Time-Restricted Feeding during Puberty Ameliorates Adiposity and Prevents Hepatic Steatosis in a Mouse Model of Childhood Obesity
Abstract
:1. Introduction
2. Materials and Methods
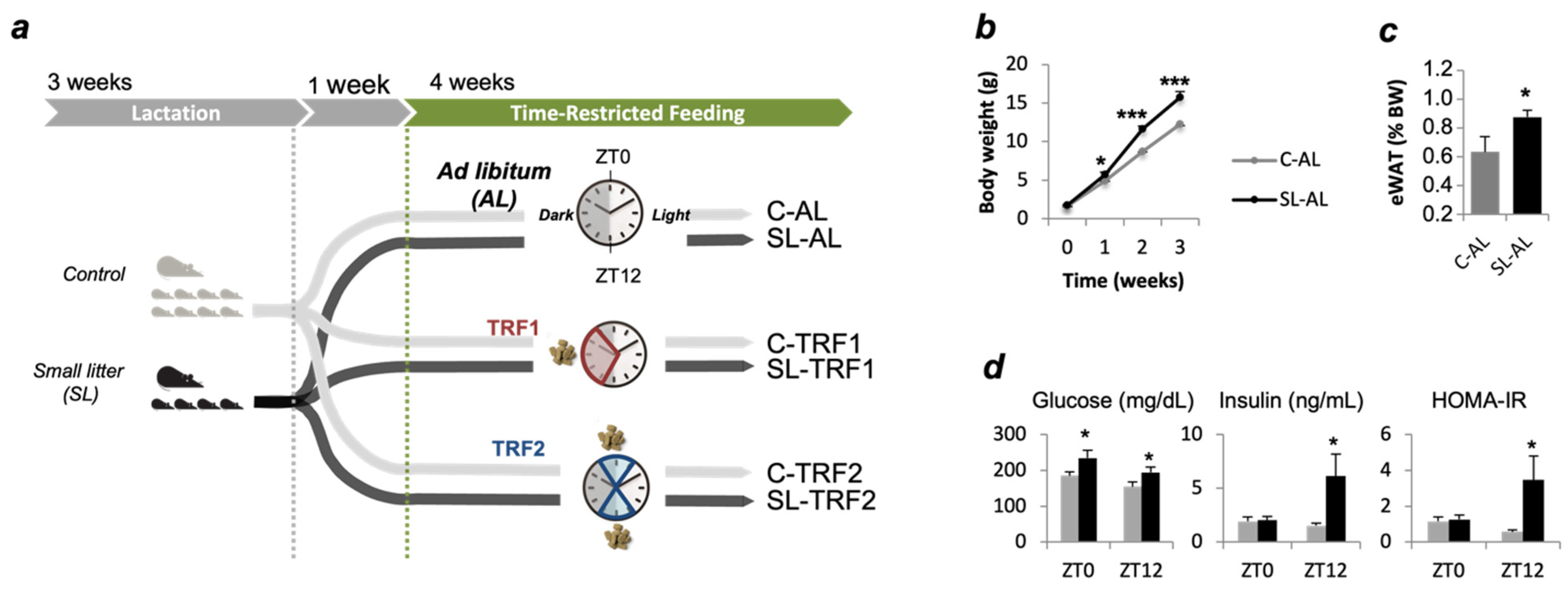
2.1. Animal Care and Experimental Design
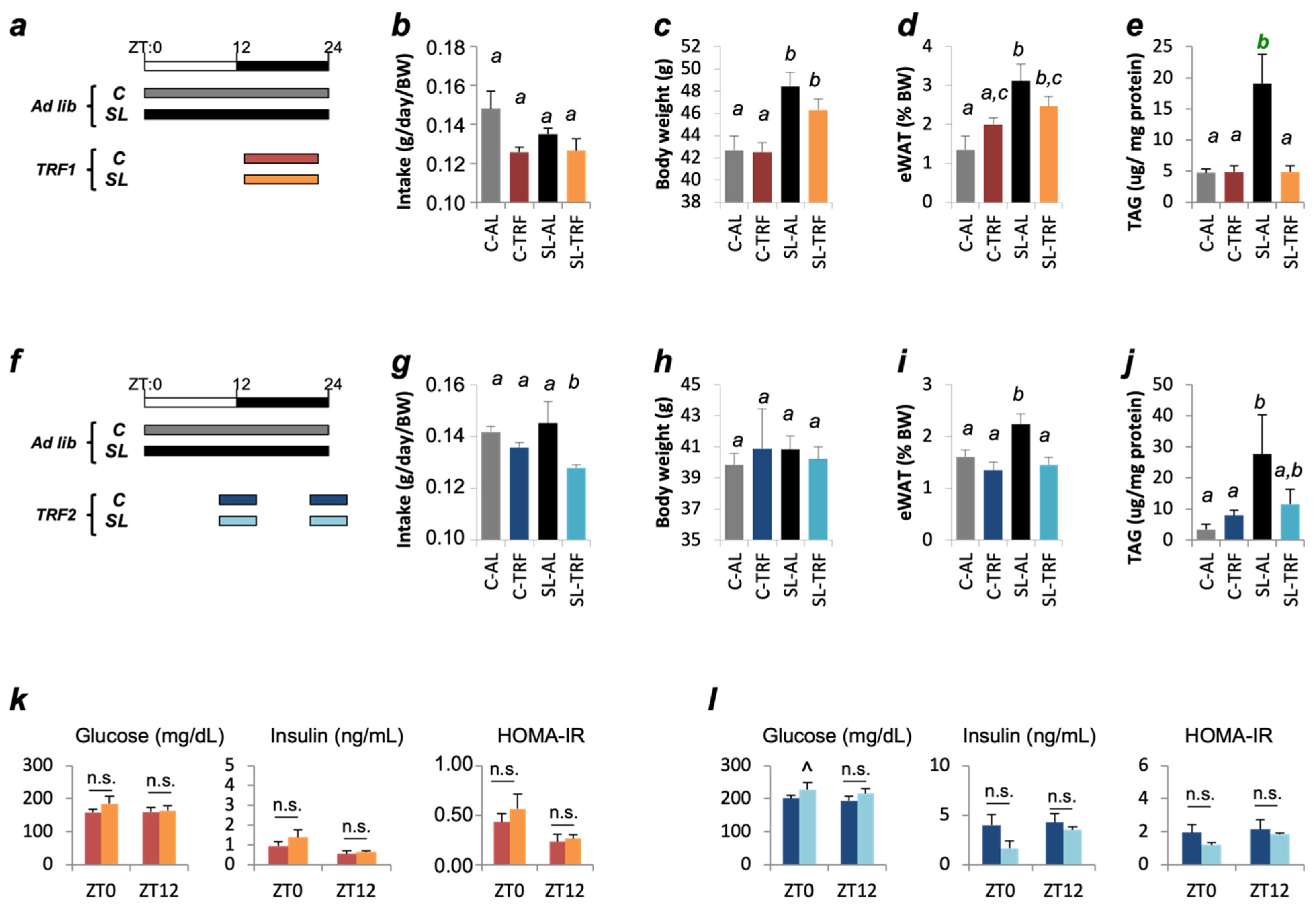
2.2. Time-Restricted Feeding (TRF)
2.3. Liver TAG Analysis
2.4. Plasma Metabolites
2.5. HOMA-IR
2.6. Real-Time Quantitative PCR (qPCR)
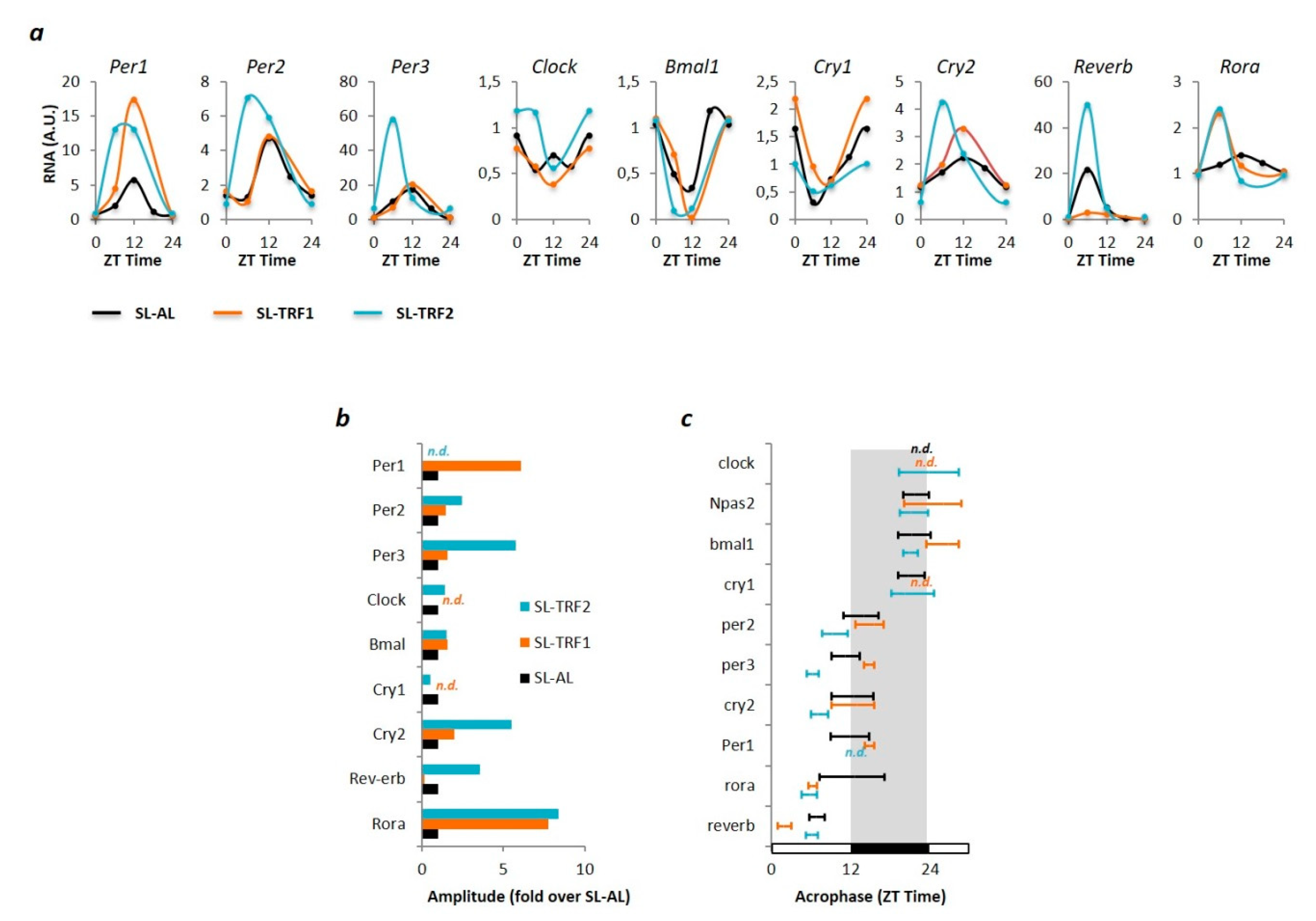
2.7. Circadian Rhythm Analysis (Cosinor Method)
2.8. Statistical Analysis
2.9. Data and Resource Availability
3. Results
3.1. TRFs Normalized Insulin Sensitivity and Hepatic TAG Content in SL Mice
3.2. TRF-Associated Metabolic Improvements Are Partially Mediated by Clock-Dependents and Clock-Independent Signals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The, N.S.; Suchindran, C.; North, K.E.; Popkin, B.M.; Gordon-Larsen, P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA J. Am. Med. Assoc. 2010, 304, 2042–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, L.J.; Langley-Evans, S.C.; McMullen, S. Childhood obesity and risk of the adult metabolic syndrome: A systematic review. Int. J. Obes. 2012, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.; Sattar, N. Childhood obesity: A ticking time bomb for cardiovascular disease. Clin. Pharmacol. Ther. 2011, 90, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 90, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric obesity—Assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Bass, R.; Eneli, I. Severe childhood obesity: An under-recognised and growing health problem. Postgrad. Med. J. 2015, 91, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; von Rosen, J.; Koletzko, B. Long-term consequences of early nutrition. Early Hum. Dev. 2006, 82, 567–574. [Google Scholar] [CrossRef]
