Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Diets
2.2. Hepatic Fat Quantification
2.3. Histological Evaluation
2.4. RNA Extraction and Quantitative Polymerase Chain Reaction
2.5. Protein Extraction and Western Blot Analysis
| Primers | Forward | Reverse | Reference |
|---|---|---|---|
| Ppara | 5′-CCCTGTTTGTGGCTGCTATAATTT-3′ | 5′-GGGAAGAGGAAGGTGTCATCTG-3′ | [34] |
| Fasn | 5′-GCTGCGGAAACTTCAGGAAAT-3′ | 5′-AGAGACGTGTCACTCCTGGACTT-3′ | [35] |
| Col1a1 | 5′-TAGGCCATTGTGTATGCAGC-3′ | 5′-ACATGTTCAGCTTTGTGGACC-3′ | [36] |
| Tnfa | 5′-AGGGTCTGGGCCATAGAACT-3′ | 5′-CCACCACGCTCTTCTGTCTAC-3′ | [36] |
| Il6 | 5′-AGTTGCCTTCTTGGGACTGA-3′ | 5′-TCCACGATTTCCCAGAGAAC-3′ | [37] |
| Il1a | 5′-CCAGAAGAAAATGAGGTCGG-3′ | 5′-AGCGCTCAAGGAGAAGACC-3′ | [38] |
| F4/80 | 5′-CATAAGCTGGGCAAGTGGTA-3′ | 5′-GGATGTACAGATGGGGGATG-3′ | [39] |
| Scd1 | 5′-AGATCTCCAGTTCTTACACGACCAC-3′ | 5′-GACGGATGTCTTCTTCCAGGTG-3′ | [40] |
| Acc1 | 5′-GATGAACCATCTCCGTTGGC-3′ | 5′-CCCAATTATGAATCGGGAGTGC-3′ | [40] |
| Cd36 | 5′-GAACCACTGCTTTCAAAAACTGG-3′ | 5′-TGCTGTTCTTTGCCACGTCA-3′ | [40] |
| Fabp4 | 5′-TGAAAGAAGTGGGAGTGGGC-3′ | 5′-CGAATTCCACGCCCAGTTTG-3′ | [41] |
| Cpt1a | 5′-CTCAGTGGGAGCGACTCTTCA-3′ | 5′-GGCCTCTGTGGTACACGACAA-3′ | [42] |
| Cbs | 5′-GCAGCGCTGTGTGGTCATC-3′ | 5′-CATCCATTTGTCACTCAGGAACTT-3′ | [43] |
| Fgf21 | 5′-CCTCTAGGTTTCTTTGCCAACAG-3′ | 5′-AAGCTGCAGGCCTCAGGAT-3′ | [44] |
| Ucp2 | 5′-GGTCGGAGATACCAGAGCAC-3′ | 5′-ATGAGGTTGGCTTTCAGGAG-3′ | This study |
| Glut2 | 5′-ACCCTGTTCCTAACCGGG-3′ | 5′-TGAACCAAGGGATTGGACC-3′ | [45] |
| G6pd | 5′-GTGGGATCCTGAGGGAAGAGT-3′ | 5′-GATGGTGGGATAGATCTTCTTCTTG-3′ | [34] |
| Srebp1c | 5′-TGACCCGGCTATTCCGTGA-3′ | 5′-CTGGGCTGAGCAATACAGTTC-3′ | [46] |
| Ucp1 | 5′-ACTGCCACACCTCCAGTCATT-3′ | 5′-CTTTGCCTCACTCAGGATTGG-3′ | [47] |
| 36bB4 | 5′-AGTCCCTGCCCTTTGTACACA-3′ | 5′-CGATCCGAGGGCCTCACTA-3′ | [48] |
2.6. Statistical Analysis
3. Results
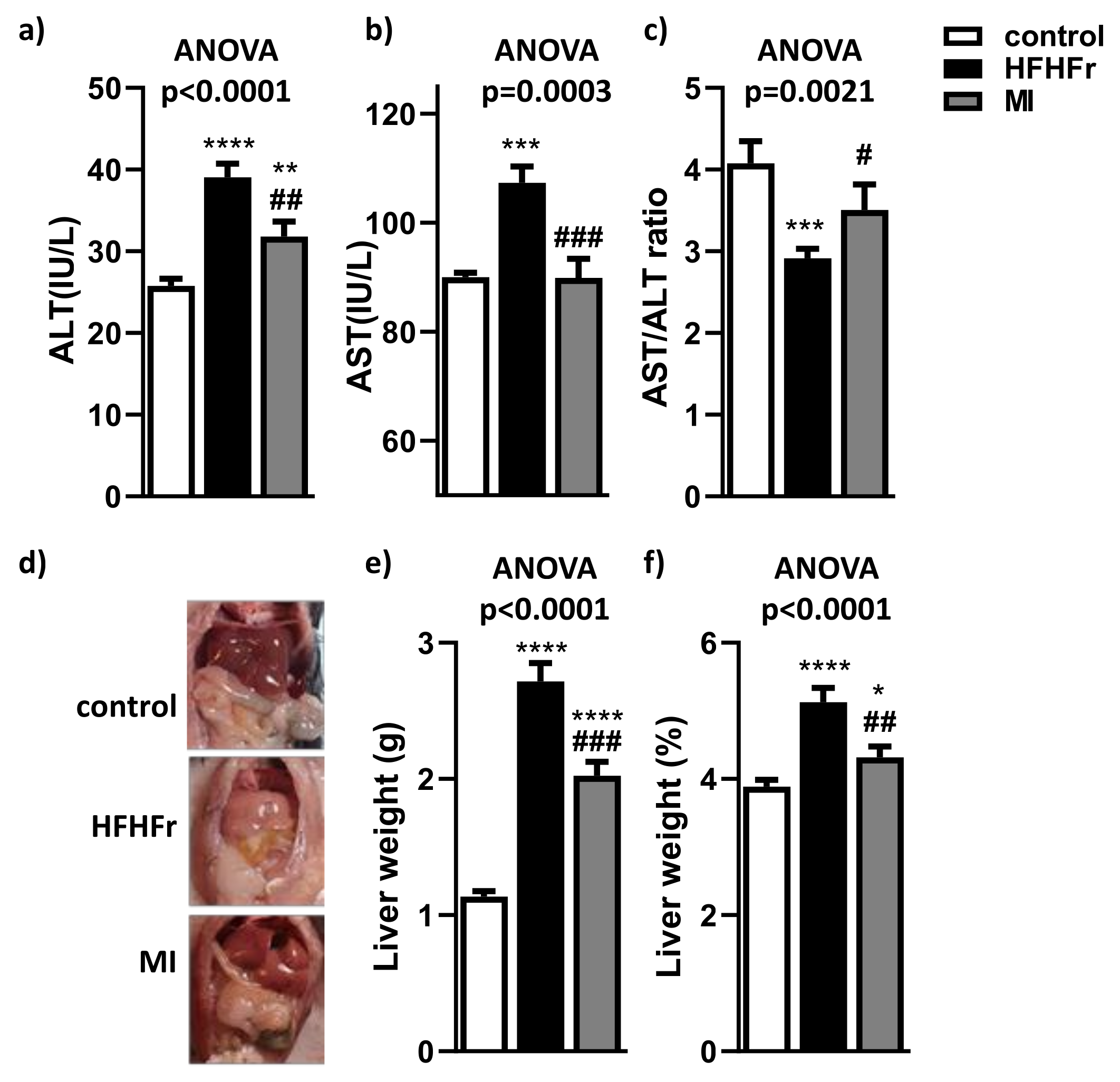
3.1. MI Supplementation Reduced Liver Injury and Macroscopic Liver Features of NAFLD
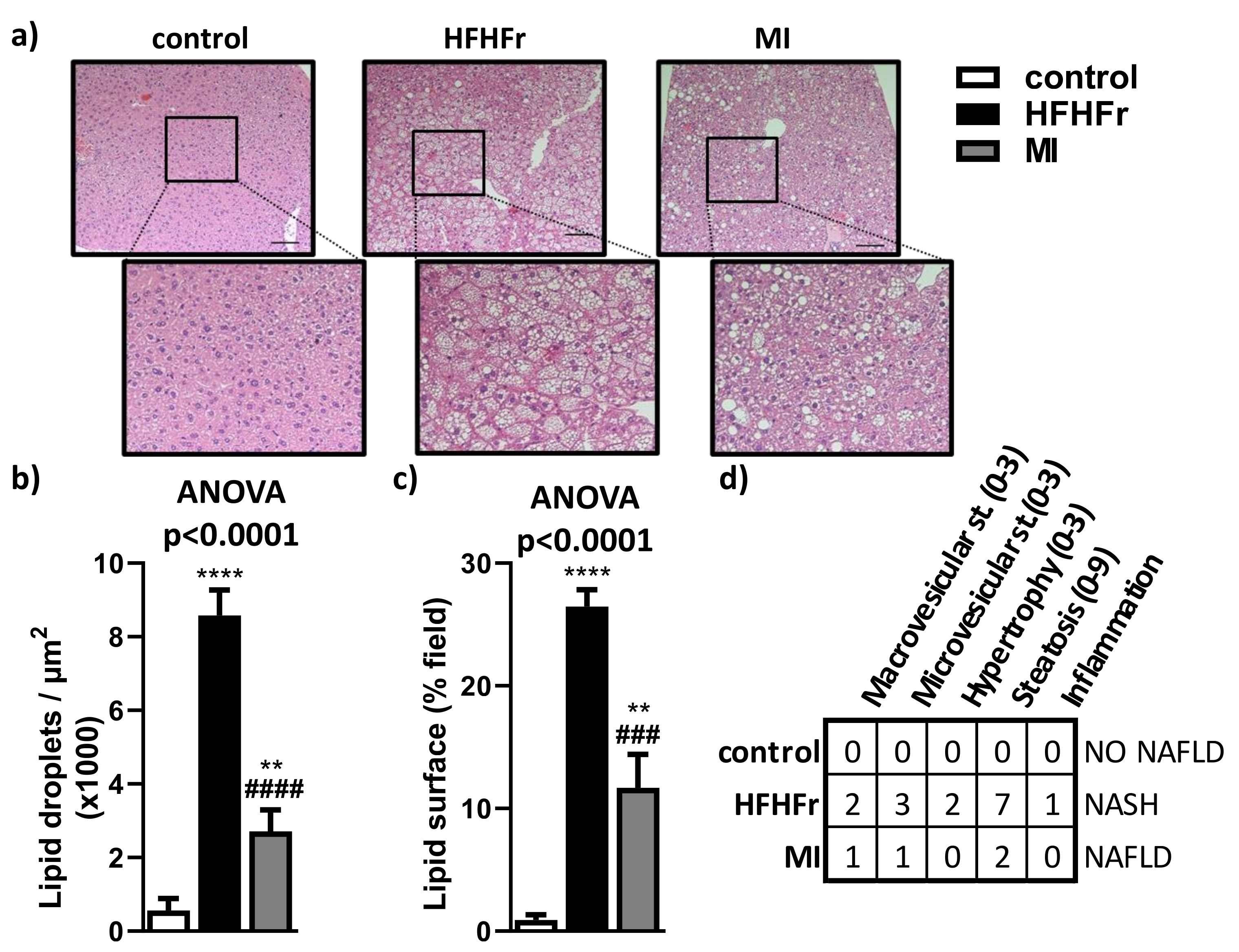
3.2. MI Supplementation Decreased Hepatic Lipid Content and Liver Steatosis
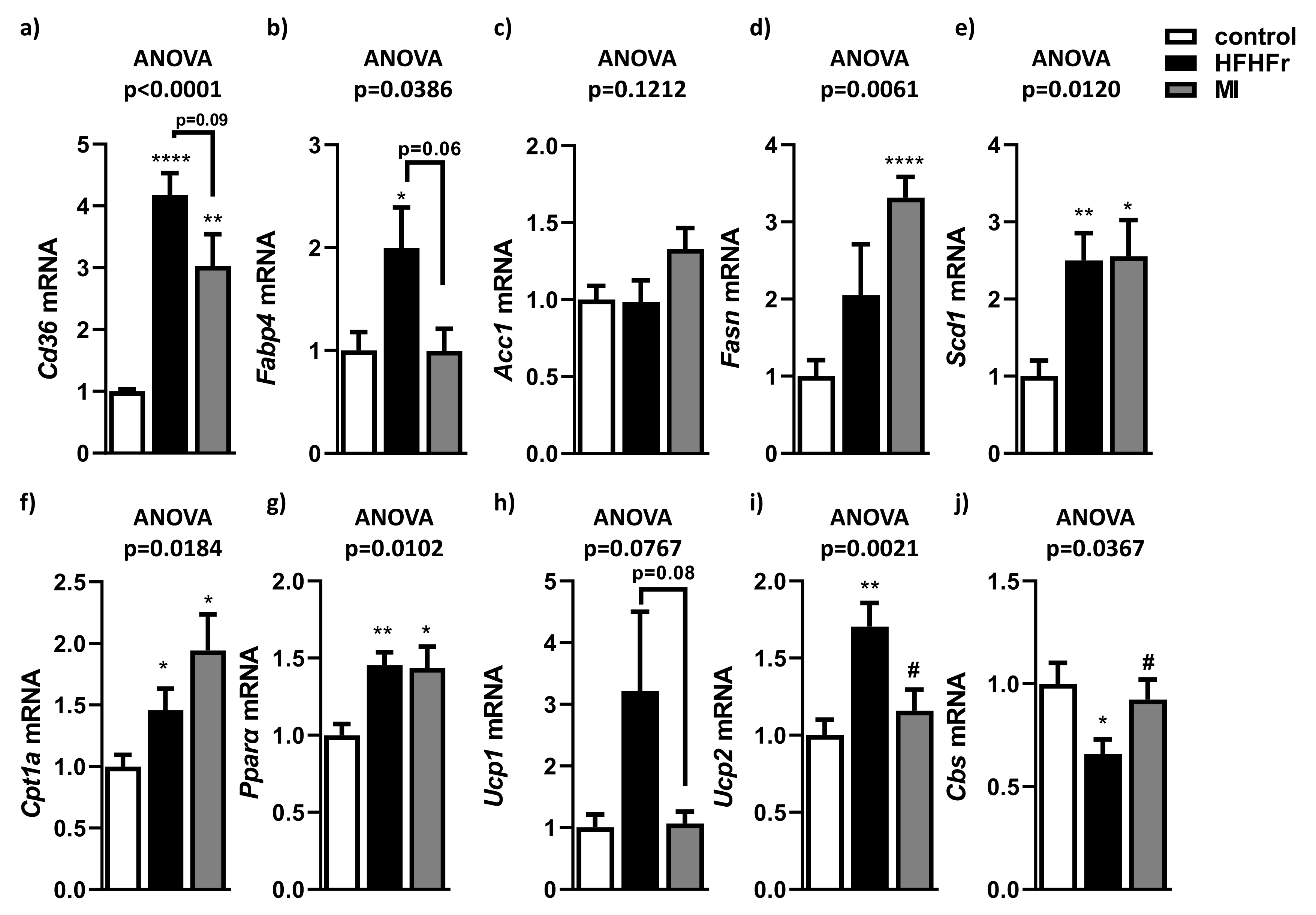
3.3. Beneficial Effects of MI Supplementation Did Not Involve Lipogenesis, Lipid Transport or Fatty Acid Oxidation Pathways
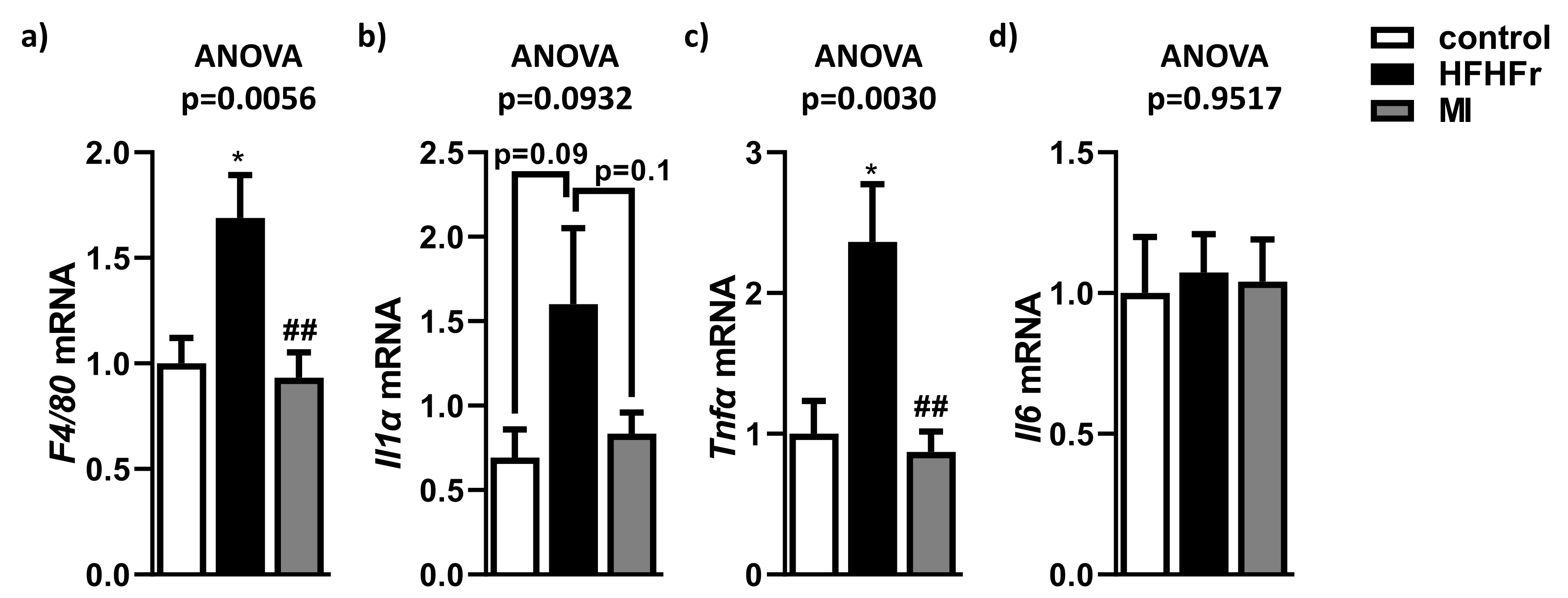
3.4. MI Supplementation Reduced Hepatic Inflammation Associated to NAFLD
