Dietary Supplements for Female Infertility: A Critical Review of Their Composition
Abstract
1. Introduction
2. Materials and Methods
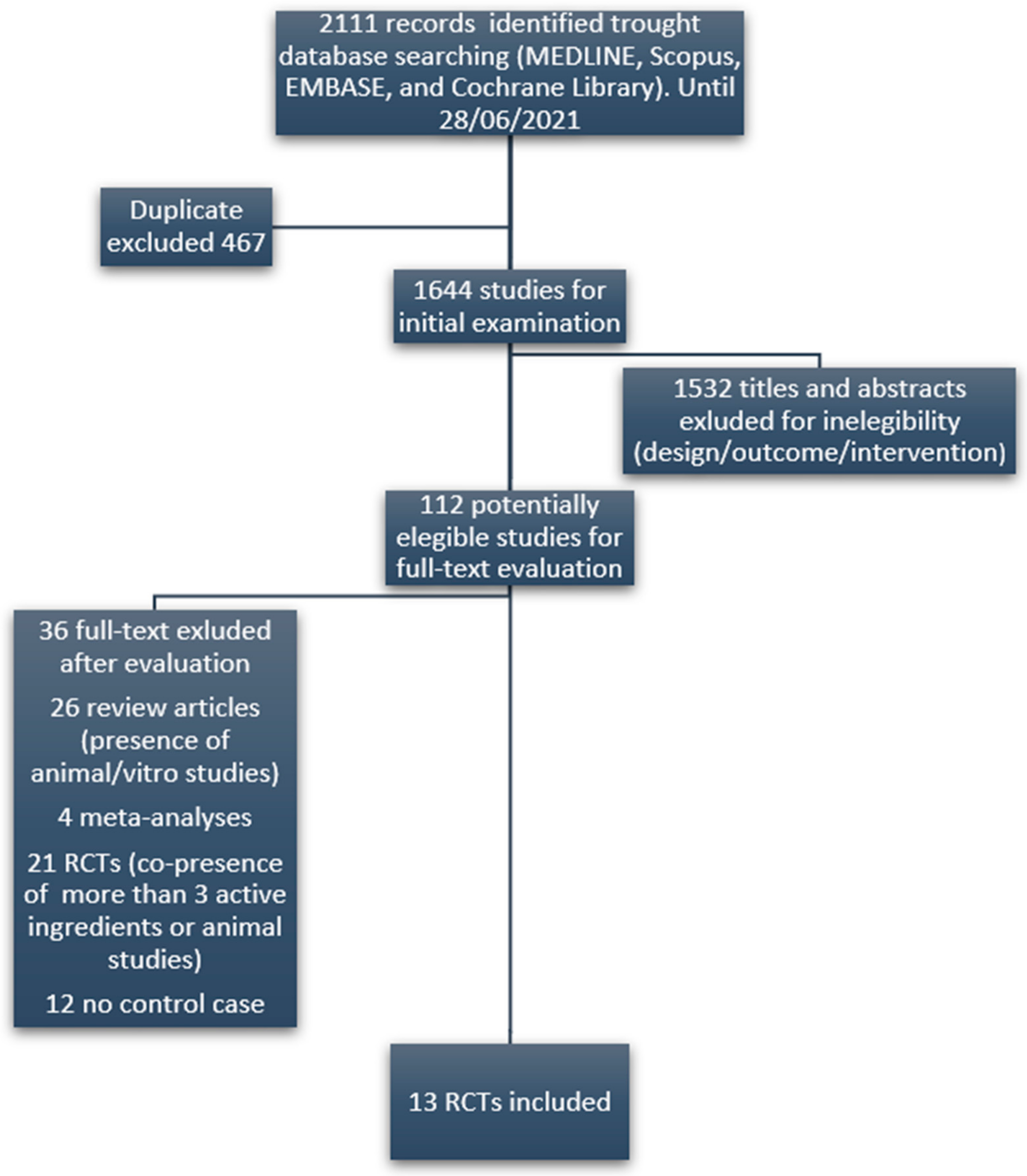
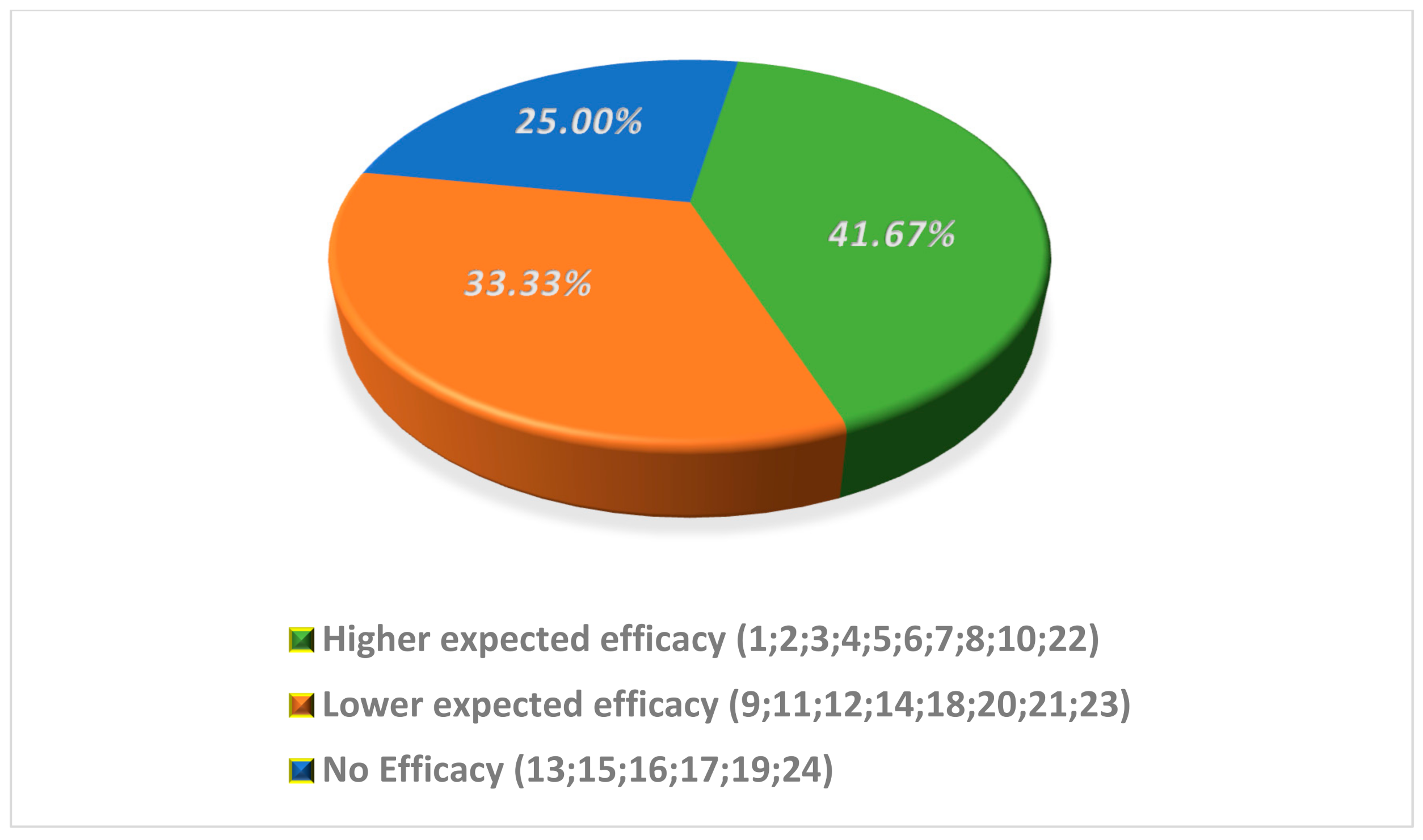
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population Division, Department of Economic Social Affairs, United Nations. World Fertility Patterns 2015—Data Booklet (ST/ESA/SER.A/370); United Nations: San Francisco, CA, USA, 2015. Available online: https://www.un.org/en/development/desa/population/publications/pdf/fertility/world-fertility-patterns-2015.pdf (accessed on 13 August 2021).
