Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample and Health Data Collection
2.3. Biochemical Analysis
2.4. Twenty-Four Hour Milk Intake
2.5. Calculated Daily Intakes of HM Components
2.6. Anthropometry and Body Composition Measurements
2.7. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Human Milk Components
3.3. Maternal and Infant Body Composition
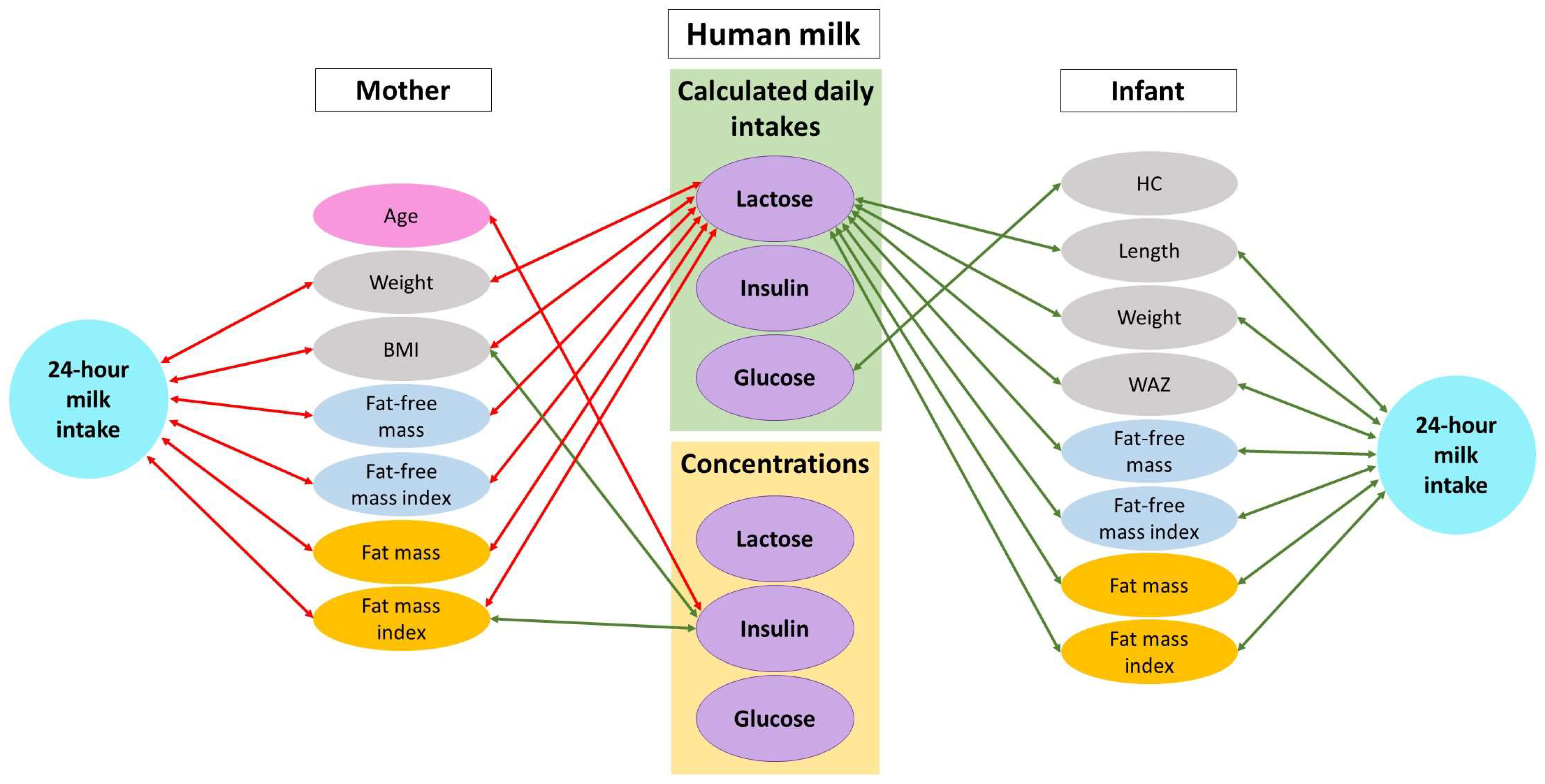
3.4. Twenty-Four Hour Milk Intake and HM Components
3.5. Twenty-Four Hour Milk Intake, HM Components, and Infant Body Composition
3.6. Maternal Characteristics and Human Milk Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Body Composition | Infant (Male) Mean ± SD (Min–Max) | Infant (Female) d Mean ± SD (Min–Max) | Infant (Pooled) Mean ± SD (Min–Max) | Mother f Mean ± SD (Min–Max) |
|---|---|---|---|---|
| Fat-free mass (kg) | 4.92 ± 0.46 (3.80–6.01) a | 4.27 ± 0.27 (3.83–4.76) | 4.57 ± 0.49 (3.80–6.01) e | 45.68 ± 8.46 (18.00–70.89) |
| Fat-free mass index (kg/m2) | 17.78 ± 1.03 (13.70–17.93) a | 14.19 ± 0.67 (12.93–15.41) | 14.92 ± 1.17 (12.93–17.93) e | 16.85 ± 2.79 (6.30–23.96) |
| Fat mass (kg) | 1.65 ± 0.35 (0.84–2.50) a | 1.51 ± 0.24 (1.06–1.94) | 1.58 ± 0.30 (0.84–2.50) e | 26.75 ± 9.42 (11.25–53.93) |
| Fat mass index (kg/m2) | 5.28 ± 0.98 (3.02–7.47) a | 5.01 ± 0.70 (3.80–6.22) | 5.14 ± 0.84 (3.02–7.47) e | 9.88 ± 3.41 (3.94–18.99) |
| Fat mass (%) | 24.88 ± 2.49 (18.05–29.76) a | 25.99 ± 1.96 (21.52–29.04) | 25.48 ± 2.27 (18.05–29.76) e | 35.91 ± 6.16 (21.88–53.35) |
| Fat mass to fat-free mass ratio | 0.33 ± 0.04 (0.22–0.42) a | 0.35 ± 0.04 (0.27–0.41) | 0.34 ± 0.04 (0.22–0.42) e | 0.58 ± 0.16 (0.37–1.14) |
| Body mass index (kg/m2) | 16.97 ± 1.32 (15.06–19.92) b | 15.98 ± 1.08 (14.05–17.99) | 16.46 ± 1.29 (14.05–19.92) f | 26.88 ± 5.21 (18.00–40.95) |
| Infant z-scores | ||||
| Weight-for-length z-score | 0.04 ± 0.92 (−1.23–2.54) b | −0.22 ± 0.83 (−1.82–1.11) | −0.09 ± 0.89 (−1.82–2.54) f | |
| Weight-for-age z-score | 0.19 ± 1.03 (−2.79–2.49) b | −0.15 ± 0.71 (−1.64.−1.15) | 0.01 ± 0.89 (−2.79–2.49) f | |
| Length-for-age z-score | 0.32 ± 1.31 (−3.09–2.83) b | 0.11 ± 1.11 (−2.25–2.18) | 0.21 ± 1.20 (−3.09–2.83) f | |
| BMI-for-age z-score | 0.10 ± 0.90 (−1.39–1.95) b | −0.28 ± 0.72 (−1.63–1.05) | −0.10 ± 0.82 (−1.63–1.95) f | |
| Head circumference-for-age z-score | 0.55 ± 0.93 (−1.34–2.08) c | 0.67 ± 0.90 (−1.07–2.56) b | 0.62 ± 0.91 (−1.34–2.56) g | |
| Infant Predictor | Intercept | SE | Slope | SE | Predictor | Infant Birth Weight |
|---|---|---|---|---|---|---|
| p-Value | p-Value | |||||
| Infant weight (kg) | ||||||
| Sex (male) | 2.259 | 0.561 | 0.638 | 0.137 | <0.001 | <0.001 |
| Birth weight (kg) | 2.143 | 0.666 | 0.001 | 0.0002 | <0.001 | NA |
| Infant length (cm) | ||||||
| Sex (male) | 47.669 | 2.208 | 1.459 | 0.540 | 0.010 | <0.001 |
| Birth weight (kg) | 47.405 | 2.340 | 0.004 | 0.001 | <0.001 | NA |
| Infant body mass index (kg/m2) | ||||||
| Sex (male) | 13.434 | 1.343 | 0.892 | 0.329 | 0.009 | 0.060 |
| Birth weight (kg) | 13.272 | 1.425 | 0.001 | 0.0004 | 0.029 | NA |
| Infant head circumference (cm) | ||||||
| Sex (male) | 36.160 | 1.350 | 0.684 | 0.298 | 0.027 | 0.003 |
| Birth weight (kg) | 36.176 | 1.413 | 0.001 | 0.0004 | 0.002 | NA |
| Infant fat-free mass (kg) | ||||||
| Sex (male) | 2.271 | 0.323 | 0.559 | 0.080 | <0.001 | <0.001 |
| Birth weight (kg) | 2.104 | 0.454 | 0.001 | 0.0001 | <0.001 | NA |
| Infant fat-free mass index (kg/m2) | ||||||
| Sex (male) | 10.763 | 0.863 | 1.433 | 0.215 | <0.001 | <0.001 |
| Birth weight (kg) | 10.332 | 1.188 | 0.001 | 0.0003 | <0.001 | NA |
| Infant fat mass (kg) | ||||||