3.5. MI Supplementation Reduced Fibrosis Markers Associated to NAFLD
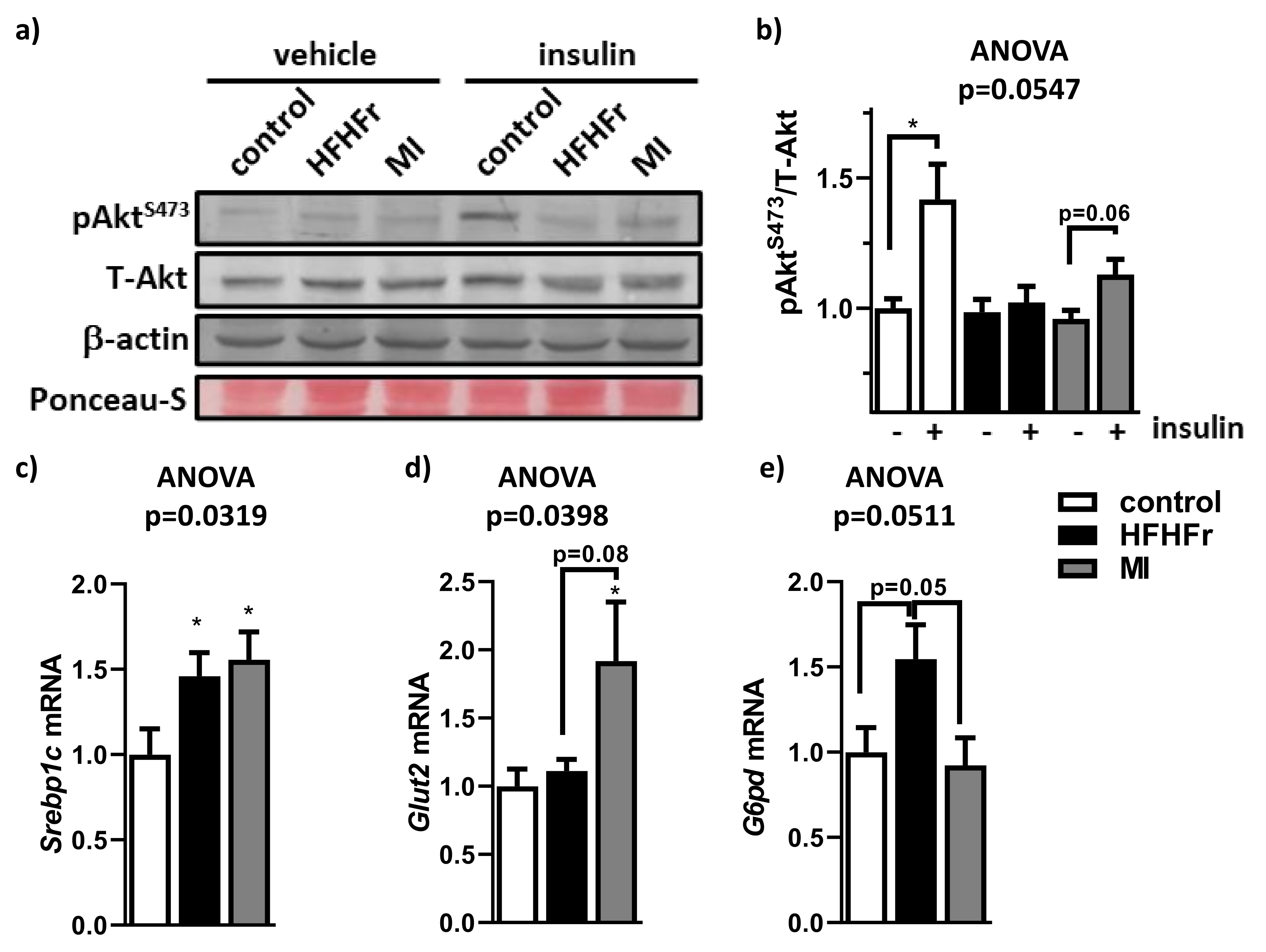
3.6. MI Supplementation Reduced Hepatic Insulin Resistance Associated to NAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quesada-Vázquez, S.; Aragonès, G.; del Bas, J.M.; Escoté, X. Diet, Gut Microbiota and Non-Alcoholic Fatty Liver Disease: Three Parts of the Same Axis. Cells 2020, 9, 176. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Argo, C.K.; Northup, P.G.; Al-Osaimi, A.M.S.; Caldwell, S.H. Systematic Review of Risk Factors for Fibrosis Progression in Non-Alcoholic Steatohepatitis. J. Hepatol. 2009, 51, 371–379. [Google Scholar] [CrossRef]
- Pai, R.K. NAFLD Histology: A Critical Review and Comparison of Scoring Systems. Curr. Hepatol. Rep. 2019, 18, 473–481. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Ural, D.; Zeybel, M.; Yuksel, H.H.; Uhlén, M.; Borén, J. The Potential Use of Metabolic Cofactors in Treatment of NAFLD. Nutrients 2019, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Review Article Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 11, 9547613. [Google Scholar] [CrossRef]
- Zhang, C.; Bjornson, E.; Arif, M.; Tebani, A.; Lovric, A.; Benfeitas, R.; Ozcan, M.; Juszczak, K.; Kim, W.; Kim, J.T.; et al. The Acute Effect of Metabolic Cofactor Supplementation: A Potential Therapeutic Strategy against Non-alcoholic Fatty Liver Disease. Mol. Syst. Biol. 2020, 16, 1–16. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Carotti, S.; Bertoccini, L.; Baroni, M.G.; Vespasiani-Gentilucci, U.; Cavallo, M.G.; Morini, S. Relationship between Adipose Tissue Dysfunction, Vitamin D Deficiency and the Pathogenesis of Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2017, 23, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Marin, V.; Gazzin, S.; Gambaro, S.E.; Ben, M.D.; Calligaris, S.; Anese, M.; Raseni, A.; Avellini, C.; Giraudi, P.J.; Tiribelli, C.; et al. Effects of Oral Administration of Silymarin in a Juvenile Murine Model of Non-Alcoholic Steatohepatitis. Nutrients 2017, 9, 1006. [Google Scholar] [CrossRef]
- Beaton, M.D. Current Treatment Options for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Can. J. Gastroenterol. 2012, 26, 353–357. [Google Scholar] [CrossRef]