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Gupta, A.S. Causes and Prevalence of Factors Causing Infertility in a Public Health Facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef]
- Noventa, M.; Quaranta, M.; Vitagliano, A.; Cinthya, V.; Valentini, R.; Campagnaro, T.; Marci, R.; Paola, R.D.; Alviggi, C.; Gangemi, M.; et al. May Underdiagnosed Nutrition Imbalances Be Responsible for a Portion of So-Called Unexplained Infertility? From Diagnosis to Potential Treatment Options. Reprod. Sci. 2016, 23, 812–822. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 2, 13–1178. [Google Scholar]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Vincenzoni, F.; Milardi, D.; Pompa, G.; Ricciardi, D.; Fruscella, E.; Mancini, F.; Pontecorvi, A.; Castagnola, M.; Marana, R. Cervical mucus proteome in endometriosis. Clin. Proteom. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Youseflu, S.; Jahanian Sadatmahalleh, S.; Mottaghi, A.; Kazemnejad, A. The association of food consumption and nutrient intake with endometriosis risk in Iranian women: A case-control study. Int. J. Reprod. BioMed. 2019, 17, 661. [Google Scholar]
- Centers for Disease Control and Prevention. 2018 Assisted Reproductive Technology Fertility Clinic Success Rates Report; US Dept of Health and Human Services: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/art/pdf/2018-report/ART-2018-Clinic-Report-Full.pdf (accessed on 13 August 2021).
- Martin, J.C.; Zhou, S.J.; Flynn, A.C.; Malek, L.; Greco, R.; Moran, L. The Assessment of Diet Quality and Its Effects on Health Outcomes Pre-pregnancy and during Pregnancy. Semin. Reprod. Med. 2016, 34, 83–92. [Google Scholar] [PubMed]
- Kermack, A.J.; Macklon, N.S. Nutritional supplementation and artificial reproductive technique (ART) outcomes. Reprod. Fertil. Dev. 2015, 27, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Macklon, N.S. Vitamin supplement usage in 400 women embarking on in vitro fertilization treatment. In Proceedings of the British Fertility Society Annual Meeting, Sheffield, UK, 8–9 January 2014. [Google Scholar]
- Federsalus. Available online: https://www.federsalus.it/wp-content/uploads/2020/06/Report_quinta-indagine-di-settore-1-1.pdf (accessed on 13 August 2021).
- Agrawal, R.; Burt, E.; Gallagher, A.M.; Butler, L.; Venkatakrishnan, R.; Peitsidis, P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: A pilot study. Reprod. Biomed. Online 2012, 24, 54–60. [Google Scholar] [CrossRef]
- Vitagliano, A.; Noventa, M.; Gizzo, S. Is it time to consider patients suffering from endometriosis-related infertility as “novel candidates” for targeted peri-conceptional D-chiro inositol supplementation? Hypothesis, rationale and some considerations. J. Assist. Reprod. Genet. 2015, 32, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Decleer, W.; Comhaire, F.; De Clerck, K.; Vanden Berghe, W.; Devriendt, G.; Osmanagaoglu, K. Preconception nutraceutical food supplementation can prevent oxidative and epigenetic DNA alterations induced by ovarian stimulation for IVF and increases pregnancy rates. Facts Views Vis. Obgyn. 2020, 12, 23–30. [Google Scholar] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Zeisel, S.H. Regulation of “nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar]
- Cawley, S.; Mullaney, L.; McKeating, A.; Farren, M.; McCartney, D.; Turner, M.J. A review of European guidelines on periconceptional folic acid supplementation. Eur. J. Clin. Nutr. 2016, 70, 143–154. [Google Scholar] [CrossRef]
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Foresta, C. Dietary Supplements for Male Infertility: A Critical Evaluation of Their Composition. Nutrients 2020, 12, 1472. [Google Scholar] [CrossRef]
- Ministero Della Salute. Available online: https://www.salute.gov.it/portale/temi/documenti/integratori/registro_integratori_per_prodotto.pdf (accessed on 10 July 2021).
