Relationship between Energy Balance and Circulating Levels of Hepcidin and Ferritin in the Fasted and Postprandial States
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Assessment of Dietary Iron Intake
2.3. Ascertainment of Diabetes Status
2.4. Mixed Meal Test
2.5. Laboratory Variables
2.6. Other Variables
2.7. Statistical Analyses
3. Results
3.1. Characteristics of Study Participants
3.2. Associations of Markers of Iron Metabolism in the Overall Cohort
3.3. Associations of Markers of Iron Metabolism in the Sub-Groups Stratified by Dietary Iron Intake
3.4. Associations of Markers of Iron Metabolism in the Sub-Groups Stratified by Diabetes Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Halaas, J.L.; Boozer, C.; Blair-West, J.; Fidahusein, N.; Denton, D.A.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8878–8883. [Google Scholar] [CrossRef] [Green Version]
- Elmquist, J.K.; Maratos-Flier, E.; Saper, C.B.; Flier, J.S. Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1998, 1, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Wierup, N.; Svensson, H.; Mulder, H.; Sundler, F. The ghrelin cell: A novel developmentally regulated islet cell in the human pancreas. Regul. Pept. 2002, 107, 63–69. [Google Scholar] [CrossRef]
- Cortelazzi, D.; Cappiello, V.; Morpurgo, P.; Ronzoni, S.; Nobile De Santis, M.; Cetin, I.; Beck-Peccoz, P.; Spada, A. Circulating levels of ghrelin in human fetuses. Eur. J. Endocrinol. 2003, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Kirsz, K.; Zieba, D.A. Ghrelin-mediated appetite regulation in the central nervous system. Peptides 2011, 32, 2256–2264. [Google Scholar] [CrossRef]
- Hosoda, H. Ghrelin and the regulation of food intake and energy balance. Mol. Interv. 2002, 2, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Borer, K.T.; Wuorinen, E.; Burant, C. Ghrelin/leptin ratio tracks energy balance, while ratings of appetite track only meal size and give paradoxical values to exercise energy expenditure. Appetite 2008, 51, 355. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Díaz-Lagares, A.; Abete, I.; Goyenechea, E.; Amil, M.; Martínez, J.A.; Casanueva, F.F. Pre-treatment circulating leptin/ghrelin ratio as a non-invasive marker to identify patients likely to regain the lost weight after an energy restriction treatment. J. Endocrinol. Investig. 2014, 37, 119–126. [Google Scholar] [CrossRef]
- Finch, C. Regulators of iron balance in humans. Blood 1994, 84, 1697–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.D. Adaptation in Iron Metabolism. Am. J. Clin. Nutr. 1990, 51, 305–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, β-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Hu, X.; Yuan, B.; Pan, X.; Meyer, H.E.; Holmboe-Ottesen, G. Association between serum ferritin, hemoglobin, iron intake, and diabetes in adults in Jiangsu, China. Diabetes Care 2006, 29, 1878–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Ma, J.; Ascherio, A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: A prospective cohort study. Am. J. Clin. Nutr. 2004, 79, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Sun, Z.Z.; Tang, Y.T.; Xu, C.; Jiao, X.Y. Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res. Clin. Pract. 2011, 93, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Traglia, M.; Campostrini, N.; Biino, G.; Corbella, M.; Sala, C.; Busti, F.; Masciullo, C.; Manna, D.; Previtali, S.; et al. Increased serum hepcidin levels in subjects with the metabolic syndrome: A population study. PLoS ONE 2012, 7, e48250. [Google Scholar] [CrossRef]
- Rodríguez-Mortera, R.; Caccavello, R.; Hermo, R.; Garay-Sevilla, M.E.; Gugliucci, A. Higher hepcidin levels in adolescents with obesity are associated with metabolic syndrome dyslipidemia and visceral fat. Antioxidants 2021, 10, 751. [Google Scholar] [CrossRef]
- Pechlaner, R.; Weiss, G.; Bansal, S.; Mayr, M.; Santer, P.; Pallhuber, B.; Notdurfter, M.; Bonora, E.; Willeit, J.; Kiechl, S. Inadequate hepcidin serum concentrations predict incident type 2 diabetes mellitus. Diabetes. Metab. Res. Rev. 2016, 32, 187–192. [Google Scholar] [CrossRef]
- Bawadi, H.; Katkhouda, R.; Al-Haifi, A.; Tayyem, R.; Elkhoury, C.F.; Jamal, Z. Energy balance and macronutrient distribution in relation to C-reactive protein and HbA1c levels among patients with type 2 diabetes. Food Nutr. Res. 2016, 60, 29904. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H. Energy balance and weight loss for diabetes remission. Diabetes Spectr. 2020, 33, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.Q.; Zhou, G.; Huang, S.N.; Mu, M.D.; Chen, Y.J.; Qian, Z.M. Ghrelin is negatively correlated with iron in the serum in human and mice. Ann. Nutr. Metab. 2018, 72, 37–42. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte iron regulates leptin and food intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, J.; Bai, Z.; Sink, S.; Zhao, C.; Lorenzo, F.R.; McClain, D.A. Iron down-regulates leptin by suppressing protein O-GlcNAc modification in adipocytes, resulting in decreased levels of O-glycosylated CREB. J. Biol. Chem. 2019, 294, 5487–5495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Hu, J.; Yang, G.; Yuan, X.; Chen, Z.; Wang, D.; Lu, Y.; Zhu, L.; Wang, G. Fasting increases iron export by modulating ferroportin 1 expression through the ghrelin/GHSR1α/MAPK pathway in the liver. Biol. Trace Elem. Res. 2021, 199, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Shahramian, I.; Akhlaghi, E.; Ramezani, A.; Rezaee, A.; Noori, N.; Sharafi, E. A study of leptin serum concentrations in patients with major beta-thalassemia. Iran. J. Pediatr. Hematol. Oncol. 2013, 3, 59–63. [Google Scholar]
- Karachaliou, F.; Vlachopapadopoulou, E.; Theochari, M.; Konstandellou, E.; Michalados, S. Leptin levels in patients with thalassemia major. Minerva Pediatr. 2006, 58, 373–378. [Google Scholar] [PubMed]