- Abeysekera, K.W.M.; Fernandes, G.S.; Hammerton, G.; Portal, A.J.; Gordon, F.H.; Heron, J.; Hickman, M. Prevalence of Steatosis and Fibrosis in Young Adults in the UK: A Population-Based Study. Lancet Gastroenterol. Hepatol. 2020, 5, 295–305. [Google Scholar] [CrossRef]
- Suárez, M.; Boqué, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Bjornson, E.; Zhang, C.; Klevstig, M.; Söderlund, S.; Ståhlman, M.; Adiels, M.; Hakkarainen, A.; Lundbom, N.; Kilicarslan, M.; et al. Personal Model-assisted Identification of NAD+ and Glutathione Metabolism as Intervention Target in NAFLD. Mol. Syst. Biol. 2017, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Agren, R.; Kampf, C.; Asplund, A.; Uhlen, M.; Nielsen, J. Genome-Scale Metabolic Modelling of Hepatocytes Reveals Serine Deficiency in Patients with Non-Alcoholic Fatty Liver Disease. Nat. Commun. 2014, 5, 3083. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. L-Carnitine Ameliorated Fatty Liver in High-Calorie Diet/STZ-Induced Type 2 Diabetic Mice by Improving Mitochondrial Function. Diabetol. Metab. Syndr. 2011, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Khodayar, M.J.; Kalantari, H.; Khorsandi, L.; Rashno, M.; Zeidooni, L. Betaine Protects Mice against Acetaminophen Hepatotoxicity Possibly via Mitochondrial Complex II and Glutathione Availability. Biomed. Pharmacother. 2018, 103, 1436–1445. [Google Scholar] [CrossRef]
- Marin, V.; Rosso, N.; Dal Ben, M.; Raseni, A.; Boschelle, M.; Degrassi, C.; Nemeckova, I.; Nachtigal, P.; Avellini, C.; Tiribelli, C.; et al. An Animal Model for the Juvenile Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. PLoS ONE 2016, 11, e0158817. [Google Scholar] [CrossRef]
- Sanches, S.C.L.; Ramalho, L.N.Z.; Augusto, M.J.; Da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. BioMed Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; Bas, J.M.; Crescenti, A.; Arola, L. Low Doses of Grape Seed Procyanidins Reduce Adiposity and Improve the Plasma Lipid Profile in Hamsters. Int. J. Obes. 2013, 37, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-sureda, V.; Peinado-onsurbe, J. A Procedure for Measuring Triacylglyceride and Cholesterol Content Using a Small Amount of Tissue. Anal. Biochem. 2005, 343, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e0115922. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Best Pr. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef]
