Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preparation of Synbiotics (Soymilk, Honey, and Lactic Acid Bacteria)
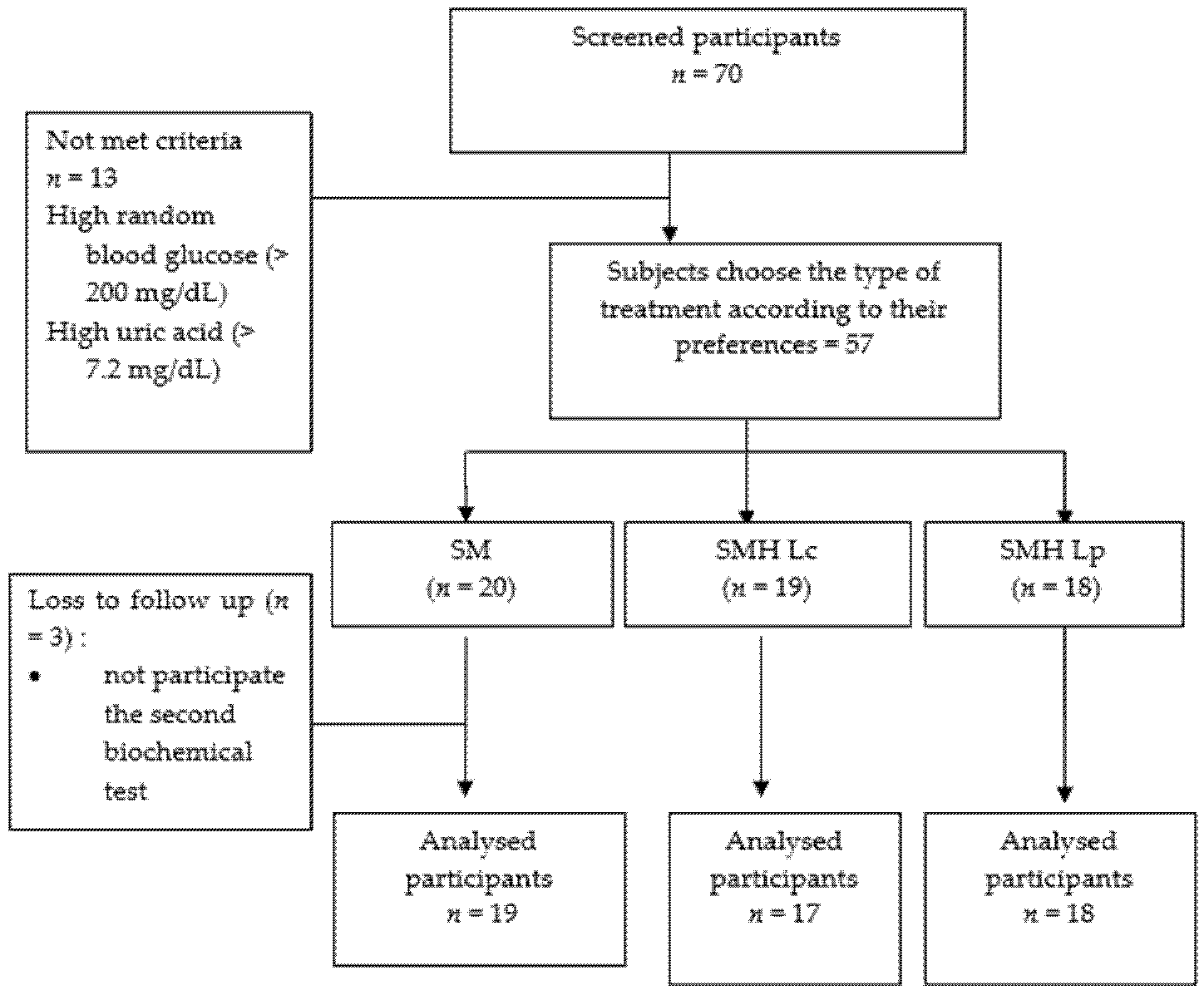
2.3. Participants
2.4. Intervention
2.5. Blood Sample Collection and Laboratory Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Baseline Data of Blood Biochemical Examination
3.3. Measurement of Blood Glucose Levels, Uric Acid, and Total Cholesterol, before and after Intervention
3.3.1. Soymilk (SM)
3.3.2. Soy-Honey Fermented Milk with Lactobacillus casei subsp. casei R-68 (SMH Lc)
3.3.3. Soy-Honey Fermented Milk with Lactobacillus plantarum 1 R 1.3.2 (SMH Lp)
3.4. Differences in Osteocalcin Levels at Beginning and End of the Intervention in the Three Groups
3.5. Differences in Osteocalcin Levels in the Three Groups after Intervention
3.6. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-garach, A.; García-fontana, B.; Muñoz-torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Facts and Statistics|International Osteoporosis Foundation. Available online: http://www.iofbonehealth.org/facts-statistics (accessed on 20 October 2018).
