Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
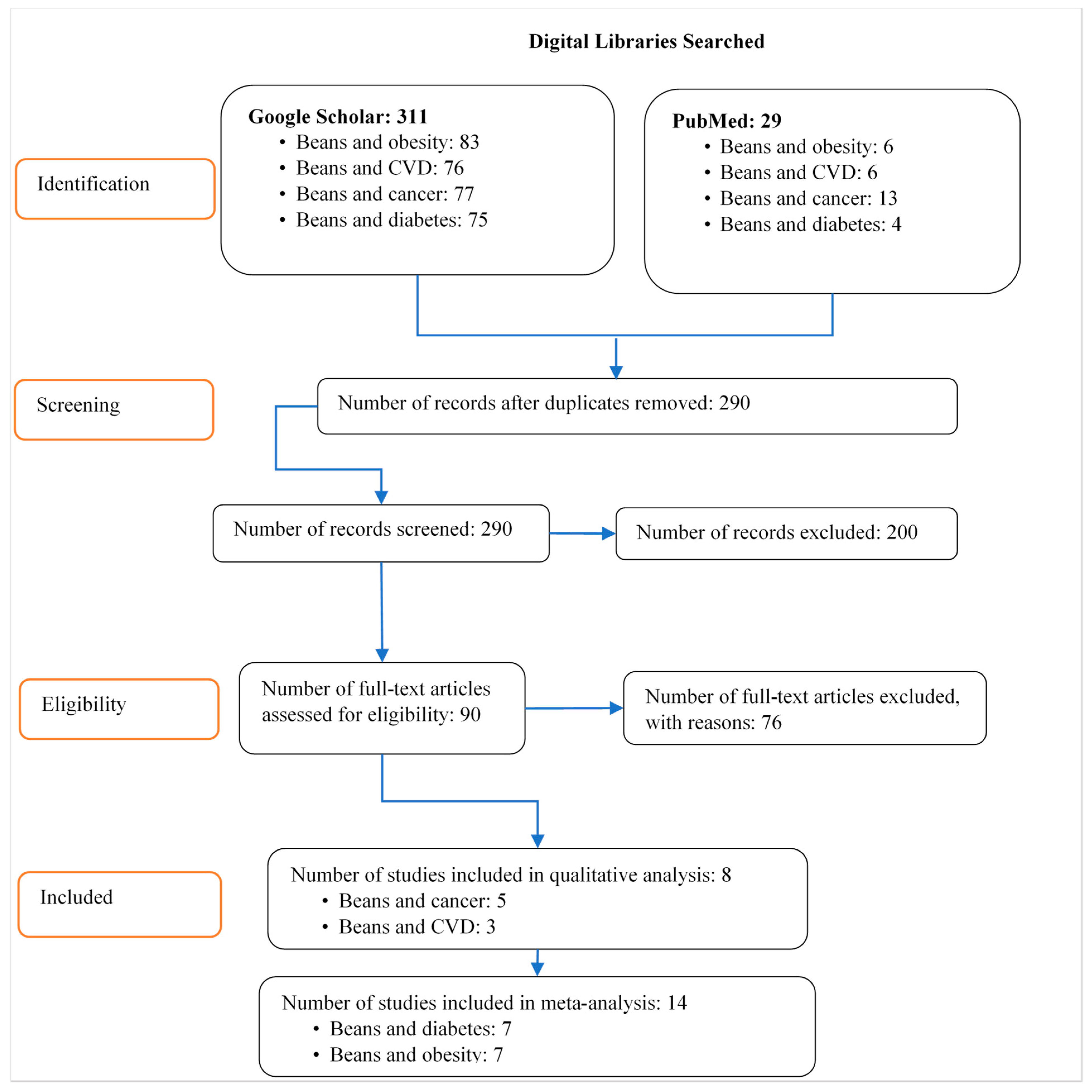
2.1. Search Strategy
2.2. Study Selection and Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Empirical Estimations and Statistical Tests
3. Results
3.1. Summary Findings of Studies
3.1.1. Common Beans and Cardiovascular Diseases (CVD)
- a.
- Common beans and cancers
- b.
- Common beans and diabetes
- c.
- Common beans and obesity
3.1.2. Empirical Estimations
- a.
- Forest plot analysis (obesity and diabetes studies)
- b.