| Birth weight (kg) | −0.062 | 0.264 | 0.001 | 0.0001 | <0.001 | NA |
| Infant fat mass index (kg/m2) | ||||||
| Birth weight (kg) | 0.967 | 0.779 | 0.001 | 0.0002 | <0.001 | NA |
| Infant fat mass (%) | ||||||
| Sex (male) | 15.374 | 2.073 | −1.616 | 0.516 | 0.003 | <0.001 |
| Birth weight (kg) | 15.860 | 2.249 | 0.003 | 0.001 | <0.001 | NA |
| Infant fat mass to fat-free mass ratio | ||||||
| Sex (male) | 0.159 | 0.037 | −0.028 | 0.009 | 0.004 | <0.001 |
| Birth weight (kg) | 0.167 | 0.040 | 0.0001 | 0.000 | <0.001 | NA |
| Weight-for-age z-score | ||||||
| Birth weight (kg) | −4.877 | 0.758 | 0.001 | 0.0002 | <0.001 | NA |
| Length-for-age z-score | ||||||
| GA (weeks) | −15.416 | 4.565 | 0.275 | 0.129 | 0.038 | <0.001 |
| Birth weight (kg) | −5.956 | 1.081 | 0.002 | 0.0003 | <0.001 | NA |
| BMI-for-age z-score | ||||||
| Birth weight (kg) | −2.213 | 0.907 | 0.001 | 0.0003 | 0.023 | NA |
| Head circumference-for-age z-score | ||||||
| Birth weight (kg) | −2.786 | 1.107 | 0.001 | 0.0003 | 0.003 | NA |
| Maternal Predictor | Intercept | SE | Slope | SE | Predictor | Infant Birth Weight |
|---|---|---|---|---|---|---|
| p-Value | p-Value | |||||
| Infant weight (kg) | ||||||
| Weight (kg) | 2.489 | 0.655 | −0.013 | 0.006 | 0.025 | <0.001 |
| BMI (kg/m2) | 2.836 | 0.646 | −0.049 | 0.015 | 0.002 | <0.001 |
| FM (kg) | 2.273 | 0.634 | −0.022 | 0.009 | 0.014 | <0.001 |
| FM (%) | 3.034 | 0.748 | −0.029 | 0.013 | 0.026 | <0.001 |
| FFMI (kg/m2) | 2.938 | 0.679 | −0.083 | 0.029 | 0.006 | <0.001 |
| FMI (kg/m2) | 2.408 | 0.628 | −0.066 | 0.023 | 0.006 | <0.001 |
| Infant body mass index (kg/m2) | ||||||
| Weight (kg) | 14.308 | 1.327 | −0.038 | 0.011 | 0.001 | <0.001 |
| BMI (kg/m2) | 14.808 | 1.372 | −0.108 | 0.032 | 0.001 | 0.001 |
| FFM (kg) | 14.535 | 1.366 | −0.066 | 0.020 | 0.002 | 0.001 |
| FM (kg) | 13.621 | 1.304 | −0.058 | 0.018 | 0.002 | 0.002 |
| FM (%) | 15.468 | 1.573 | −0.072 | 0.027 | 0.010 | 0.011 |
| FFMI (kg/m2) | 15.160 | 1.423 | −0.198 | 0.060 | 0.002 | 0.001 |
| FMI (kg/m2) | 13.876 | 1.328 | −0.150 | 0.048 | 0.003 | 0.004 |
| Infant head circumference (cm) | ||||||
| FFM (kg) | 37.037 | 1.396 | −0.044 | 0.019 | 0.023 | <0.001 |
| FFMI (kg/m2) | 37.791 | 1.431 | −0.152 | 0.054 | 0.007 | <0.001 |
| Infant fat-free mass (kg) | ||||||
| BMI (kg/m2) | 2.549 | 0.456 | −0.029 | 0.011 | 0.009 | <0.001 |
| FM (kg) | 2.197 | 0.438 | −0.013 | 0.006 | 0.032 | <0.001 |
| FM (%) | 2.743 | 0.521 | −0.020 | 0.009 | 0.030 | <0.001 |
| FFMI (kg/m2) | 2.646 | 0.510 | −0.051 | 0.024 | 0.042 | <0.001 |
| FMI (kg/m2) | 2.293 | 0.435 | −0.042 | 0.016 | 0.012 | <0.001 |
| FM/FFM | 2.456 | 0.468 | −0.715 | 0.337 | 0.039 | <0.001 |
| Infant fat-free mass index (kg/m2) | ||||||
| Weight (kg) | 11.053 | 1.161 | −0.025 | 0.010 | 0.015 | <0.001 |
| BMI (kg/m2) | 11.613 | 1.174 | −0.084 | 0.028 | 0.004 | <0.001 |
| FM (kg) | 10.634 | 1.116 | −0.043 | 0.015 | 0.007 | <0.001 |
| FM (%) | 12.242 | 1.343 | −0.061 | 0.024 | 0.013 | <0.001 |
| FFMI (kg/m2) | 11.849 | 1.327 | −0.141 | 0.063 | 0.030 | <0.001 |
| FMI (kg/m2) | 10.885 | 1.118 | −0.122 | 0.041 | 0.005 | <0.001 |
| FM/FFM | 11.372 | 1.210 | −2.113 | 0.871 | 0.019 | <0.001 |
| Infant fat mass (kg) | ||||||
| Weight (kg) | 0.073 | 0.263 | −0.005 | 0.002 | 0.041 | <0.001 |
| BMI (kg/m2) | 0.194 | 0.265 | −0.017 | 0.006 | 0.010 | <0.001 |
| FFMI (kg/m2) | 0.337 | 0.288 | −0.037 | 0.014 | 0.009 | <0.001 |
| FMI (kg/m2) | 0.030 | 0.258 | −0.020 | 0.010 | 0.038 | <0.001 |
| Infant fat mass index (kg/m2) | ||||||
| Weight (kg) | 1.409 | 0.768 | −0.015 | 0.007 | 0.023 | <0.001 |
| BMI (kg/m2) | 1.720 | 0.785 | −0.049 | 0.018 | 0.010 | <0.001 |