- Dedoussis, G.V.; Kyrtsonis, M.C.; Andrikopoulos, N.E.; Voskaridou, E.; Loutradis, A. Inverse correlation of plasma leptin and soluble transferrin receptor levels in beta-thalassemia patients. Ann. Hematol. 2002, 81, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, N.; Orbak, Z.; Karakelloglu, C.; Akcay, F. The effect of therapy on plasma ghrelin and leptin levels, and appetite in children with iron deficiency anemia. J. Pediatr. Endocrinol. Metab. 2019, 32, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.W.; Latham, M.C.; Stephenson, L.S.; Kinoti, S.N.; Pertet, A.M. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J. Nutr. 1994, 124, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Bharmal, S.H.; Pendharkar, S.A.; Singh, R.G.; Cameron-Smith, D.; Petrov, M.S. Associations between ketone bodies and fasting plasma glucose in individuals with post-pancreatitis prediabetes. Arch. Physiol. Biochem. 2020, 126, 308–319. [Google Scholar] [CrossRef]
- Petrov, M.S. Post-pancreatitis diabetes mellitus: Prime time for secondary disease. Eur. J. Endocrinol. 2021, 184, R137–R149. [Google Scholar] [CrossRef]
- McKenzie, S.J.L.; Premkumar, R.; Askelund, K.J.; Pendharkar, S.A.; Phillips, A.R.J.; Windsor, J.A.; Petrov, M.S. The effect of enteral nutrition on adipokines in patients with acute pancreatitis. J. Nutr. Sci. 2015, 4, e33. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.M.; Premkumar, R.; Phillips, A.R.; Windsor, J.A.; Petrov, M.S. Ghrelin and gastroparesis as early predictors of clinical outcomes in acute pancreatitis. Pancreatology 2016, 16, 181–188. [Google Scholar] [CrossRef]
- Kennedy, J.I.; Askelund, K.J.; Premkumar, R.; Phillips, A.R.; Murphy, R.; Windsor, J.A.; Petrov, M.S. Leptin is associated with persistence of hyperglycemia in acute pancreatitis. Medicine 2016, 95, e2382. [Google Scholar] [CrossRef]
- Singh, R.G.; Pendharkar, S.A.; Gillies, N.A.; Miranda-Soberanis, V.; Plank, L.D.; Petrov, M.S. Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin. Exp. Med. 2017, 17, 477–487. [Google Scholar] [CrossRef]
- Maraví Poma, E.; Zubia Olascoaga, F.; Petrov, M.S.; Navarro Soto, S.; Laplaza Santos, C.; Morales Alava, F.; Darnell Martin, A.; Gorraiz López, B.; Bolado Concejo, F.; Casi Villarroya, M.; et al. SEMICYUC 2012. Recommendations for intensive care management of acute pancreatitis. Med. Intensiva 2013, 37, 163–179. [Google Scholar] [CrossRef]
- Kimita, W.; Petrov, M.S. Iron metabolism and the exocrine pancreas. Clin. Chim. Acta 2020, 511, 167–176. [Google Scholar] [CrossRef]
- Li, X.; Kimita, W.; Cho, J.; Ko, J.; Bharmal, S.H.; Petrov, M.S. Dietary fibre intake in type 2 and new-onset prediabetes/diabetes after acute pancreatitis: A nested cross-sectional study. Nutrients 2021, 13, 1112. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US) Panel on Micronutrients Iron. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001; ISBN 10: 0-309-07290-5. [Google Scholar]