- Yu, J.; Cao, G.; Yuan, S.; Luo, C.; Yu, J.; Cai, M. Probiotic Supplements and Bone Health in Postmenopausal Women: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2021, 11, e041393. [Google Scholar] [CrossRef]
- Tu, M.-Y.; Chen, H.-L.; Tung, Y.-T.; Kao, C.-C.; Hu, F.-C.; Chen, C.-M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020, 18, 273–284. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakłos-Szyda, M.; Gałązka-Czarnecka, I.; Grzelczyk, J.; Budryn, G. Cicer Arietinum, L. Sprouts’ Influence on Mineralization of Saos-2 and Migration of MCF-7 Cells. Molecules 2020, 25, 4490. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Cai, J.S.; Zhang, J.G.; Hu, F.; Wei, Z.J. B-Vitamin Enriched Fermented Soymilk: A Novel Strategy for Soy-Based Functional Foods Development. Trends Food Sci. Technol. 2020, 105, 43–55. [Google Scholar] [CrossRef]
- Stijepić, M.; Glušac, J.; Djurdjević-Milošević, D. Effect of Honey Addition on Rheological Properties of Probiotic Soy Yoghurt. Glas. Hem. Tehnol. I Ekol. Repub. Srp. 2013, 4. [Google Scholar] [CrossRef]
- Nath, A.H.; Ukkuru, M.P.; Kumari, M.K. Development of a Probiotic Honey Beverage. Int. J. Appl. Pure Sci. Agric. 2015, 1, 130–139. [Google Scholar]
- Abou-Dobara, M.I.; Ismail, M.M.; Refat, N.M. Preparation of Functional Fermented Dairy Product Containing High Levels of Omega-6, Omega-9, Antioxidants Activity and Probiotic. Diabetes Manag. 2017, 7, 306–318. [Google Scholar]
- Diez-Perez, A.; Naylor, K.E.; Abrahamsen, B.; Agnusdei, D.; Brandi, M.L.; Cooper, C.; Dennison, E.; Eriksen, E.F.; Gold, D.T.; Guañabens, N.; et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the Screening of Adherence to Oral Bisphosphonates. Osteoporos. Int. 2017, 28, 767–774. [Google Scholar] [CrossRef]
- Tournis, S.; Makris, K. Clinical Use of Bone Turnover Markers in Osteoporosis. In Reference Module in Biomedical Sciences; Elsevier: Iraklion, Greece, 2018; pp. 1–17. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Ghasemi Nasab, M.; Riasatian, M.; Sadeghi, F. Soy Isoflavones Prevent Bone Resorption and Loss, a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, D.; Lal, A.K. Serum Osteocalcin as a Diagnostic Biomarker for Primary Osteoporosis in Women. J. Clin. Diagn. Res. 2015, 9, RC04–RC07. [Google Scholar] [CrossRef]
- Song, L. Calcium and Bone Metabolism Indices. In Advances in Clinical Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 82, pp. 1–46. [Google Scholar] [CrossRef]
- Tian, L.; Yang, R.; Wei, L.; Liu, J.; Yang, Y.; Shao, F.; Li, T.; Wang, Y.; Guo, T. Prevalence of Osteoporosis and Related Lifestyle and Metabolic Factors of Postmenopausal Women and Elderly Men. Medicine 2017, 43, e8294. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-S.; Liao, J.-W.; Pan, T.-M. Effect of Bioactive Compounds in Lactobacilli-Fermented Soy Skim Milk on Femoral Bone Microstructure of Aging Mice. J. Sci. Food Agric. 