| FFM (kg) | 1.564 | 0.810 | −0.027 | 0.013 | 0.047 | <0.001 |
| FM (kg) | 1.119 | 0.757 | −0.022 | 0.010 | 0.042 | <0.001 |
| FFMI (kg/m2) | 2.148 | 0.851 | −0.110 | 0.041 | 0.009 | <0.001 |
| FMI (kg/m2) | 1.235 | 0.763 | −0.059 | 0.028 | 0.041 | <0.001 |
| Infant fat mass (%) | ||||||
| Age (years) | 19.908 | 2.888 | −0.119 | 0.056 | 0.039 | <0.001 |
| Infant fat mass to fat-free mass ratio | ||||||
| Age (years) | 0.236 | 0.052 | −0.002 | 0.001 | 0.0495 | <0.001 |
| Weight-for-length z-score | ||||||
| Weight (kg) | 0.083 | 0.961 | −0.028 | 0.008 | 0.001 | 0.069 |
| BMI (kg/m2) | 0.310 | 1.020 | −0.069 | 0.024 | 0.005 | 0.15 |
| FFM (kg) | 0.264 | 0.985 | −0.049 | 0.015 | 0.002 | 0.070 |
| FM (kg) | −0.431 | 0.953 | −0.041 | 0.013 | 0.002 | 0.15 |
| FM (%) | 0.738 | 1.159 | −0.046 | 0.020 | 0.023 | 0.40 |
| FFMI (kg/m2) | 0.524 | 1.059 | −0.126 | 0.045 | 0.007 | 0.15 |
| FMI (kg/m2) | −0.285 | 0.983 | −0.098 | 0.036 | 0.008 | 0.25 |
| Weight-for-age z-score | ||||||
| Weight (kg) | −4.451 | 0.739 | −0.016 | 0.006 | 0.015 | <0.001 |
| BMI (kg/m2) | −4.128 | 0.743 | −0.053 | 0.017 | 0.004 | <0.001 |
| FM (kg) | −4.728 | 0.721 | −0.025 | 0.010 | 0.014 | <0.001 |
| FM (%) | −3.969 | 0.861 | −0.030 | 0.015 | 0.048 | <0.001 |
| FFMI (kg/m2) | −4.044 | 0.783 | −0.087 | 0.033 | 0.011 | <0.001 |
| FMI (kg/m2) | −4.594 | 0.722 | −0.070 | 0.026 | 0.010 | <0.001 |
| Length-for-age z-score | ||||||
| Age (year) | −3.828 | 1.382 | −0.063 | 0.027 | 0.024 | <0.001 |
| BMI-for-age z-score | ||||||
| Weight (kg) | −1.744 | 0.894 | −0.017 | 0.008 | 0.025 | 0.003 |
| BMI (kg/m2) | −1.518 | 0.923 | −0.049 | 0.021 | 0.027 | 0.004 |
| FFM (kg) | −1.588 | 0.905 | −0.033 | 0.014 | 0.020 | 0.003 |
| FM (kg) | −2.058 | 0.877 | −0.026 | 0.012 | 0.032 | 0.005 |
| FM (%) | −1.134 | 1.031 | −0.035 | 0.018 | 0.0495 | 0.012 |
| FFMI (kg/m2) | −1.284 | 0.946 | −0.098 | 0.040 | 0.019 | 0.003 |
| FMI (kg/m2) | −1.942 | 0.887 | −0.068 | 0.032 | 0.041 | 0.007 |
| Head circumference-for-age z-score | ||||||
| FFM (kg) | −2.112 | 1.093 | −0.034 | 0.015 | 0.023 | <0.001 |
| FFMI (kg/m2) | −1.733 | 1.152 | −0.099 | 0.043 | 0.026 | <0.001 |
References
- Pacheco, A.R.; Barile, D.; Underwood, M.A.; Mills, D.A. The impact of the milk glycobiome on the neonate gut microbiota. Annu. Rev. Anim. Biosci. 2015, 3, 419–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.-J.; Hartmann, P.E. 25 years of research in human lactation: From discovery to translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef] [PubMed]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient balance and micronutrient amounts through growth and development. Ital. J. Pediatrics 2021, 47, 109. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatric Clin. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in human milk and body composition of term infants during the first 12 months of lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C.; Chomtho, S.; Fewtrell, M.S. Programming of body composition by early growth and nutrition. Proc. Nutr. Soc. 2007, 66, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lonnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24h and throughout the first year of lactation. Br. J. Nutr. 2007, 88, 29–37. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Stromberg, R.; Jornvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 2013, 8, e53876. [Google Scholar] [CrossRef] [Green Version]