- Petrov, M.S.; Basina, M. Diagnosis of endocrine disease: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur. J. Endocrinol. 2021, 184, R151–R163. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Pendharkar, S.; Singh, R.G.; Cho, J.; Petrov, M.S. Glucose counter-regulation after acute pancreatitis. Pancreas 2019, 48, 670–681. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; Stuart, C.E.; Alarcon Ramos, G.C.; Ko, J.; Petrov, M.S. Oxyntomodulin may distinguish new-onset diabetes after acute pancreatitis from type 2 diabetes. Clin. Transl. Gastroenterol. 2020, 11, e00132. [Google Scholar] [CrossRef]
- Cervantes, A.; Singh, R.G.; Kim, J.U.; DeSouza, S.V.; Petrov, M.S. Relationship of anthropometric indices to abdominal body composition: A multi-ethnic New Zealand magnetic resonance imaging study. J. Clin. Med. Res. 2019, 11, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.F.; Hassan, M.; Nazar, H.S.; Gillani, S.; Afzal, N.; Qayyum, I. Effect of body mass index on serum leptin levels. J. Ayub Med. Coll. Abbottabad 2011, 23, 40–43. [Google Scholar]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeely, M.J.; Boyko, E.J.; Weigle, D.S.; Shofer, J.B.; Chessler, S.D.; Leonnetti, D.L.; Fujimoto, W.Y. Association between baseline plasma leptin levels and subsequent development of diabetes in Japanese Americans. Diabetes Care 1999, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, P.U. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism 1976, 25, 1567–1574. [Google Scholar] [CrossRef]
- Fumeron, F.; Péan, F.; Driss, F.; Balkau, B.; Tichet, J.; Marre, M.; Grandchamp, B.; Insulin Resistance Syndrome (DESIR) Study Group. Ferritin and transferrin are both predictive of the onset of hyperglycemia in men and women over 3 years: The data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. Diabetes Care 2006, 29, 2090–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Apekey, T.A.; Walley, J.; Kain, K. Ferritin levels and risk of type 2 diabetes mellitus: An updated systematic review and meta-analysis of prospective evidence. Diabetes. Metab. Res. Rev. 2013, 29, 308–318. [Google Scholar] [CrossRef]
- Welsh, P.; Murray, H.; Buckley, B.; de Craen, A.; Ford, I.; Jukema, J.; Macfarlane, P.; Packard, C.; Stott, D.; Westendorp, R.; et al. Leptin predicts diabetes but not cardiovascular disease: Results from a large prospective study in an elderly population. Diabetes Care 2009, 32, 308–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, B.M.; Dulin, W.E. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 1970, 6, 268–273. [Google Scholar] [CrossRef]
- Cho, J.; Scragg, R.; Petrov, M.S. Risk of mortality and hospitalization after post-pancreatitis diabetes mellitus vs type 2 diabetes mellitus: A population-based matched cohort study. Am. J. Gastroenterol. 2019, 114, 804–812. [Google Scholar] [CrossRef]