- Random-effects meta-regression
4. Discussion and Conclusions
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Aseete, P.; Katungi, E.; Bonabana-Wabbi, J.; Birachi, E.; Ugen, M.A. Consumer demand heterogeneity and valuation of value-added pulse products: A case of precooked beans in Uganda. Agric. Food Secur. 2018, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- FAO. The Global Economy of Pulse; Rawal, V., Navarro, D., Eds.; FAO: Rome, Italy, 2019. [Google Scholar]
- Nakazi, F.; Njuki, J.; Ugen, M.A.; Aseete, P.; Katungi, E.; Birachi, E.; Kabanyoro, R.; Mugagga, I.J.; Nanyonjo, G. Is bean really a women’s crop? Men and women’s participation in bean production in Uganda. Agric. Food Secur. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Nchanji, E.B.; Ssekandi, W.; Nanyongo, G.; Bantebya, G. Gendered Varietal and Trait Preferences of Common Bean Value Chain Actors in Uganda: Implications for breeding. GREAT Virtual Symposium. Available online: https://www.youtube.com/watch?v=S9GoZYvik2I (accessed on 20 October 2021).
- Ogecha, J.; Arinaitwe, W.; Muthomi, J.; Aritua, V.; Obanyi, J. Incidence and Severity of Common Bean (Phaseolus vulgaris L.) Pests in Agro-Ecological Zones and Farming Systems of Western Kenya. East. Afr. Agric. For. J. 2019, 83, 191–205. [Google Scholar] [CrossRef]
- Letaa, E.; Katungi, E.; Kabungo, C.; Ndunguru, A.A. Impact of improved common bean varieties on household food security on adopters in Tanzania. J. Dev. Eff. 2020, 12, 89–108. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Wenger, M.J.; Scott, S.P.; Rhoten, S.E.; Lung’aho, M.G.; Haas, J.D. Consumption of Iron-Biofortified Beans Positively Affects Cognitive Performance in 18- to 27-Year-Old Rwandan Female College Students in an 18-Week Randomized Controlled Efficacy Trial. J. Nutr. 2017, 147, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Luna, S.V.; Pompano, L.M.; Lung’aho, M.; Gahutu, J.B.; Haas, J.D. Increased iron status during a feeding trial of iron-biofortified beans increases physical work efficiency in Rwandan women. J. Nutr. 2020, 150, 1093–1099. [Google Scholar] [CrossRef]
- Haas, J.D.; Luna, S.V.; Lung’aho, M.G.; Wenger, M.J.; Murray-Kolb, L.E.; Beebe, S.; Gahutu, J.B.; Egli, I.M. Consuming Iron Biofortified Beans Increases Iron Status in Rwandan Women after 128 Days in a Randomized Controlled Feeding Trial. J. Nutr. 2016, 146, 1586–1592. [Google Scholar] [CrossRef]
- McDermott, J.; Wyatt, A.J. The role of pulses in sustainable and healthy food systems. Ann. N. Y. Acad. Sci. 2017, 1392, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Nchanji, E.B.; Collins, O.A.; Katungi, E.; Nduguru, A.; Kabungo, C.; Njuguna, E.M.; Ojiewo, C.O. What Does Gender Yield Gap Tell Us about Smallholder Farming in Developing Countries? Sustainability 2021, 13, 77. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Yang, H.; Gu, J.; Wang, J.; Ren, F. Regular intake of white kidney beans extract (Phaseolus vulgaris L.) induces weight loss compared to placebo in obese human subjects. Food Sci. Nutr. 2020, 8, 1315–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary guidance for pulses: The challenge and opportunity to be part of both the vegetable and protein food groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Bean Consumption Accounts for Differences in Body Fat and Waist Circumference: A Cross-Sectional Study of 246 Women. J. Nutr. Meta 2020. [Google Scholar] [CrossRef]
- Castilla, J.C.; Ávila, A.R.; Orozco, A.C.; Martínez, J.C.; Ortiz, G.D. Biologically Active Compounds in the Common Bean (Phaseolus vulgaris L.) and their Health Benefit. In Seeds as Functional Foods and Nutraceuticals: New Frontiers in Food Science; Nova Publishers: New York, NY, USA, 2014. [Google Scholar]
- Marventano, S.; Izquierdo Pulido, M.; Sánchez-González, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Onakpoya, I.; Aldaas, S.; Terry, R.; Ernst, E. The efficacy of Phaseolus vulgaris as a weight-loss supplement: A systematic review and meta-analysis of randomised clinical trials. Br. J. Nutr. 2011, 106, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Padhi, E.M.T.; Ramdath, D.D. A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. J. Funct. Foods 2017, 38, 635–643. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Kendall, C.W.C.; Esfahani, A.; Wong, J.M.W.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J.A. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary legume consumption reduces risk of colorectal cancer: Evidence from a meta-analysis of cohort studies. Sci. Rep. 2015, 5, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryne, C.N.; Veenstra, J.M.; Deschambault, B.R.; Benali, M.; Marcotte, M.; Boye, J.I.; Tosh, S.M.; Farnworth, E.R.; Wright, A.J.; Duncan, A.M. Spray-dried pulse consumption does not affect cardiovascular disease risk or glycemic control in healthy males. Food Res. Int. 2012, 48, 131–139. [Google Scholar] [CrossRef]