- Huang, Y.; de Boer, W.B.; Adams, L.A.; Macquillan, G.; Rossi, E.; Rigby, P.; Raftopoulos, S.C.; Bulsara, M.; Jeffrey, G.P. Image Analysis of Liver Collagen Using Sirius Red Is More Accurate and Correlates Better with Serum Fibrosis Markers than Trichrome. Liver Int. 2013, 33, 1249–1256. [Google Scholar] [CrossRef]
- Flint, M.H.; Lyons, M.F.; Meaney, M.F.; Williams, D.E. The Masson Staining of Collagen—An Explanation of an Apparent Paradox. Histochem. J. 1975, 7, 529–546. [Google Scholar] [CrossRef]
- Yang, J.; Neira, S.; Elisa, F.; Gil-iturbe, E.; Castilla-madrigal, R.; Fern, M.; Mart, J.A.; Moreno-aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef]
- Ballak, D.B.; Van Diepen, J.A.; Moschen, A.R.; Jansen, H.J.; Hijmans, A.; Groenhof, G.J.; Leenders, F.; Bufler, P.; Boekschoten, M.V.; Müller, M.; et al. IL-37 Protects against Obesity-Induced Inflammation and Insulin Resistance. Nat. Commun. 2014, 5, 4711. [Google Scholar] [CrossRef]
- Antraco, V.J.; Hirata, B.K.S.; de Jesus Simão, J.; Cruz, M.M.; da Silva, V.S.; de Sá, R.D.C.; Abdala, F.M.; Armelin-Correa, L.; Alonso-Vale, M.I.C. Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (Nash) and Stimulate Adipogenesis. Nutrients 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- López-Yoldi, M.; Fernández-Galilea, M.; Laiglesia, L.M.; Larequi, E.; Prieto, J.; Martínez, J.A.; Bustos, M.; Moreno-Aliaga, M.J. Cardiotrophin-1 Stimulates Lipolysis through the Regulation of Main Adipose Tissue Lipases. J. Lipid Res. 2014, 55, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Escote, X.; Ceperuelo-Mallafre, V.; Megia, A.; Caubet, E.; Naf, S.; Gomez, J.M.; Gonzalez-Clemente, J.M.; Vicente, V.; Vendrell, J. Relation between Human LPIN1, Hypoxia and Endoplasmic Reticulum Stress Genes in Subcutaneous and Visceral Adipose Tissue. Int. J. Obes. 2010, 34, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S.; et al. Hepatic Fatty Acid Transporter Cd36 Is a Common Target of LXR, PXR, and PPARγ in Promoting Steatosis. Gastroenterology 2008, 134, 556–567. [Google Scholar] [CrossRef]
- Zhao, X.; Li, R.; Liu, Y.; Zhang, X.; Zhang, M.; Zeng, Z.; Wu, L.; Gao, X.; Lan, T.; Wang, Y. Polydatin Protects against Carbon Tetrachloride-Induced Liver Fibrosis in Mice. Arch. Biochem. Biophys. 2017, 629, 1–7. [Google Scholar] [CrossRef]