- Forsgard, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Ryan, C.A.; Hussey, S.; Murphy, B.; Fitzgerald, G.F.; Stanton, C. Role of gut microbiota in early infant development. Clin. Med. Pediatrics 2009, 3, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; van Tol, E.A.; Vervoort, J.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.E.; Patinkin, Z.W.; Pyle, L.; de la Houssaye, B.; Davidson, B.S.; Geraghty, S.; Morrow, A.L.; Krebs, N. Markers of oxidative stress in human milk do not differ by maternal BMI but are related to infant growth trajectories. Matern. Child Health J. 2017, 21, 1367–1376. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Sonksen, P.; Sonksen, J. Insulin: Understanding its action in health and disease. Br. J. Anaesth. 2000, 85, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Rong, S.S.; Sun, X.; Ding, G.; Wan, W.; Zou, L.; Wu, S.; Li, M.; Wang, D. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. Br. J. Nutr. 2018, 120, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Fields, D.A.; George, B.; Williams, M.; Whitaker, K.; Allison, D.B.; Teague, A.; Demerath, E.W. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatric Obes. 2017, 12 (Suppl. 1), 78–85. [Google Scholar] [CrossRef] [Green Version]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatric Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.; Goruk, S.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.; Sears, M.R.; Field, C.J.; Azad, M.B. Adiponectin, leptin and insulin in breast milk: Associations with maternal characteristics and infant body composition in the first year of life. Int. J. Obes. 2018, 42, 36–43. [Google Scholar] [CrossRef]
- Goran, M.I.; Martin, A.A.; Alderete, T.L.; Fujiwara, H.; Fields, D.A. Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients 2017, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.Q. Biology of glucose transport in the mammary gland. J. Mammary Gland. Biol. Neoplasia 2014, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Hay, W.W.; Fennessey, P. Physiological significance of the concentration of human milk glucose. Protoplasma 1990, 159, 118–128. [Google Scholar] [CrossRef]
- Nagel, E.M.; Kummer, L.; Jacobs, D.R., Jr.; Foster, L.; Duncan, K.; Johnson, K.; Harnack, L.; Haapala, J.; Kharoud, H.; Gallagher, T.; et al. Human milk glucose, leptin, and insulin predict cessation of full breastfeeding and initiation of formula use. Breastfeed. Med. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Boylan, M.; Hart, S.L.; Román-Shriver, C.; Spallholz, J.E.; Pence, B.C.; Sawyer, B.G. Glucose and insulin levels are increased in obese and overweight mothers’ breast-milk. Food Nutr. Sci. 2011, 2, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Tie, W.J.; Kent, J.C.; Tat Lai, C.; Rea, A.; Hepworth, A.R.; Murray, K.; Geddes, D.T. Reproducibility of the creamatocrit technique for the measurement of fat content in human milk. Food Chem. 2021, 356, 129708. [Google Scholar] [CrossRef]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [Green Version]
- Kent, J.C.; Hepworth, A.R.; Sherriff, J.L.; Cox, D.B.; Mitoulas, L.R.; Hartmann, P.E. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Organization WHO. Child Growth Standards Software. WHO Anthro Survey Analyser and Other Tools (version 3.2.2). Available online: https://www.who.int/childgrowth/software/en/ (accessed on 16 June 2021).