- Cho, J.; Scragg, R.; Petrov, M.S. Postpancreatitis diabetes confers higher risk for pancreatic cancer than type 2 diabetes: Results from a nationwide cancer registry. Diabetes Care 2020, 43, 2106–2112. [Google Scholar] [CrossRef]
- Woodmansey, C.; McGovern, A.P.; McCullough, K.A.; Whyte, M.B.; Munro, N.M.; Correa, A.C.; Gatenby, P.A.C.; Jones, S.A.; de Lusignan, S. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (Type 3c): A retrospective cohort study. Diabetes Care 2017, 40, 1486–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Scragg, R.; Pandol, S.J.; Goodarzi, M.O.; Petrov, M.S. Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: A nationwide cohort study. Diabetes Care 2019, 42, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.S.; Svane, H.M.L.; Nicolaisen, S.K.; Kristensen, J.K.; Drewes, A.M.; Brandslund, I.; Beck-Nielsen, H.; Nielsen, J.S.; Thomsen, R.W. Clinical and biochemical characteristics of postpancreatitis diabetes mellitus: A cross-sectional study from the Danish Nationwide DD2 cohort. J. Diabetes 2021. [Online ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Nguyen, N.N.; Cervantes, A.; Alarcon Ramos, G.C.; Cho, J.; Petrov, M.S. Associations between intra-pancreatic fat deposition and circulating levels of cytokines. Cytokine 2019, 120, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.E.; Ko, J.; Modesto, A.E.; Alarcon Ramos, G.C.; Bharmal, S.H.; Cho, J.; Singh, R.G.; Petrov, M.S. Implications of tobacco smoking and alcohol consumption on ectopic fat deposition in individuals after pancreatitis. Pancreas 2020, 49, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Skudder-Hill, L.; Cho, J.; Bharmal, S.H.; Petrov, M.S. The relationship between abdominal fat phenotypes and insulin resistance in non-obese individuals after acute pancreatitis. Nutrients 2020, 12, 2883. [Google Scholar] [CrossRef]
- Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Batterham, R.L.; Park, A.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 2003, 88, 4696–4701. [Google Scholar] [CrossRef]
- Schepp, W.; Dehne, K.; Riedel, T.; Schmidtler, J.; Schaffer, K.; Classen, M. Oxyntomodulin: A cAMP-dependent stimulus of rat parietal cell function via the receptor for glucagon-like peptide-1 (7-36)NH2. Digestion 1996, 57, 398–405. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 2006, 55, 3387–3393. [Google Scholar] [CrossRef] [Green Version]
- Chand, S.K.; Singh, R.G.; Pendharkar, S.A.; Petrov, M.S. Iron: A strong element in the pathogenesis of chronic hyperglycaemia after acute pancreatitis. Biol. Trace Elem. Res. 2018, 183, 71–79. [Google Scholar] [CrossRef]