- Mazzolini, G.; Atorrasagasti, C.; Onorato, A.; Peixoto, E.; Schlattjan, M.; Sowa, J.P.; Sydor, S.; Gerken, G.; Canbay, A. SPARC Expression Is Associated with Hepatic Injury in Rodents and Humans with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2018, 8, 725. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, W.; Bousquenaud, M.; Cattin, S.; Zaric, J.; Sun, L.K.; Rüegg, C. Emodin Inhibits Inflammation, Carcinogenesis, and Cancer Progression in the AOM/DSS Model of Colitis-Associated Intestinal Tumorigenesis. Front. Oncol. 2021, 10, 564674. [Google Scholar] [CrossRef]
- Grabner, G.F.; Fawzy, N.; Schreiber, R.; Pusch, L.M.; Bulfon, D.; Koefeler, H.; Eichmann, T.O.; Lass, A.; Schweiger, M.; Marsche, G.; et al. Metabolic Regulation of the Lysosomal Cofactor Bis(Monoacylglycero)Phosphate in Mice. J. Lipid Res. 2020, 61, 995–1003. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Qian, J.; Tang, Y. Fasting Exacerbates Hepatic Growth Differentiation Factor 15 to Promote Fatty Acid β-Oxidation and Ketogenesis via Activating XBP1 Signaling in Liver. Redox Biol. 2018, 16, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Li, X.; Guo, F.; Li, L.; Zeng, X.; Ma, L.; Fu, P. Genetic Inhibition of FABP4 Attenuated Endoplasmic Reticulum Stress and Mitochondrial Dysfunction in Rhabdomyolysis-Induced Acute Kidney Injury. Life Sci. 2021, 268, 119023. [Google Scholar] [CrossRef]
- Kimura, R.; Takahashi, N.; Lin, S.; Goto, T.; Murota, K.; Nakata, R.; Inoue, H.; Kawada, T. DHA Attenuates Postprandial Hyperlipidemia via Activating PPARα in Intestinal Epithelial Cells. J. Lipid Res. 2013, 54, 3258–3268. [Google Scholar] [CrossRef]
- Markó, L.; Szijártó, I.A.; Filipovic, M.R.; Kaßmann, M.; Balogh, A.; Park, J.-K.; Przybyl, L.; N’diaye, G.; Krämer, S.; Anders, J.; et al. Role of Cystathionine Gamma-Lyase in Immediate Renal Impairment and Inflammatory Response in Acute Ischemic Kidney Injury. Sci. Rep. 2016, 6, 27517. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Small, S.M.; Peltekian, L.; Kessler, R.L.; Potthoff, M.J. FGF21 Resistance Is Not Mediated by Downregulation of Beta-Klotho Expression in White Adipose Tissue. Mol. Metab. 2017, 6, 602–610. [Google Scholar] [CrossRef]