- Metges, C.C. Early nutrition and later obesity: Animal models provide insights into mechanisms. In Early Nutrition Programming and Health Outcomes in Later Life. Advances in Experimental Medicine and Biology; Koletzko, B., Decsi, T., Molnár, D., de la Hunty, A., Eds.; Srpinger: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Reynolds, C.M.; Gray, C.; Li, M.; Segovia, S.A.; Vickers, M.H. Early life nutrition and energy balance disorders in offspring in later life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef] [PubMed]
- Adair, L.S. Long-term consequences of nutrition and growth in early childhood and possible preventive interventions. In Proceedings of the Nestle Nutrition Institute Workshop Series, Basel, Switzerland, 27 January 2014. [Google Scholar]
- Parra-Vargas, M.; Ramon-Krauel, M.; Lerin, C.; Jimenez-Chillaron, J.C. Size Does Matter: Litter Size Strongly Determines Adult Metabolism in Rodents. Cell Metab. 2020, 32, 334–340. [Google Scholar] [CrossRef]
- Pentinat, T.; Ramon-Krauel, M.; Cebria, J.; Diaz, R.; Jimenez-Chillaron, J.C. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology 2010, 151, 5617–5623. [Google Scholar] [CrossRef]
- Ramon-Krauel, M.; Pentinat, T.; Bloks, V.W.; Cebrià, J.; Ribo, S.; Pérez-Wienese, R.; Vilà, M.; Palacios-Marin, I.; Fernández-Pérez, A.; Vallejo, M.; et al. Epigenetic programming at the Mogat1 locus may link neonatal overnutrition with long-term hepatic steatosis and insulin resistance. FASEB J. 2018, 32, 6025–6037. [Google Scholar] [CrossRef]