- Chand, S.K.; Singh, R.G.; Pendharkar, S.A.; Bharmal, S.H.; Petrov, M.S. Interplay between innate immunity and iron metabolism after acute pancreatitis. Cytokine 2018, 103, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.B.; Koistinen, H.A.; Zierath, J.R.; Sweeney, G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002, 16, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Seufert, J. Leptin effects on pancreatic β-cell gene expression and function. Diabetes 2004, 53, S152–S158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Overall Cohort (n = 76) | Postprandial Sub-Cohort (n = 34) | p-Value |
|---|---|---|---|
| Age (years) | 52.8 (14.9) | 58.7 (6.4) | 0.011 |
| Men n (%) | 51 (67) | 24 (71) | 0.850 |
| Body mass index (kg/m2) | 29.3 (6.3) | 28.5 (6.4) | 0.461 |
| Waist circumference (cm) | 99.6 (14.8) | 99.2 (15.9) | 0.973 |
| Hip circumference (cm) | 104.5 (14.9) | 101.8 (13.3) | 0.199 |
| Waist-to-hip ratio | 0.96 (0.08) | 0.97 (0.07) | 0.131 |
| Fasting plasma leptin (ng/mL) | 5.4 (2.5–16.2) | 4.1 (2.4–13.7) a | 0.010 |
| Fasting plasma ghrelin (pg/mL) | 16.2 (8.2–25.5) | 14.4 (9.0–27.7) a | 0.801 |
| Dietary iron intake (mg/day) | 9.4 (7.5–13.3) | 10.4 (8.1–13.5) | 0.592 |
| Energy intake (kcal/day) | 1628.0 (1226.5–2135.7) | 1983.9 (1288.7–2382.3) | 0.404 |
| Alcohol (g/day) | 1.6 (0–10.0) | 3.3 (0–10.7) | 0.531 |
| Basal metabolic rate (kcal/day) | 1626.9 (296.0) | 1595.0 (307.0) | 0.522 |
| Fasting blood glucose (mmol/L) | 5.5 (5.1–6.5) | 5.5 (5.3–7.1) | 0.215 |
| Haemoglobin A1c (mmol/mol) | 38 (35–41) | 40 (37–44) | 0.202 |
| Recurrence of acute pancreatitis | 0.760 | ||
| No | 55 (72) | 23 (68) | |
| Yes | 21 (28) | 11 (32) | |
| Aetiology | 0.441 | ||
| Biliary | 34 (41) | 17(51) | |
| Alcohol-related | 16 (20) | 6 (18) | |
| Other | 26 (39) | 10 (30) | |
| Use of antidiabetic medications | 0.563 | ||
| No | 66 | 30 | |
| Yes | 10 | 4 | |
| Time since first pancreatitis attack (months) | 19 (14–27) | 19.6 (10–23) | 0.060 |
| Marker | State | Model | Hepcidin | Ferritin | ||||
|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |||
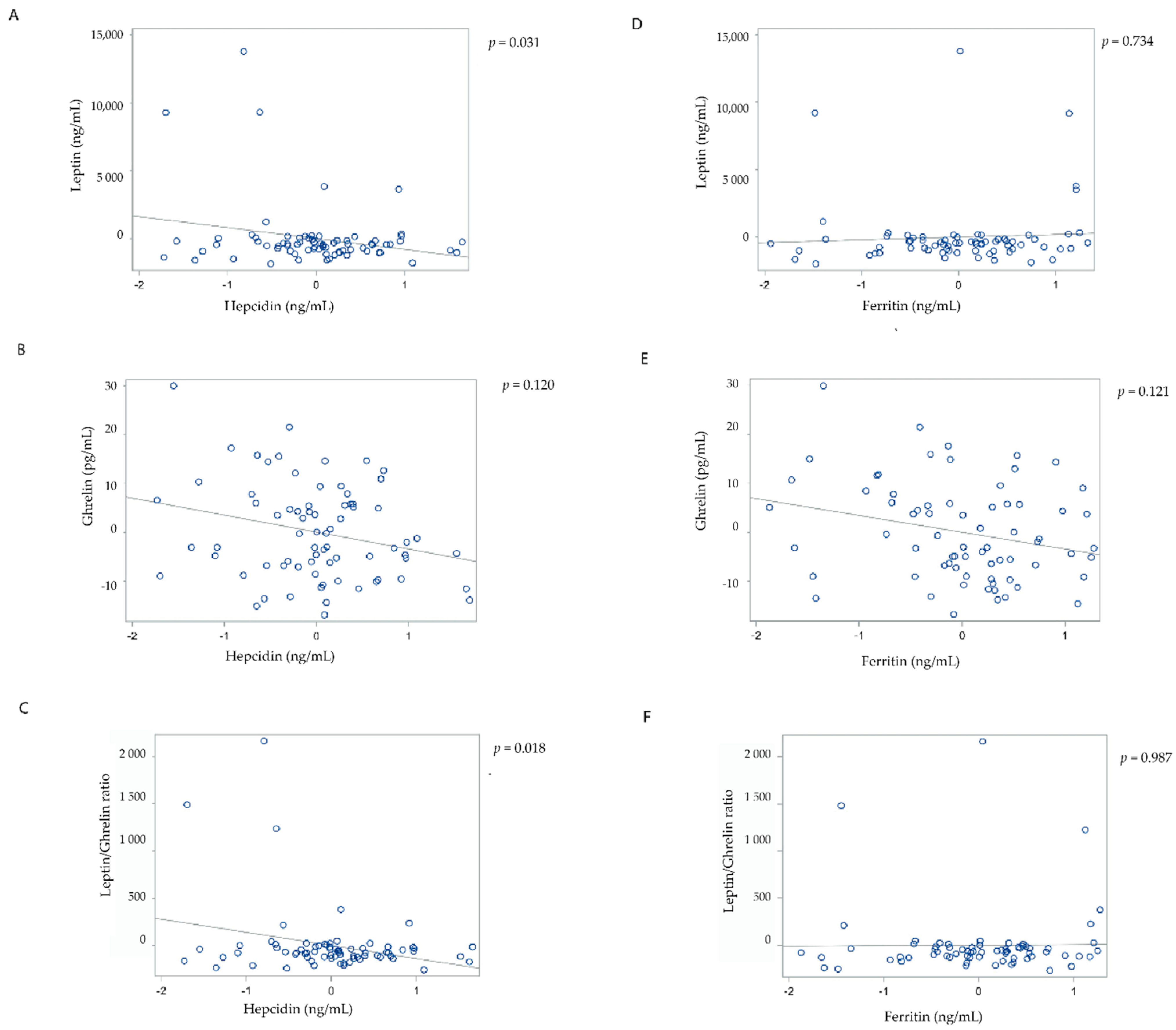
| Leptin | Fasted | 1 | −839.02 | 343.39 | 0.017 | −84.02 | 327.21 | 0.798 |
| 2 | −766.37 | 380.79 | 0.048 | 183.02 | 382.64 | 0.634 | ||
| 3 | −797.52 | 384.13 | 0.042 | 163.51 | 387.23 | 0.674 | ||
| 4 | −883.45 | 400.94 | 0.031 | 138.11 | 403.84 | 0.734 | ||
| Postprandial | 1 | 39.79 | 53.48 | 0.463 | 84.05 | 33.39 | 0.444 | |
| 2 | −11.84 | 64.91 | 0.857 | 97.40 | 43.02 | 0.247 | ||
| 3 | −13.59 | 66.44 | 0.840 | 98.49 | 44.14 | 0.171 | ||
| 4 | −12.21 | 71.67 | 0.866 | 83.95 | 45.71 | 0.420 | ||
| Ghrelin | Fasted | 1 | −2.31 | 1.53 | 0.136 | −2.19 | 1.42 | 0.126 |
| 2 | −2.53 | 1.69 | 0.139 | −2.83 | 1.67 | 0.094 | ||
| 3 | −1.84 | 1.57 | 0.247 | −2.07 | 1.56 | 0.189 | ||
| 4 | −2.47 | 1.57 | 0.120 | −2.46 | 1.56 | 0.121 | ||
| Postprandial | 1 | 177.59 | 81.60 | 0.037 | 28.02 | 72.70 | 0.703 | |
| 2 | 176.17 | 92.70 | 0.067 | 23.87 | 88.23 | 0.789 | ||
| 3 | 178.64 | 98.09 | 0.079 | 15.01 | 92.35 | 0.872 | ||