- Gridneva, Z.; Rea, A.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Relationships between breastfeeding patterns and maternal and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Farajian, S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: A review of evidence. Adv. Biomed. Res. 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Development of visceral and subcutaneous-abdominal adipose tissue in breastfed infants during first year of lactation. Nutrients 2021, 13, 3294. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M.; Wong, W.W.; Smith, E.O.; Ellis, K.J. Body composition during the first 2 years of life: An updated reference. Pediatric Res. 2000, 47, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [Green Version]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Fields, D.A.; Schneider, C.R.; Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity 2016, 24, 1213–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyenet, S.J.; Schwartz, M.W. Clinical review: Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieffer, T.J.; Habener, J.F. The adipoinsular axis: Effects of leptin on pancreatic beta-cells. Am. J. Physiol. -Endocrinol. Metab. 2000, 278, E1–E14. [Google Scholar] [CrossRef]
- Emmett, P.M.; Rogers, I.S. Properties of human milk and their relationship with maternal nutrition. Early Hum. Dev. 1997, 49, S7–S28. [Google Scholar] [CrossRef]
- Henrichs, J.; Schenk, J.J.; Roza, S.J.; van den Berg, M.P.; Schmidt, H.G.; Steegers, E.A.; Hofman, A.; Jaddoe, V.W.; Verhulst, F.C.; Tiemeier, H. Maternal psychological distress and fetal growth trajectories: The Generation R Study. Psychol. Med. 2010, 40, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Bray, P.F.; Shields, W.D.; Wolcott, G.J.; Madsen, J.A. Occipitofrontal head circumference—An accurate measure of intracranial volume. J. Pediatrics 1969, 75, 303–305. [Google Scholar] [CrossRef]
- Rollins, J.D.; Collins, J.S.; Holden, K.R. United States head circumference growth reference charts: Birth to 21 years. J. Pediatrics 2010, 156, 907–913.e2. [Google Scholar] [CrossRef] [PubMed]
- Kinnala, A.; Suhonen-Polvi, H.; Aarimaa, T.; Kero, P.; Korvenranta, H.; Ruotsalainen, U.; Bergman, J.; Haaparanta, M.; Solin, O.; Nuutila, P.; et al. Cerebral metabolic rate for glucose during the first six months of life: An FDG positron emission tomography study. Arch. Dis. Child.-Fetal Neonatal Ed. 1996, 74, F153–F157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewey, K.G.; Heinig, M.J.; Nommsen, L.A.; Lonnerdal, B. Maternal versus infant factors related to breast milk intake and residual milk volume: The DARLING study. Pediatrics 1991, 87, 829–837. [Google Scholar] [PubMed]
- Kent, J.C.; Mitoulas, L.; Cox, D.B.; Owens, R.A.; Hartmann, P.E. Breast volume and milk production during extended lactation in women. Exp. Physiol. 1999, 84, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, E.R.J. Growth in exclusively breastfed infants. J. Pediatr. 2019, 95 (Suppl. 1), 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.; Herath, M.P.; Beckett, J.M.; Ahuja, K.D.K.; Byrne, N.M.; Hills, A.P. Exclusivity of breastfeeding and body composition: Learnings from the baby-bod study. Int. Breastfeed. J. 2021, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Chomtho, S.; Wells, J.C.; Davies, P.S.; Lucas, A.; Fewtrell, M.S. Early growth and body composition in infancy. Adv. Exp. Med. Biol. 2009, 646, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Pettitt, D.J.; Hanson, R.L.; Imperatore, G.; Bennett, P.H.; Knowler, W.C. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care 1999, 22, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Gillman, M.W.; Hennekens, C.H.; Speizer, F.E.; Manson, J.E. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann. Intern. Med. 1999, 130, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Carberry, A.E.; Colditz, P.B.; Lingwood, B.E. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatric Res. 2010, 68, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, B.; Lof, M.; Forsum, E. Body composition in full-term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010, 99, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds-methods comparison. Pediatric Res. 2017, 81, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Gianni, M.L.; Orsi, A.; Piemontese, P.; Amato, O.; Liotto, N.; Morlacchi, L.; Taroni, F.; Fields, D.A.; Catalano, P.M.; et al. Quality of growth in exclusively breast-fed infants in the first six months of life: An Italian study. Pediatric Res. 2010, 68, 542–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powe, C.E.; Knott, C.D.; Conklin-Brittain, N. Infant sex predicts breast milk energy content. Am. J. Hum. Biol. 2010, 22, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Roth, E.; Lo, Y.J.; Hurst, C.; Vollner, J.; Kendell, A. In poor families, mothers’ milk is richer for daughters than sons: A test of Trivers-Willard hypothesis in agropastoral settlements in Northern Kenya. Am. J. Phys. Anthropol. 2012, 149, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Song, J.H.; Song, S.; Kang, N.M. Do gender and birth height of infant affect calorie of human milk? An association study between human milk macronutrient and various birth factors. J. Matern.-Fetal Neonatal Med. 2017, 30, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.A. No evidence for sex biases in milk macronutrients, energy, or breastfeeding frequency in a sample of Filipino mothers. Am. J. Phys. Anthropol. 2013, 152, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hanley, A.J.; Sermer, M.; Zinman, B.; O’Connor, D.L. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am. J. Clin. Nutr. 2012, 95, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Shehadeh, N.; Khaesh-Goldberg, E.; Shamir, R.; Perlman, R.; Sujov, P.; Tamir, A.; Makhoul, I.R. Insulin in human milk: Postpartum changes and effect of gestational age. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, F214–F216. [Google Scholar] [CrossRef] [Green Version]
- Young, B.E.; Patinkin, Z.; Palmer, C.; de la Houssaye, B.; Barbour, L.A.; Hernandez, T.; Friedman, J.E.; Krebs, N.F. Human milk insulin is related to maternal plasma insulin and BMI: But other components of human milk do not differ by BMI. Eur. J. Clin. Nutr. 2017, 71, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, S.; Koh, H.E.; Patterson, B.W.; Yoshino, M.; LaForest, R.; Gropler, R.J.; Klein, S.; Mittendorfer, B. Obesity is associated with increased basal and postprandial beta-cell insulin secretion even in the absence of insulin resistance. Diabetes 2020, 69, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, T.J.; Trengove, N.J.; Graham, D.F.; Hartmann, P.E. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int. J. Endocrinol. 2012, 2012, 296368. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Elahi, D.; Tobin, J.D.; Andres, R. The effect of age on insulin resistance and secretion: A review. Semin. Nephrol. 1996, 16, 289–298. [Google Scholar] [PubMed]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between maternal body composition and appetite hormones and macronutrients in human milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef]
- Barbosa, L.; Butte, N.F.; Villalpando, S.; Wong, W.W.; Smith, E.O. Maternal energy balance and lactation performance of Mesoamerindians as a function of body mass index. Am. J. Clin. Nutr. 1997, 66, 575–583. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Oledzka, G.; Szostak-Wegierek, D.; Weker, H.; Wesolowska, A. Maternal nutrition and body composition during breastfeeding: Association with human milk composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.; Jung, J.A.; Kim, H.; Jo, A.; Kang, S.; Lee, S.W.; Yi, H.; Kim, J.; Yim, J.G.; Jung, B.M. Macronutrient composition of human milk from Korean mothers of full term infants born at 37–42 gestational weeks. Nutr. Res. Pract. 2015, 9, 433–438. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Ning, Y.; You, L.; Ma, D.; Zheng, Y.; Yang, X.; Li, W.; Wang, J.; Wang, P. Breast milk macronutrient composition and the associated factors in urban Chinese mothers. Chin. Med. J. 2014, 127, 1721–1725. [Google Scholar] [PubMed]