- Winham, D.M.; Hutchins, A.M.; Johnston, C.S. Pinto bean consumption reduces biomarkers for heart disease risk. J. Am. Coll. Nutr. 2007, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Albert, P.S.; Zhang, Z.; Bagshaw, D.; Kris-Etherton, P.M.; Ulbrecht, J.; Miller, C.K.; Bobe, G.; Colburn, N.H.; Lanza, E. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J. Nutr. 2010, 140, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Perera, T.; Young, M.R.; Zhang, Z.; Murphy, G.; Colburn, N.H.; Lanza, E.; Hartman, T.J.; Cross, A.J.; Bobe, G. Identification and monitoring of metabolite markers of dry bean consumption in parallel human and mouse studies. Mol. Nutr. Food Res. 2015, 59, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Baxter, B.A.; Oppel, R.C.; Ryan, E.P. Navy beans impact the stool metabolome and metabolic pathways for colon health in cancer survivors. Nutrients 2019, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Bazan, M.; Rao, S.; Bailey, S.M.; Wdowik, M.; et al. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr. Cancer 2016, 68, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Ivanov, I.; Dougherty, E.R.; Hartman, T.J.; Lanza, E.; Bobe, G.; Colburn, N.H.; Lupton, J.R.; Davidson, L.A.; Chapkin, R.S. Noninvasive detection of candidate molecular biomarkers in subjects with a history of insulin resistance and colorectal adenomas. Cancer Prev. Res. (Phila) 2009, 2, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.; Johansson, E.; Ekström, L.; Björck, I. Effects of a Brown Beans Evening Meal on Metabolic Risk Markers and Appetite Regulating Hormones at a Subsequent Standardized Breakfast: A Randomized Cross-Over Study. PLoS ONE 2013, 8, e59985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedilla-Alonso, B.; Pedrosa, M.M.; Cuadrado, C.; Brito, M.; Asensio-S-Manzanera, C.; Asensio-Vegas, C. Composition of two Spanish common dry beans (Phaseolus vulgaris), “Almonga” and “Curruquilla”, and their postprandial effect in type 2 diabetics. J. Sci. Food Agric. 2013, 93, 1076–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 109, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Reverri, E.J.; Randolph, J.M.; Steinberg, F.M.; Tissa Kappagoda, C.; Edirisinghe, I.; Burton-Freeman, B.M. Black beans, fiber, and antioxidant capacity pilot study: Examination of whole foods vs. functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients 2015, 7, 6139–6154. [Google Scholar] [CrossRef]
- Winham, D.M.; Hutchins, A.M. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr. Res. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Kazemi, M.; McBreairty, L.E.; Chizen, D.R.; Pierson, R.A.; Chilibeck, P.D.; Zello, G.A. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: A randomized controlled trial. Nutrients 2018, 10, 1387. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S. Effect of Pinto, Black and Dark Red Kidney Bean Consumption as Part of a Meal on Postprandial Glucose in Adults with Type 2 Diabetes. Master’s Thesis, Arizona State University, Phoenix, RI, USA, 2011. [Google Scholar]
- Udani, J.; Singh, B.B. Blocking carbohydrate absorption and weight loss: A clinical trial using a proprietary fractionated white bean extract. Altern. Ther. Health Med. 2007, 13, 32–37. [Google Scholar]
- Støa Birketvedt, G.; Langbakk, B.; Florholmen, J. A dietary supplement with bean extract decreases body weight, body fat, waist circumference and blood pressure in overweight and obese subjects. Curr. Top. Nutraceutical Res. 2004, 3, 137–142. [Google Scholar]
- Grube, B.; Chong, W.F.; Chong, P.W.; Riede, L. Weight reduction and maintenance with IQP-PV-101: A 12-week randomized controlled study with a 24-week open label period. Obesity 2014, 22, 645–651. [Google Scholar] [CrossRef]
- Maruyama, C.; Araki, R.; Kawamura, M.; Kondo, N.; Kigawa, M.; Kawai, Y.; Takanami, Y.; Miyashita, K.; Shimomitsu, T. Azuki bean juice lowers serum triglyceride concentrations in healthy young women. J. Clin. Biochem. Nutr. 2008, 43, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Celleno, L.; Tolaini, M.V.; D’Amore, A.; Perricone, N.V.; Preuss, H.G. A Dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int. J. Med Sci. 2007, 4, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, W.L.; Michael White, C.; Cappelleri, J.C.; Kluger, J.; Coleman, C.I. Understanding heterogeneity in meta-analysis: The role of Meta-regression. Int. J. Clin. Pract. 2009, 63, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Inthout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res. Methodol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables, 6th ed.; Medpharm GmbH Scientific Publishers: Stuttgart, Germany, 2008. [Google Scholar]
- Selvin, E.; Parrinello, C.M. Age-related differences in glycaemic control in diabetes. Diabetologia 2013, 56, 2549–2551. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Health. Aim for a healtht weight: Key Recommendations. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/recommen.htm (accessed on 20 October 2021).