- Stolarczyk, E.; Le Gall, M.; Even, P.; Houllier, A.; Serradas, P.; Brot-laroche, E.; Leturque, A. Loss of Sugar Detection by GLUT2 Affects Glucose Homeostasis in Mice. PLoS ONE 2007, 2, e1288. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, H.H.; Hwang, J.-Y.; Park, C.; Cho, B.Y.; Park, Y.J. Effects of Pioglitazone on Nonalcoholic Fatty Liver Disease in the Absence of Constitutive Androstane Receptor Expression. PPAR Res. 2018, 2018, 9568269. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Zhao, J.; Du, M. AMPKα1 Regulates Idh2 Transcription through H2B O-GlcNAcylation during Brown Adipogenesis. Acta Biochim. Biophys. Sin. 2021, 53, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Dunoyer-Geindre, S.; Kruithof, E.K.O. Epigenetic Control of Tissue-Type Plasminogen Activator Synthesis in Human Endothelial Cells. Cardiovasc. Res. 2011, 90, 457–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singhal, G.; Kumar, G.; Chan, S.; Fisher, F.M.; Ma, Y.; Vardeh, H.G.; Nasser, I.A.; Flier, J.S.; Maratos-Flier, E. Deficiency of Fibroblast Growth Factor 21 (FGF21) Promotes Hepatocellular Carcinoma (HCC) in Mice on a Long Term Obesogenic Diet. Mol. Metab. 2018, 13, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Uncoupling protein-2 and non-alcoholic fatty liver disease. Front. Biosci. 2005, 10, 2082–2096. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Belardinilli, F.; Bailetti, D.; Sponziello, M.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Pastori, D.; Ceci, F.; Montali, A.; et al. Evaluation of Polygenic Determinants of Non-Alcoholic Fatty Liver Disease (NAFLD) By a Candidate Genes Resequencing Strategy. Sci. Rep. 2018, 8, 3702. [Google Scholar] [CrossRef]
- Liao, Y.J.; Chen, T.L.; Lee, T.S.; Wang, H.A.; Wang, C.K.; Liao, L.Y.; Liu, R.S.; Huang, S.F.; Chen, Y.M.A. Glycine N-Methyltransferase Deficiency Affects Niemann-Pick Type C2 Protein Stability and Regulates Hepatic Cholesterol Homeostasis. Mol. Med. 2012, 18, 412–422. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Söderlund, S.; Matikainen, N.; Ståhlman, M.; Bergh, P.; Adiels, M.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571. [Google Scholar] [CrossRef]
- Clapper, J.R.; Hendricks, M.D.; Gu, G.; Wittmer, C.; Dolman, C.S.; Herich, J.; Athanacio, J.; Villescaz, C.; Ghosh, S.S.; Heilig, J.S.; et al. Diet-Induced Mouse Model of Fatty Liver Disease and Nonalcoholic Steatohepatitis Reflecting Clinical Disease Progression and Methods of Assessment. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Tarzamani, M.K.; Farhang, S.