- Diana, A.; Haszard, J.J.; Houghton, L.A.; Gibson, R.S. Breastmilk intake among exclusively breastfed Indonesian infants is negatively associated with maternal fat mass. Eur. J. Clin. Nutr. 2019, 73, 1206–1208. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.M.; Renault, K.M.; Norgaard, K.; Nilas, L.; Jensen, J.E.; Hyldstrup, L.; Michaelsen, K.F.; Cortes, D.; Pryds, O. Newborn regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014, 103, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, G.; Ponsonby, A.L.; Wells, J.C.; Pezic, A.; Cochrane, J.; Dwyer, T. Maternal and infant factors associated with neonatal adiposity: Results from the Tasmanian Infant Health Survey (TIHS). Int. J. Obes. 2012, 36, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, H.R.; Dinger, M.K.; Knehans, A.W.; Thompson, D.M.; Fields, D.A. Impact of maternal body mass index on neonate birthweight and body composition. Am. J. Obstet. Gynecol. 2008, 198, 416.e1–416.e6. [Google Scholar] [CrossRef] [PubMed]
- Sewell, M.F.; Huston-Presley, L.; Super, D.M.; Catalano, P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am. J. Obstet. Gynecol. 2006, 195, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Mascie-Taylor, C.G.; Begum, H.A. Maternal anthropometry as a predictor of birth weight. Public Health Nutr. 2007, 10, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, N.; Stuart, B.; Donnelly, V.; Kennelly, M.M.; Turner, M.J. The influence of maternal body composition on birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 14–17. [Google Scholar] [CrossRef]
| Characteristics | Mean ± SD (Min–Max) or n (%) |
|---|---|
| Maternal age at infant birth (years) | 32.7 ± 4.6 (25.1–46.4) |
| Maternal ethnicity | |
| Caucasian | 48 (84.2) |
| Asian | 5 (8.8) |
| Other | 4 (70) |
| Parity | 2.1 ± 0.8 (1–4) |
| Mode of delivery | |
| Vaginal | 36 (63.2) |
| Elective caesarean section | 14 (24.6) |
| Emergency caesarean section | 7 (12.3) |
| Maternal height (cm) | 164.7 ± 6.9 (148.0–178.0) |
| Maternal weight (kg) | 72.8 ± 15.3 (47.5–119.8) a |
| Infant gestational age (weeks) | 39.3 ± 1.1 (37.0–41.2) |
| Infant sex | |
| Male | 27 (47.4) |
| Female | 30 (52.6) |
| Infant birth weight (kg) | 3.505 ± 0.413 (2.610–4.705) |
| Infant birth length (cm) | 50.8 ± 2.1 (46.0–55.0) |
| Infant weight (kg) | 6.150 ± 0.756 (4.638–8.410) a |
| Infant length (cm) | 61.1 ± 2.6 (55.5–67.0) a |
| Infant head circumference (cm) | 40.8 ± 1.2 (38.0–43.0) b |
| 24-h milk intake (mL) | 792.8 ± 176.1 (511.7–1304.9) c |
| Components | Concentration a Mean ± SD (Min–Max) | CDI b Mean ± SD (Min–Max) |
|---|---|---|
| Lactose | 86.56 ± 7.91 (g/L) (69.91–106.09) | 68.35 ± 16.63 (g/day) (39.91–129.27) |
| Insulin | 1.48 ± 1.14 (ng/mL) (0.46–5.90) | 1.16 ± 0.78 (ng/day) (0.30–4.17) |
| Glucose | 0.26 ± 0.09 (g/L) (0.09–0.47) | 0.20 ± 0.09 (g/day) (0.05–0.49) |
| Predictor | Intercept | SE | Slope | SE | Predictor | Infant Birth Weight |
|---|---|---|---|---|---|---|
| p-Value | p-Value | |||||
| Infant weight (kg) | ||||||
| Lactose intake (g/day) | 1.779 | 0.643 | 0.017 | 0.005 | 0.001 | <0.001 |
| 24-h milk intake (mL) | 1.672 | 0.638 | 0.002 | 0.0004 | <0.001 | <0.001 |