- Faraoni, D.; Schaefer, S.T. Randomized controlled trials vs. observational studies: Why not just live together? BMC Anesthesiol. 2016, 16, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Study Type | Health Issue | Study Subjects | M | F | Control | Treatment | Age | Wks | Qty | Bean Form | Variety | Study Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bazzano et al., 2001 [25] | Cohort | CVD | Healthy | 3493 | 5685 | - | - | 25–74 | 12 | 98.6 g/day | grain | pinto, red | Beans lower risk of CVD by 11 percent. |
| Cryne et al., 2012 [26] | Randomized crossover | CVD | Hypercholesterolemic adults | 21 | 21 | - | - | 19–40 | 4 | 100 g/day | grain | navy, pinto | Bean consumption does not affect serum lipids, homocysteine, or glycemic parameters. |
| Winham et al., 2007 [27] | Randomized crossover | CVD | Hypercholesterolemic adult men | 7 | 9 | - | - | 22–63 | 8 | 180 g/day | grain | pinto | Serum total cholesterol decreased by 19 ± 5 mean. |
| Hartman et al., 2010 [28] | Randomized crossover | Cancer | Adenomas | 64 | 0 | - | - | 35–75 | 4 | 251 g/day | grain | navy, pinto, kidney, black | sTNFRI/II concentrations increased by 23.8 percent. |
| Perera et al., 2015 [29] | Randomized crossover | Cancer | Non-smoking males | 46 | 0 | - | - | 35–75 | 4 | 250 g/day | grain | navy | Serum pipecolic acid and S-methyl cysteine increased. |
| Baxter et al., 2019 [30] | Cohort | Cancer | Overweight and obese CRC survivors | 6 | 13 | - | - | 60–65 | 4 | 35 g/day | grain | navy | The relative stool abundance of ophthalmate increased 5.25-fold for navy bean, indicating glutathione regulation. |
| Borresen et al., 2016 [31] | Cohort | Cancer | Colorectal cancer (CRC) survivors | 12 | 17 | - | - | 59–64 | 4 | 35 g/day | grain | navy | SAA levels at week 4 improved levels associated with CRC chemoprevention. |
| Zhao et al., 2009 [32] | Randomized crossover | Cancer | Adenomas | 23 | 0 | - | - | 35–75 | 4 | 250 g/day | grain | navy, kidney, pinto | Gene products (RNA) isolated from a stool after bean consumption had diagnostic value in assessing colon cancer risk. |
| Nilsson et al., 2013 [33] | Randomized crossover | Diabetes | Healthy young adults | 6 | 19 | −2.1 | −2.5 | 23.8 | 2 | 101 g/day | grain | brown | Brown beans lowered blood glucose by 215 percent and insulin by 216 percent. |
| Olmedilla-Alonso et al., 2013 [34] | Randomized crossover | Diabetes | Type 2 diabetics | 7 | 5 | −0.28 | −2.48 | 50–76 | 0.3 | 275 g/day | grain | white, cream | Only white ‘Almonga’ rendered a significant reduction in the triglyceridemic response. |
| Spadafranca et al., 2013 [35] | Randomized, double blind | Diabetes | Normal weight | 6 | 6 | 0.4 | −0.5 | 20–26 | 0.2 | 100 mg/day | extract | navy, pinto | PVE lowered postprandial glucose +15·4%, insulin +981, and C-peptide excursions in 30 min. |
| Reverri et al., 2015 [36] | Randomized crossover | Diabetes | Metabolic syndrome (MetS) | 6 | 6 | 0.9 | 1.1 | 35–63 | 1.2 | - | extract | black | Meals with black beans reduced postprandial insulin concentrations. |
| Winham and Hutchins, 2007 [37] | Randomized crossover | Diabetes | Diabetics | 10 | 12 | −0.3 | −1.6 | 24–67 | 8 | 180 g/day | grain | navy | Total cholesterol serum (TC) for baked beans was −5.6 ± 1.5 percent |