- Ribas-Aulinas, F.; Ribo, S.; Parra-Vargas, M.; Fernández-Pérez, A.; Cebrià, J.; Guardiola-Perello, M.; Ramon-Krauel, M.; Lerin, C.; Diaz, R.; Kalko, S.G.; et al. Neonatal overfeeding during lactation rapidly and permanently misaligns the hepatic circadian rhythm and programmes adult NAFLD. Mol. Metab. 2021, 45, 101162. [Google Scholar] [CrossRef]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: From mice to men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidmar, A.P.; Goran, M.I.; Raymond, J.K. Time-Limited Eating in Pediatric Patients with Obesity-A Case Series. J. Food Sci. Nutr. Res. 2020, 2, 236. [Google Scholar] [CrossRef]
- Vidmar, A.P.; Goran, M.I.; Naguib, M.; Fink, C.; Wee, C.P.; Hegedus, E.; Lopez, K.; Gonzalez, J.; Raymond, J.K. Time limited eating in adolescents with obesity (time LEAd): Study protocol. Contemp. Clin. Trials 2020, 95, 106082. [Google Scholar] [CrossRef] [PubMed]
- Roza, N.A.V.; Possignolo, L.F.; Palanch, A.C.; Gontijo, J.A.R. Effect of long-term high-fat diet intake on peripheral insulin sensibility, blood pressure, and renal function in female rats. Food Nutr. Res. 2016, 60, 28536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornélissen, G.; Halberg, F.; Stebbings, J.; Halberg, E.; Carandente, F.; Hsi, B. Chronobiometry with pocket calculators and computer systems. Ric. Clin. Lab. 1980, 10, 333–385. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.; Arbogast, B.; Cornelissen Guillaume, G.; Lee, J.K.; Halberg, F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 1982, 9, 397–439. [Google Scholar] [PubMed]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian clock genes in the metabolism of non-alcoholic fatty liver disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Gabory, A. Epigenetic mechanisms involved in developmental nutritional programming. World J. Diabetes 2011, 2, 164. [Google Scholar] [CrossRef]
- Lundell, L.S.; Parr, E.B.; Devlin, B.L.; Ingerslev, L.R.; Altıntaş, A.; Sato, S.; Sassone-Corsi, P.; Barrès, R.; Zierath, J.R.; Hawley, J.A. Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat. Commun. 2020, 11, 4643. [Google Scholar] [CrossRef]
- Deota, S.; Panda, S. New Horizons: Circadian Control of Metabolism Offers Novel Insight Into the Cause and Treatment of Metabolic Diseases. J. Clin. Endocrinol. Metab. 2021, 106, e1488–e1493. [Google Scholar] [CrossRef]
- Sherman, H.; Frumin, I.; Gutman, R.; Chapnik, N.; Lorentz, A.; Meylan, J.; le Coutre, J.; Froy, O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J. Cell. Mol. Med. 2011, 15, 2745–2759. [Google Scholar] [CrossRef] [Green Version]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinouchi, K.; Magnan, C.; Ceglia, N.; Liu, Y.; Cervantes, M.; Pastore, N.; Huynh, T.; Ballabio, A.; Baldi, P.; Masri, S.; et al. Fasting Imparts a Switch to Alternative Daily Pathways in Liver and Muscle. Cell Rep. 2018, 25, 3299–3314.