagi, T., Naito, Y., & Fukui, M. (2021). Unique Habitual Food Intakes in the Gut Microbiota Cluster Associated with Type 2 Diabetes Mellitus. Nutrients, 13(11), 3816. https://doi.org/10.3390/nu13113816