2012, 92, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Choman, M.; Blanton, C.; Gabaldón, A. The Effect of a Synbiotic Diet on Bone Structure in Aging Male Mice. FASEB J. 2015, 29, 738.1. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of Probiotics, Prebiotics, and Synbiotics on Mineral Metabolism in Ovariectomized Rats—Impact of Bacterial Mass, Intestinal Absorptive Area and Reduction of Bone Turn-Over. NFS J. 2016, 3, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Jansson, P.A.; Curiac, D.; Lazou Ahrén, I.; Hansson, F.; Martinsson Niskanen, T.; Sjögren, K.; Ohlsson, C. Probiotic Treatment Using a Mix of Three Lactobacillus Strains for Lumbar Spine Bone Loss in Postmenopausal Women: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus Subtilis C-3102 on Bone Mineral Density in Healthy Postmenopausal Japanese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Desfita, S.; Sari, W.; Yusuf, Y.; Pato, U. Fermented Soy Milk and Honey Potential to Improve Bone Health of Menopausal Women, 1st ed.; Deepublish: Pekanbaru, Indonesia, 2020. (In Indonesian) [Google Scholar]
- Pato, U.; Yusuf, Y.; Nainggolan, Y.P. Effect of Lactobacillus Casei Subsp. Casei R-68 Isolated from Dadih on the Procarcinogenic Enzyme Activity and Fecal Microflora Count of Rats Challenged with Pathogenic Bacteria. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 1656–1662. [Google Scholar] [CrossRef]
- Indrati, R.; Utami, T.; Marsono, Y. Binding of Bile Salts by Fermented Soymilk and Its Stability against Pepsin and Pancreatin. J. Teknol. Dan Ind. Pangan 2013, 24, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slačanac, V.; Lučan, M.; Hardi, J.; Krstanović, V.; Koceva Komlenić, D. Fermentation of Honey-Sweetened Soymilk with Bifidobacterium Lactis Bb-12 and Bifidobacterium Longum Bb-46: Fermentation Activity of Bifidobacteria and in Vitro Antagonistic Effect against Listeria Monocytogenes FSL N1-017. Czech J. Food Sci. 2012, 30, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M.; Gaya, P.; Rodríguez, E.; Langa, S.; Peirotén, Á.; Medina, M.; Arqués, J.L. Probiotic Bacteria for Healthier Aging: Immunomodulation and Metabolism of Phytoestrogens. Biomed Res. Int. 2017, 2017, 5939818. [Google Scholar] [CrossRef]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal Microbiota: A Potential Target for the Treatment of Postmenopausal Osteoporosis. Nat. Publ. Gr. 2017, 5, 17046. [Google Scholar] [CrossRef]
- Yan, J.; Charles, J.F. Gut Microbiome and Bone: To Build, Destroy, or Both? Curr. Osteoporos. Rep. 2017, 15, 376–384. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and Health Benefits of Major Isoflavone Aglycones and Their Metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Pan, M.; El-Nezami, H.S.; Wan, J.M.F.; Wang, M.F.; Habimana, O.; Lee, J.C.Y.; Louie, J.C.Y.; Shah, N.P. Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, Prebiotics, and Synbiotics Regulate the Intestinal Microbiota Differentially and Restore the Relative Abundance of Specific Gut Microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Ślizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Feizollahzadeh, S.; Ghiasvand, R.; Rezaei, A.; Khanahmad, H.; sadeghi, A.; Hariri, M. Effect of Probiotic Soy Milk on Serum Levels of Adiponectin, Inflammatory Mediators, Lipid Profile, and Fasting Blood Glucose Among Patients with Type II Diabetes Mellitus. Probiotics Antimicrob. Proteins 2017, 9, 41–47. [Google Scholar] [CrossRef]
- Nagino, T.; Kano, M.; Masuoka, N.; Kaga, C.; Anbe, M.; Miyazaki, K.; Kamachi, K.; Isozaki, M.; Suzuki, C.; Kasuga, C.; et al. Intake of a Fermented Soymilk Beverage Containing Moderate Levels of Isoflavone Aglycones Enhances Bioavailability of Isoflavones in Healthy Premenopausal Japanese Women: A Double-Blind, Placebo-Controlled, Single-Dose, Crossover Trial. Biosci. Microbiota Food Health 2015, 35, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.M.; Ho, C.S.; Chen, Y.M.; Woo, J. Can Soy Intake Affect Serum Uric Acid Level? Pooled Analysis from Two 6-Month Randomized Controlled Trials among Chinese Postmenopausal Women with Prediabetes or Prehypertension. Eur. J. Nutr. 2015, 54, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Yasutake, T.; Matsumoto, M.; Mizuno, R.; Fujita, K.; Sasuga, Y. Effect of Lactic Acid Bacteria-Fermented Soy Milk Extract (LEX) on Urinary 3-Indoxyl Sulfate in Japanese Healthy Adult Women: An Open-Label Pilot Study. Nutr. Diet. Suppl. 2020, 12, 301–309. [Google Scholar] [CrossRef]
| Characteristics | All | SM | SMH Lc | SMH Lp |
|---|---|---|---|---|
| (n = 54) | (n = 19) | (n = 17) | (n = 18) | |
| Age (year) | 59.72 ± 7.68 | 65.16 ± 8.25 | 55.88 ± 4.53 | 57.61 ± 6.32 |
| Education | ||||
| Elementary school (%) | 48.1 (26) | 63.2 (12) | 47.1 (8) | 33.3 (6) |
| Yunior High School (%) | 14.8 (8) | 10.5 (2) | 11.8 (2) | 22.2 (4) |
| Senior High School (%) | 35.2 (19) | 21.1 (4) | 41.2 (7) | 44.4 (8) |
| College (%) | 1.9 (1) | 5.3 (1) | 0 (0) | 0 (0) |
| Profession | ||||
| House Wife (%) | 90.7 (49) | 94.7 (18) | 94.1 (16) | 83.3 (15) |
| Employee (%) | 1.9 (1) | 5.3 (1) | 0 (0) | 0 (0) |
| Self-employee (%) | 7.4 (4) | 0 (0) | 5.8(1) | 16.7 (3) |
| Weight (kg) | 59.44 ± 12.50 | 56.24 ± 14.31 | 61.65 ± 11.89 | 60.73 ± 10.91 |
| Height (cm) | 149.19 ± 5.95 | 148.47 ± 4.61 | 149.71 ± 8.12 | 149.44 ± 4.99 |
| BMI (kg/m2) | 26.69 ± 5.24 | 25.51 ± 6.19 | 27.44 ± 4.19 | 27.23 ± 5.09 |
| Time after menopause (year) | 9.09 ± 6.88 | 11.79 ± 9.07 | 6.53 ± 2.76 | 8.67 ± 6.18 |
| Calcium intake (mg) | 239.86 ± 169.35 | 232.68 ± 175.92 | 268.54 ± 199.28 | 220.33 ± 134.11 |
| Phospor intake (mg) | 586.88 ± 174 | 577.04 ± 179.88 | 605.87 ± 205.06 | 579.33 ± 141.51 |
| Magnesium intake (mg) | 177.34 ± 49.75 | 166.61 ± 39.46 | 182.89 ± 56.28 | 183.42 ± 53.73 |
| Variable | n | Mean | SD | p-Value * |
|---|---|---|---|---|
| Random Blood Glucose (mg/dL) | ||||
| SM | 19 | 104.95 | 19.72 | 0.68 |
| SMH Lc | 17 | 108.59 | 19.31 | |