- Kuchakulla, M.; Soni, Y.; Patel, P.; Parekh, N.; Ramasamy, R. A Systematic Review and Evidence-based Analysis of Ingredients in Popular Male Fertility Supplements. Urology 2019, 4295, 31006–31014. [Google Scholar] [CrossRef]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.; Rader, D.J.; Rubin, G.D.; et al. American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and In-tervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Macedo, M.; Lozano, G. Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, L.; Stracquadanio, M.; Pagano, I.; Carbonaro, A.; Palumbo, M.; Gulino, F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: A double blind trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 509–514. [Google Scholar] [PubMed]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; Fusi, F.; Occhi, F.; De Santis, L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil. Steril. 2009, 91, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Carlomagno, G.; Rizzo, P.; Raffone, E.; Roseff, S. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 452–457. [Google Scholar] [PubMed]
- Mendoza, N.; Diaz-Ropero, M.P.; Aragon, M. Comparison of the effect of two combinations of myo-inositol and D-chiro-inositol in women with polycystic ovary syndrome undergoing ICSI: A randomized controlled trial. Gynecol. Endocrinol. 2019, 35, 695–700. [Google Scholar] [CrossRef]
- Badawy, A.; State, O.; Abdelgawad, S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: A cross-over trial. Acta Obstet. Gynecol. Scand. 2007, 86, 218–222. [Google Scholar] [CrossRef]
- Nasr, A. Effect of N-acetyl-cysteine after ovarian drilling in clomiphene citrate-resistant PCOS women: A pilot study. Reprod. Biomed. Online 2010, 20, 403–409. [Google Scholar] [CrossRef][Green Version]
- Cheraghi, E.; Mehranjani, M.S.; Shariatzadeh, M.A.; Esfahani, M.H.; Ebrahimi, Z. N-Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: An alternative to metformin. Reprod. Fertil. Dev. 2016, 28, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Hashimoto, S.; Matsumoto, H. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol. Endocrinol. 2018, 34, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Hamed, A.H.; Saso, S.; Thabet, H.H. Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 180, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Dastorani, M.; Aghadavod, E.; Mirhosseini, N. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod. Biol. Endocrinol. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Noventa, M.; Vitagliano, A.; Quaranta, M.; Borgato, S.; Abdulrahim, B.; Gizzo, S. Preventive and Therapeutic Role of Dietary Inositol Supplementation in Periconceptional Period and During Pregnancy: A Summary of Evidences and Future Applications. Reprod. Sci. 2016, 23, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Espinola, M.S.B.; Dewailly, D.; Ozay, A.C.; Prapas, N.; Vazquez-Levin, M.; Wdowiak, A.; Unfer, V. Expert Group on Inositols in Preclinical and Clinical Research. Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends Endocrinol. Metab. 2020, 31, 570–579. [Google Scholar] [CrossRef]
- Gateva, A.; Unfer, V.; Kamenov, Z. The use of inositol(s) isomers in the management of polycystic ovary syndrome: A comprehensive review. Gynecol. Endocrinol. 2018, 34, 545–550. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the prevention of gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Arch Gynecol. Obstet. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Chiu, T.T.; Rogers, M.S.; Briton-Jones, C.; Haines, C. Effects of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Hum. Reprod. 2003, 18, 408–416. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitagliano, A.; Noventa, M.; Ambrosini, G.; D’Anna, R. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: A systematic review and meta-analysis of randomized controlled trials. Arch Gynecol. Obstet. 2018, 298, 675–684. [Google Scholar] [CrossRef]