| 4 | 203.55 | 107.53 | 0.070 | 8.28 | 100.95 | 0.936 | ||
| Leptin/ghrelin ratio | Fasted | 1 | −135.64 | 51.64 | 0.011 | −25.01 | 50.84 | 0.624 |
| 2 | −131.09 | 57.03 | 0.025 | 4.20 | 59.49 | 0.944 | ||
| 3 | −136.32 | 57.65 | 0.021 | 0.62 | 60.48 | 0.992 | ||
| 4 | −148.26 | 61.20 | 0.018 | −1.02 | 63.92 | 0.987 | ||
| Postprandial | 1 | 508.93 | 3474.50 | 0.884 | −658.64 | 1324.33 | 0.623 | |
| 2 | −1703.07 | 4307.24 | 0.696 | −1581.14 | 1818.22 | 0.394 | ||
| 3 | −1924.32 | 4549.60 | 0.676 | −1587.83 | 1933.07 | 0.421 | ||
| 4 | −1501.89 | 4261.40 | 0.728 | −939.35 | 1902.81 | 0.628 | ||
| Marker | Iron Intake | Model | Hepcidin | Ferritin | ||||
|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |||
| Leptin | “Insufficient” iron intake | 1 | −560.17 | 527.67 | 0.299 | 384.06 | 457.41 | 0.409 |
| 2 | −299.91 | 693.95 | 0.670 | 995.04 | 571.63 | 0.096 | ||
| 3 | −289.77 | 708.71 | 0.690 | 998.47 | 583.61 | 0.103 | ||
| 4 | −456.75 | 718.98 | 0.533 | 975.97 | 634.17 | 0.141 | ||
| “Sufficient” iron intake | 1 | −678.34 | 460.41 | 0.148 | 86.43 | 400.90 | 0.830 | |
| 2 | −673.04 | 454.84 | 0.147 | −3.74 | 456.76 | 0.994 | ||
| 3 | −714.47 | 469.01 | 0.136 | −22.07 | 476.69 | 0.963 | ||
| 4 | −496.29 | 529.24 | 0.354 | 53.15 | 482.07 | 0.913 | ||
| Ghrelin | “Insufficient” iron intake | 1 | −0.56 | 2.72 | 0.838 | −0.66 | 2.44 | 0.788 |
| 2 | −1.68 | 3.48 | 0.635 | −1.81 | 3.23 | 0.582 | ||
| 3 | −1.68 | 3.52 | 0.640 | −1.64 | 3.30 | 0.625 | ||
| 4 | −2.89 | 3.37 | 0.403 | −2.99 | 3.16 | 0.358 | ||
| “Sufficient” iron intake | 1 | −4.11 | 2.22 | 0.070 | −3.94 | 1.88 | 0.042 | |
| 2 | −3.98 | 2.30 | 0.091 | −4.14 | 2.26 | 0.075 | ||
| 3 | −2.33 | 1.95 | 0.239 | −2.21 | 1.95 | 0.263 | ||
| 4 | −3.20 | 2.17 | 0.148 | −2.06 | 1.99 | 0.308 | ||
| Leptin/ghrelin ratio | “Insufficient” iron intake | 1 | −76.15 | 71.96 | 0.301 | 60.77 | 65.09 | 0.360 |
| 2 | −50.91 | 93.83 | 0.593 | 139.10 | 82.51 | 0.107 | ||
| 3 | −50.89 | 95.83 | 0.601 | 137.54 | 84.90 | 0.121 | ||
| 4 | −74.00 | 97.20 | 0.456 | 119.70 | 88.82 | 0.195 | ||
| “Sufficient” iron intake | 1 | −107.56 | 724.54 | 0.145 | 7.78 | 64.33 | 0.904 | |
| 2 | −105.27 | 71.71 | 0.150 | −6.99 | 73.44 | 0.925 | ||
| 3 | −111.16 | 73.89 | 0.141 | −9.88 | 76.52 | 0.898 | ||
| 4 | −696.89 | 85.15 | 0.418 | 10.67 | 77.88 | 0.892 | ||
| Marker | Model | Diabetes Status | Hepcidin | Ferritin | ||||
|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |||
| Leptin | 1 | Normoglycaemia | −1964.10 | 1549.34 | 0.226 | 927.80 | 1697.09 | 0.595 |
| 2 | −2813.72 | 2088.47 | 0.205 | 1484.94 | 2131.15 | 0.504 | ||
| 3 | −2809.74 | 2187.83 | 0.228 | 1464.94 | 2332.29 | 0.547 | ||
| 4 | −3051.06 | 2258.88 | 0.210 | 1550.02 | 2407.59 | 0.540 | ||
| 1 | T2DM | 185.92 | 283.34 | 0.519 | 108.88 | 283.22 | 0.705 | |
| 2 | 400.71 | 265.85 | 0.149 | 362.17 | 272.18 | 0.201 | ||