| SMH Lp | 18 | 110.83 | 21.97 | |
| Uric acid(mg/dL) | ||||
| SM | 19 | 4.87 | 1.04 | 0.76 |
| SMH Lc | 17 | 4.82 | 1.13 | |
| SMH Lp | 18 | 5.07 | 1.00 | |
| Cholesterol (mg/dL) | ||||
| SM | 19 | 228.53 | 51.52 | 0.28 |
| SMH Lc | 17 | 236.82 | 34.72 | |
| SMH Lp | 18 | 214.78 | 33.23 | |
| Osteocalcin (ng/mL) | ||||
| SM | 19 | 31.60 | 12.47 | 0.55 |
| SMH Lc | 17 | 29.71 | 9.26 | |
| SMH Lp | 18 | 33.76 | 10.46 |
| Variable | n | Mean | SD | p-Value |
|---|---|---|---|---|
| SM | ||||
| Random Blood Glucose (mg/dL) | ||||
| Pre-test | 19 | 104.95 | 19.72 | 0.28 |
| Post-test | 19 | 110.58 | 19.13 | |
| Uric Acid (mg/dL) | ||||
| Pre-test | 19 | 4.87 | 1.04 | 0.57 |
| Post-test | 19 | 4.78 | 0.99 | |
| Cholesterol (mg/dL) | ||||
| Pre-test | 19 | 228.53 | 51.52 | 0.52 |
| Post-test | 19 | 224.21 | 48.55 | |
| SMH Lc | ||||
| Random Blood Glucose (mg/dL) | ||||
| Pre-test | 17 | 108.59 | 19.31 | 0.75 |
| Post-test | 17 | 110.12 | 24.66 | |
| Uric Acid (mg/dL) | ||||
| Pre-test | 17 | 4.81 | 1.13 | 0.75 |
| Post-test | 17 | 4.77 | 1.21 | |
| Cholesterol (mg/dL) | ||||
| Pre-test | 17 | 236.82 | 34.72 | 0.02 * |
| Post-test | 17 | 222.24 | 34.42 | |
| SMH Lp | ||||
| Random Blood Glucose (mg/dL) | ||||
| Pre-test | 18 | 110.83 | 21.97 | 0.12 |
| Post-test | 18 | 122.94 | 32.54 | |
| Uric Acid (mg/dL) | ||||
| Pre-test | 18 | 5.07 | 1.01 | 0.69 |
| Post-test | 18 | 5.01 | 1.18 | |
| Cholesterol (mg/dL) | ||||
| Pre-test | 18 | 214.78 | 33.23 | 0.69 |
| Post-test | 18 | 215.89 | 34.36 |
| Variable | n | Mean | SD | p-Value |
|---|---|---|---|---|
| SM | ||||
| Osteocalcin | ||||
| Pre-test | 19 | 31.59 | 12.46 | 0.40 |
| Post-test | 19 | 30.64 | 11.44 | |
| SMH Lc | ||||
| Osteocalcin | ||||
| Pre-test | 17 | 29.71 | 9.26 | 0.21 |
| Post-test | 17 | 27.74 | 8.81 | |
| SMH Lp | ||||
| Osteocalcin | ||||
| Pre-test | 18 | 33.76 | 10.46 | 0.02 * |
| Post-test | 18 | 29.34 | 12.54 |
| Variable | n | Mean | SD | p-Value * |
|---|---|---|---|---|
| SM | 19 | 30.64 | 11.44 | 0.73 |
| SMH Lc | 17 | 27.74 | 8.81 | |
| SMH Lp | 18 | 29.34 | 12.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desfita, S.; Sari, W.; Yusmarini, Y.; Pato, U.; Zakłos-Szyda, M.; Budryn, G. Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients 2021, 13, 3581. https://doi.org/10.3390/nu13103581
Desfita S, Sari W, Yusmarini Y, Pato U, Zakłos-Szyda M, Budryn G. Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients. 2021; 13(10):3581. https://doi.org/10.3390/nu13103581
Chicago/Turabian StyleDesfita, Sri, Wulan Sari, Yusmarini Yusmarini, Usman Pato, Małgorzata Zakłos-Szyda, and Grażyna Budryn. 2021. "Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women" Nutrients 13, no. 10: 3581. https://doi.org/10.3390/nu13103581
APA StyleDesfita, S., Sari, W., Yusmarini, Y., Pato, U., Zakłos-Szyda, M., & Budryn, G. (2021). Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients, 13(10), 3581. https://doi.org/10.3390/nu13103581