| Kazemi et al., 2018 [38] | Cohort | Diabetes | Polycystic ovary syndrome (PCOS) | 0 | 95 | −4 | −5.5 | 18–35 | 16 | 225 g/day | grain | pinto, black, kidney | The total area under the curve reduced for insulin response to a 75 g oral glucose tolerance test. |
| Thompson, 2011 [39] | Cohort | Diabetes | Type 2 diabetics | 9 | 8 | −31.5 | −41.9 | 35–70 | 24 | 50 g/day | grain | pinto, black, dark red kidney | Glucose lowered for pinto, black, and red bean (compared to control) at 90, 120, and 150 min post-treatment. |
| Udani and Singh, 2007 [40]. | Randomized, double blind | Obesity | Obese | 17 | 8 | 2.6% | 3.4% | 18–40 | 4 | 2000 mg/day | extract | white kidney | Weight decreased by 6.0 lbs and waist size decreased by 2.2 inches. |
| Wang et al., 2020 [14] | Randomized, double blind | Obesity | Obese | 29 | 27 | 0.9% | 2.7% | 18–65 | 4.5 | 2400 mg/day | extract | white kidney | Weight decreased by 2.24 kg (an average of 0.448 kg per week). |
| Birketvedt et al., 2002 [41] | Randomized, double blind | Obesity | Overweight and obese volunteers | 21 | 31 | 0.1% | 3.2% | 22–66 | 12 | 900 mg/day | extract | white kidney | Serum cholesterol decreased by 6 percent in the supplement group. |
| Grube et al., 2014 [42] | Randomized, double blind | Obesity | BMI between 25 and 35 kg/m2 | 13 | 87 | 1.4% | 3.5% | 18–60 | 12 | 500 mg/day | extract | white kidney | The IQP-PV-101 group lost a mean of 2.91 ± 62.63 kg in weight. |
| Maruyama et al., 2008 [43] | Randomized, double blind | Obesity | Healthy women | 0 | 33 | +1.1% | 0.2% | 21.3 | 6.7 | 750 g/day | extract | adzuki | Triglyceride concentrations in the adzuki group decreased by 0.170 mmol/liter (15.4%). |
| Celleno et al., 2007 [44] | Randomized, double blind | Obesity | Overweight | 17 | 42 | 0.5% | 3.9% | 20–45 | 4 | 445 mg/day | extract | white kidney | Weight decreased by 2.93 kg and waist circumference decreased by 4.8 cm. |
| Winham and Hutchins, 2007 [37] | Randomized crossover | Obesity | Healthy | 10 | 12 | - | - | 24–67 | 8 | 180 g/day | grain | navy | Serum LDL-C decreased by −5.4 ± 2.3 percent. |
| Obesity | Diabetes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderators | β | SE | t | p > z | 95% Conf. Interval | β | SE | Z | p > z | 95% Conf. Interval | ||
| Age | 0.19 | 0.05 | 3.50 | 0.07 | −0.04 | 0.42 | −0.13 | 0.05 | −2.56 | 0.13 | −0.35 | 0.09 |
| Number of weeks | −0.26 | 0.09 | −2.97 | 0.10 | −0.65 | 0.12 | −0.19 | 0.09 | −2.07 | 0.17 | −0.59 | 0.21 |
| Quantity of beans | −0.001 | 0.001 | −4.22 | 0.05 | −0.003 | 0.001 | 0.02 | 0.01 | 1.72 | 0.23 | −0.03 | 0.07 |
| Constant | −1.14 | 1.53 | −0.75 | 0.53 | −7.74 | 5.45 | 1.20 | 2.02 | 0.60 | 0.61 | −7.47 | 9.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nchanji, E.B.; Ageyo, O.C. Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients 2021, 13, 3701. https://doi.org/10.3390/nu13113701
Nchanji EB, Ageyo OC. Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients. 2021; 13(11):3701. https://doi.org/10.3390/nu13113701
Chicago/Turabian StyleNchanji, Eileen Bogweh, and Odhiambo Collins Ageyo. 2021. "Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials" Nutrients 13, no. 11: 3701. https://doi.org/10.3390/nu13113701
APA StyleNchanji, E. B., & Ageyo, O. C. (2021). Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients, 13(11), 3701. https://doi.org/10.3390/nu13113701