- Gomes, S.; Lopes, C.; Pinto, E. Folate and folic acid in the periconceptional period: Recommendations from official health organizations in thirty-six countries worldwide and WHO. Public Health Nutr. 2016, 19, 176–189. [Google Scholar] [CrossRef]
- Canfield, M.A.; Collins, J.S.; Botto, L.D.; Williams, L.J.; Mai, C.T.; Kirby, R.S. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: Findings from a multi-state population-based study. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.A.; Houghton, L.A.; Jones, G.T.; van Rij, A.M.; Morgan, K.; McLennan, I.S. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab. 2012, 97, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.O.; Seifer, D.B.; Weedon, J.; Adeyemi, O.; Holman, S.; Anastos, K. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil. Steril. 2012, 98, 228–234. [Google Scholar] [CrossRef]
- Cozzolino, M. Is it realistic to consider vitamin D as a follicular and serum marker of human oocyte quality? J. Assist. Reprod. Genet. 2019, 36, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Busnelli, A.; Pellegrini, L.; Riviello, E.; Vitagliano, A. How vitamin D level influences in vitro fertilization outcomes: Results of a systematic review and meta-analysis. Fertil. Steril. 2020, 114, 1014–1025. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and Reproduction Revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Taketani, T.; Tamura, H.; Takasaki, A.; Lee, L.; Kizuka, F.; Tamura, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Shimamura, K. Protective role of melatonin in progesterone production by human luteal cells. J. Pineal Res. 2011, 51, 207–213. [Google Scholar] [CrossRef]
- Eryilmaz, O.G.; Devran, A.; Sarikaya, E.; Aksakal, F.N.; Mollamahmutoglu, L.; Cicek, N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J. Assist. Reprod. Genet. 2011, 28, 815–820. [Google Scholar] [CrossRef]
- Zheng, M.; Tong, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Melatonin concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures. Gynecol. Endocrinol. 2018, 34, 446–450. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency. EFSA J. 2011, 9, 2241. [Google Scholar]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M.C.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine inhibits lipid accumulation in mouse embryonic adipocytes. Redox Biol. 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Skopiński, P. N-acetylcysteine as an anti-oxidant and anti-inflammatory drug and its some clinical applications. Cent. J. Immunol. 2012, 37, 57–66. [Google Scholar]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar]
- de Andrade, K.; Moura, F.; dos Santos, J.; de Araújo, O.; de Farias Santos, J.; Goulart, M.; De Andrade, K.Q.; Moura, F.A.; Dos Santos, J.M.; De Araújo, O.R.P. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- Thakker, D.; Raval, A.; Patel, I.; Walia, R. N-acetylcysteine for polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Obstet. Gynecol. Int. 2015, 2015, 817849. [Google Scholar] [CrossRef]
- Elgindy, E.A.; El-Huseiny, A.M.; Mostafa, M.I.; Gaballah, A.M.; Ahmed, T.A. N-acetyl cysteine: Could it be an effective adjuvant therapy in ICSI cycles? A preliminary study. Reprod. Biomed. Online 2010, 20, 789–796. [Google Scholar] [CrossRef]