| 3 | 351.80 | 275.21 | 0.218 | 332.78 | 275.19 | 0.244 | ||
| 4 | 126.98 | 366.45 | 0.734 | 191.25 | 317.29 | 0.556 | ||
| 1 | NODAP | −818.31 | 346.00 | 0.024 | −336.33 | 301.00 | 0.272 | |
| 2 | −752.19 | 389.38 | 0.062 | −214.77 | 412.01 | 0.606 | ||
| 3 | −754.03 | 395.13 | 0.066 | −212.73 | 418.32 | 0.615 | ||
| 4 | −806.09 | 395.44 | 0.050 | −302.96 | 415.91 | 0.472 | ||
| Ghrelin | 1 | Normoglycaemia | −1.69 | 3.86 | 0.669 | −3.59 | 3.65 | 0.345 |
| 2 | 3.55 | 5.04 | 0.496 | −5.18 | 4.50 | 0.280 | ||
| 3 | 3.58 | 5.23 | 0.510 | −6.08 | 4.76 | 0.237 | ||
| 4 | 3.78 | 5.08 | 0.476 | −5.53 | 4.73 | 0.281 | ||
| 1 | T2DM | −7.50 | 2.78 | 0.014 | −5.94 | 3.47 | 0.104 | |
| 2 | −7.82 | 2.96 | 0.018 | −6.40 | 3.77 | 0.110 | ||
| 3 | −6.24 | 2.56 | 0.028 | −5.24 | 3.17 | 0.121 | ||
| 4 | −7.02 | 3.70 | 0.080 | −4.41 | 4.17 | 0.311 | ||
| 1 | NODAP | 0.03 | 1.93 | 0.986 | −1.02 | 1.58 | 0.525 | |
| 2 | −0.39 | 2.12 | 0.856 | −2.12 | 2.10 | 0.321 | ||
| 3 | 0.61 | 3.12 | 1.856 | −2.10 | 2.13 | 0.332 | ||
| 4 | −1.27 | 2.00 | 0.531 | −2.19 | 1.96 | 0.274 | ||
| Leptin/ghrelin ratio | 1 | Normoglycaemia | −295.69 | 232.25 | 0.224 | 118.84 | 255.85 | 0.651 |
| 2 | −439.54 | 305.44 | 0.178 | 190.01 | 317.45 | 0.564 | ||
| 3 | −439.25 | 320.27 | 0.200 | 191.90 | 347.43 | 0.596 | ||
| 4 | −478.67 | 328.58 | 0.179 | 202.71 | 357.65 | 0.589 | ||
| 1 | T2DM | 15.98 | 26.43 | 0.553 | 71.70 | 30.43 | 0.816 | |
| 2 | 29.69 | 25.74 | 0.266 | 25.44 | 30.39 | 0.416 | ||
| 3 | 23.34 | 26.34 | 0.389 | 20.86 | 30.37 | 0.503 | ||
| 4 | −8.92 | 35.63 | 0.806 | −8.41 | 37.23 | 0.825 | ||
| 1 | NODAP | −133.41 | 51.65 | 0.014 | −63.75 | 45.06 | 0.166 | |
| 2 | −121.13 | 58.02 | 0.045 | −49.16 | 61.58 | 0.431 | ||
| 3 | −121.44 | 58.85 | 0.048 | −48.81 | 62.51 | 0.441 | ||
| 4 | −129.40 | 59.14 | 0.037 | −62.19 | 62.30 | 0.326 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimita, W.; Bharmal, S.H.; Ko, J.; Cho, J.; Petrov, M.S. Relationship between Energy Balance and Circulating Levels of Hepcidin and Ferritin in the Fasted and Postprandial States. Nutrients 2021, 13, 3557. https://doi.org/10.3390/nu13103557
Kimita W, Bharmal SH, Ko J, Cho J, Petrov MS. Relationship between Energy Balance and Circulating Levels of Hepcidin and Ferritin in the Fasted and Postprandial States. Nutrients. 2021; 13(10):3557. https://doi.org/10.3390/nu13103557
Chicago/Turabian StyleKimita, Wandia, Sakina H. Bharmal, Juyeon Ko, Jaelim Cho, and Maxim S. Petrov. 2021. "Relationship between Energy Balance and Circulating Levels of Hepcidin and Ferritin in the Fasted and Postprandial States" Nutrients 13, no. 10: 3557. https://doi.org/10.3390/nu13103557
APA StyleKimita, W., Bharmal, S. H., Ko, J., Cho, J., & Petrov, M. S. (2021). Relationship between Energy Balance and Circulating Levels of Hepcidin and Ferritin in the Fasted and Postprandial States. Nutrients, 13(10), 3557. https://doi.org/10.3390/nu13103557