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. N-Acetylcysteine Improves Liver Function in Patients with Non-Alcoholic Fatty Liver Disease. Hepat. Mon. 2010, 10, 12–16. [Google Scholar] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Malaguarnera, M.; Avitabile, T.; Li Volti, G.; Galvano, F. L-Carnitine Supplementation to Diet: A New Tool in Treatment of Nonalcoholic Steatohepatitis—A Randomized and Controlled Clinical Trial. Am. J. Gastroenterol. 2010, 105, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, W.; Yan, C.; Yang, R.; Chen, Q.; Xu, H.; Huang, Y. Gypenosides Improve the Intestinal Microbiota of Non-Alcoholic Fatty Liver in Mice and Alleviate Its Progression. Biomed. Pharmacother. 2019, 118, 109258. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, Q.; Sun, Y.; Chen, Y.; Ren, B.; Liu, X. Sesamol Ameliorates Diet-Induced Obesity in C57BL/6J Mice and Suppresses Adipogenesis in 3T3-L1 Cells via Regulating Mitochondria-Lipid Metabolism. Mol. Nutr. Food Res. 2017, 61, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Toita, R.; Kang, J.H. Long-Term Profile of Serological Biomarkers, Hepatic Inflammation, and Fibrosis in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Toxicol. Lett. 2020, 332, 1–6. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Cabré, M.; Basaure, P.; Reverte, I.; Domingo, J.L.; Teresa Colomina, M. Adulthood Dietary Exposure to a Common Pesticide Leads to an Obese-like Phenotype and a Diabetic Profile in ApoE3 Mice. Environ. Res. 2015, 142, 169–176. [Google Scholar] [CrossRef]
- El-Boghdady, N.A.; Abdeltawab, N.F.; Nooh, M.M. Resveratrol and Montelukast Alleviate Paraquat-Induced Hepatic Injury in Mice: Modulation of Oxidative Stress, Inflammation, and Apoptosis. Oxidative Med. Cell. Longev. 2017, 2017, 9396425. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, J.S.; Shon, C.H.; Lee, C.Y.; Ryu, J.M.; Son, D.J.; Hwang, B.Y.; Yoo, H.S.; Cho, Y.C.; Lee, J.; et al. Fermented Korean Red Ginseng Extract Enriched in Rd and Rg3 Protects against Non-Alcoholic Fatty Liver Disease through Regulation of MTORC1. Nutrients 2019, 11, 2963. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Sengottuvelan, M.; Nalini, N. Protective Effect of Glycine Supplementation on the Levels of Lipid Peroxidation and Antioxidant Enzymes in the Erythrocyte of Rats with Alcohol-Induced Liver Injury. Cell Biochem. Funct. 