| Infant length (cm) | ||||||
| Lactose intake (g/day) | 46.010 | 2.345 | 0.047 | 0.017 | 0.010 | <0.001 |
| 24-h milk intake (mL) | 45.905 | 2.388 | 0.004 | 0.002 | 0.016 | <0.001 |
| Head circumference (cm) | ||||||
| Glucose intake (g/day) | 35.338 | 1.495 | 4.304 | 1.885 | 0.028 | 0.004 |
| Infant fat-free mass (kg) | ||||||
| Lactose intake (g/day) | 1.841 | 0.440 | 0.011 | 0.003 | 0.001 | <0.001 |
| 24-h milk intake (mL) | 1.776 | 0.438 | 0.001 | 0.0003 | <0.001 | <0.001 |
| Infant fat-free mass index (kg/m2) | ||||||
| Lactose intake (g/day) | 9.757 | 1.257 | 0.026 | 0.009 | 0.009 | 0.011 |
| 24-h milk intake (mL) | 9.591 | 1.252 | 0.003 | 0.001 | 0.005 | 0.013 |
| Infant fat mass (kg) | ||||||
| Lactose intake (g/day) | −0.081 | 0.267 | 0.005 | 0.002 | 0.011 | <0.001 |
| 24-h milk intake (mL) | −0.118 | 0.266 | 0.001 | 0.0002 | 0.006 | <0.001 |
| Infant fat mass index (kg/m2) | ||||||
| Lactose intake (g/day) | 0.956 | 0.815 | 0.014 | 0.006 | 0.028 | <0.001 |
| 24-h milk intake (mL) | 0.855 | 0.813 | 0.001 | 0.001 | 0.017 | <0.001 |
| Weight-for-age z-score | ||||||
| Lactose intake (g/day) | −5.200 | 0.792 | 0.017 | 0.006 | 0.007 | <0.001 |
| 24-h milk intake (mL) | −5.330 | 0.785 | 0.002 | 0.001 | 0.003 | <0.001 |
| Maternal Predictor | Intercept | SE | Slope | SE | Predictor | Infant Birth Weight |
|---|---|---|---|---|---|---|
| p-Value | p-Value | |||||
| Calculated daily intakes of HM components | ||||||
| Lactose (g/day) | ||||||
| Weight (kg) | 47.115 | 19.137 | −0.462 | 0.163 | 0.007 | 0.009 |
| BMI (kg/m2) | 55.258 | 19.195 | −1.501 | 0.459 | 0.002 | 0.007 |
| FFM (kg) | 50.552 | 20.228 | −0.775 | 0.327 | 0.023 | 0.014 |
| FM (kg) | 39.098 | 19.043 | −0.677 | 0.268 | 0.016 | 0.020 |
| FFMI (kg/m2) | 63.565 | 20.691 | −3.000 | 0.987 | 0.004 | 0.007 |
| FMI (kg/m2) | 42.531 | 19.061 | −1.909 | 0.721 | 0.011 | 0.024 |
| Concentrations of HM components | ||||||
| Insulin (ng/mL) | ||||||
| Age (years) | 2.392 | 1.666 | −0.073 | 0.032 | 0.027 | 0.24 |
| BMI (kg/m2) | −1.090 | 1.389 | 0.067 | 0.032 | 0.044 | 0.55 |
| FMI (kg/m2) | −0.543 | 1.325 | 0.100 | 0.048 | 0.043 | 0.42 |
| 24-h milk intake (mL) | ||||||
| Weight (kg) | 552.379 | 197.950 | −5.256 | 1.688 | 0.003 | 0.004 |
| BMI (kg/m2) | 630.592 | 201.169 | −16.005 | 4.811 | 0.002 | 0.005 |
| FFM (kg) | 590.075 | 210.553 | −8.743 | 3.403 | 0.014 | 0.008 |
| FM (kg) | 461.557 | 197.438 | −7.740 | 2.781 | 0.008 | 0.011 |
| FFMI (kg/m2) | 714.516 | 217.715 | −31.497 | 10.383 | 0.004 | 0.005 |
| FMI (kg/m2) | 495.925 | 199.567 | −20.608 | 7.548 | 0.009 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, A.S.; Stinson, L.F.; Rea, A.; Lai, C.T.; Payne, M.S.; Murray, K.; Geddes, D.T.; Gridneva, Z. Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding. Nutrients 2021, 13, 3724. https://doi.org/10.3390/nu13113724
Cheema AS, Stinson LF, Rea A, Lai CT, Payne MS, Murray K, Geddes DT, Gridneva Z. Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding. Nutrients. 2021; 13(11):3724. https://doi.org/10.3390/nu13113724
Chicago/Turabian StyleCheema, Ali S., Lisa F. Stinson, Alethea Rea, Ching Tat Lai, Matthew S. Payne, Kevin Murray, Donna T. Geddes, and Zoya Gridneva. 2021. "Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding" Nutrients 13, no. 11: 3724. https://doi.org/10.3390/nu13113724
APA StyleCheema, A. S., Stinson, L. F., Rea, A., Lai, C. T., Payne, M. S., Murray, K., Geddes, D. T., & Gridneva, Z. (2021). Human Milk Lactose, Insulin, and Glucose Relative to Infant Body Composition during Exclusive Breastfeeding. Nutrients, 13(11), 3724. https://doi.org/10.3390/nu13113724