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Ye, Y.; Mao, Y.; Liao, W.; Xu, W. Time-restricted feeding during childhood has persistent effects on mice commensal microbiota. Ann. Transl. Med. 2019, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Mao, Y.; Xu, G.; Liao, W.; Ren, J.; Yang, H.; Yang, J.; Sun, L.; Chen, H.; Wang, W.; et al. Time-restricted feeding causes irreversible metabolic disorders and gut microbiota shift in pediatric mice. Pediatr. Res. 2019, 85, 518–526. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Aulinas, F.; Parra-Vargas, M.; Ramon-Krauel, M.; Diaz, R.; Lerin, C.; Cambras, T.; Jimenez-Chillaron, J.C. Time-Restricted Feeding during Puberty Ameliorates Adiposity and Prevents Hepatic Steatosis in a Mouse Model of Childhood Obesity. Nutrients 2021, 13, 3579. https://doi.org/10.3390/nu13103579
Ribas-Aulinas F, Parra-Vargas M, Ramon-Krauel M, Diaz R, Lerin C, Cambras T, Jimenez-Chillaron JC. Time-Restricted Feeding during Puberty Ameliorates Adiposity and Prevents Hepatic Steatosis in a Mouse Model of Childhood Obesity. Nutrients. 2021; 13(10):3579. https://doi.org/10.3390/nu13103579
Chicago/Turabian StyleRibas-Aulinas, Francesc, Marcela Parra-Vargas, Marta Ramon-Krauel, Ruben Diaz, Carles Lerin, Trinitat Cambras, and Josep C. Jimenez-Chillaron. 2021. "Time-Restricted Feeding during Puberty Ameliorates Adiposity and Prevents Hepatic Steatosis in a Mouse Model of Childhood Obesity" Nutrients 13, no. 10: 3579. https://doi.org/10.3390/nu13103579
APA StyleRibas-Aulinas, F., Parra-Vargas, M., Ramon-Krauel, M., Diaz, R., Lerin, C., Cambras, T., & Jimenez-Chillaron, J. C. (2021). Time-Restricted Feeding during Puberty Ameliorates Adiposity and Prevents Hepatic Steatosis in a Mouse Model of Childhood Obesity. Nutrients, 13(10), 3579. https://doi.org/10.3390/nu13103579