- Cheraghi, E.; Mehranjani, M.S.; Shariatzadeh, S.M.A.; Nasr Esfahani, M.H.; Alani, B. N-Acetylcysteine Compared to Metformin, Improves The Expression Profile of Growth Differentiation Factor-9 and Receptor Tyrosine Kinase c-Kit in The Oocytes of Patients with Polycystic Ovarian Syndrome. Int. J. Fertil. Steril. 2018, 11, 270–278. [Google Scholar]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Akarsu, S.; Gode, F.; Isik, A.Z.; Dikmen, Z.G.; Tekindal, M.A. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J. Assist. Reprod. Genet. 2017, 34, 599–605. [Google Scholar] [CrossRef]
- Turi, A.; Giannubilo, S.R.; Bruge, F.; Principi, F.; Battistoni, S.; Santoni, F.A.L. Coenzyme Q10 content in follicular fluid and its relationship with oocyte fertilization and embryo grading. Arch. Gynecol. Obstet. 2012, 1173–1176. [Google Scholar] [CrossRef]
- Zhang, M.; ShiYang, X.; Zhang, Y.; Miao, Y.; Chen, Y.; Cui, Z.; Xiong, B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic. Biol. Med. 2019, 143, 84–94. [Google Scholar] [CrossRef]
| Active Ingredients | References | Outcome Evaluated | Employed Daily Dose | Minimal Effective Dose (mED) |
|---|---|---|---|---|
| Melatonin—A/B | [29] Pacchiarotti et al. | Oocyte and embryo quality | 3 mg | 3 mg |
| [30] Espino et al. | Ox status, oocyte quality | 3 mg | ||
| [30] Espino et al. | Ox status, oocyte quality | 6 mg | ||
| Folic acid—A/B | [31] Ciotta et al. | Oocyte quality | 400 mcg | 400 mcg |
| [32] Papaleo et al. | Oocyte quality | 400 mcg | ||
| [29] Pacchiarotti et al. | Oocyte and embryo quality | 400 mcg | ||
| Myo-inositol—A/B | [31] Ciotta et al. | Oocyte quality | 4 g | 4 g |
| [33] Unfer et al. | Oocyte and Embryo quality | 4 g | ||
| [32] Papaleo et al. | Oocyte quality | 4 g | ||
| [29] Pacchiarotti et al. | Oocyte and embryo quality | 4 g | ||
| D-chiro-inositol—B/C | [34] Mendoza et al. | Pregnancy rate | 300 mg | 300 mg |
| NAC—A/B | [35] Badawy et al. | Ovulation rate | 1.2 g | 1.2 g |
| [36] Nasr et al. | Pregnancy rate | 1.2 g | ||
| [37] Cheraghi et al. | Oocyte and Embryo quality | 1.8 g | ||
| CQ10—B/C | [38] Xu et al. | Fertilization rate and embryo quality | 600 mg | 600 mg |
| Carnitine—A/B | [39] Kitano et al. | Embryo quality | 1 g | 1 g |
| [40] Ismail et al. | Pregnancy rate | 3 g | ||
| Vitamin D—B/C | [41] Dastorani et al. | AMH level | 89 mcg | 89 mcg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitagliano, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Di Nisio, A.; Grande, G.; Foresta, C.; Garolla, A. Dietary Supplements for Female Infertility: A Critical Review of Their Composition. Nutrients 2021, 13, 3552. https://doi.org/10.3390/nu13103552
Vitagliano A, Petre GC, Francini-Pesenti F, De Toni L, Di Nisio A, Grande G, Foresta C, Garolla A. Dietary Supplements for Female Infertility: A Critical Review of Their Composition. Nutrients. 2021; 13(10):3552. https://doi.org/10.3390/nu13103552
Chicago/Turabian StyleVitagliano, Amerigo, Gabriel Cosmin Petre, Francesco Francini-Pesenti, Luca De Toni, Andrea Di Nisio, Giuseppe Grande, Carlo Foresta, and Andrea Garolla. 2021. "Dietary Supplements for Female Infertility: A Critical Review of Their Composition" Nutrients 13, no. 10: 3552. https://doi.org/10.3390/nu13103552
APA StyleVitagliano, A., Petre, G. C., Francini-Pesenti, F., De Toni, L., Di Nisio, A., Grande, G., Foresta, C., & Garolla, A. (2021). Dietary Supplements for Female Infertility: A Critical Review of Their Composition. Nutrients, 13(10), 3552. https://doi.org/10.3390/nu13103552