2004, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-Based Treatment Ameliorates NAFLD by Modulating Fatty Acid Oxidation, Glutathione Synthesis, and the Gut Microbiome. Sci. Transl. Med. 2020, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the Mitochondrial Unfolded Protein Response by Nicotinamide Adenine Dinucleotide Repletion Reverses Fatty Liver Disease in Mice. Hepatology 2016, 63, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.B.; Li, G.Q.; Song, J.; Xu, T.Y.; Li, Z.Y.; Guan, Y.F.; et al. Hepatic NAD+ deficiency as a Therapeutic Target for Non-Alcoholic Fatty Liver Disease in Ageing. Br. J. Pharmacol. 2016, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Baixauli, J.; Puigbò, P.; Torrell, H.; Palacios-Jordan, H.; Ripoll, V.J.R.; Caimari, A.; Bas, J.M.D.; Baselga-Escudero, L.; Mulero, M. A Pilot Study for Metabolic Profiling of Obesity-Associated Microbial Gut Dysbiosis in Male Wistar Rats. Biomolecules 2021, 11, 303. [Google Scholar] [CrossRef]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene Expression in Human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, 1281–1287. [Google Scholar] [CrossRef]
- Steneberg, P.; Sykaras, A.G.; Backlund, F.; Straseviciene, J.; Söderström, I.; Edlund, H. Hyperinsulinemia Enhances Hepatic Expression of the Fatty Acid Transporter Cd36 and Provokes Hepatosteatosis and Hepatic Insulin Resistance. J. Biol. Chem. 2015, 290, 19034–19043. [Google Scholar] [CrossRef]
- Cordero, P.; Gomez-Uriz, A.M.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary Supplementation with Methyl Donors Reduces Fatty Liver and Modifies the Fatty Acid Synthase DNA Methylation Profile in Rats Fed an Obesogenic Diet. Genes Nutr. 2013, 8, 105–113. [Google Scholar] [CrossRef]
- Lai, I.K.; Dhakal, K.; Gadupudi, G.S.; Li, M.; Ludewig, G.; Robertson, L.W.; Olivier, A.K. N-Acetylcysteine (NAC) Diminishes the Severity of PCB 126-Induced Fatty Liver in Male Rodents. Toxicology 2012, 302, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (Nafld). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic Recruitment of Macrophages Promotes Nonalcoholic Steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Mustafa, G.; Alam, M.; Ahmad, N. Insulin Resistance in Development and Progression of Nonalcoholic Fatty Liver Disease. World J. Gastrointest. Pathophysiol. 2016, 7, 211. [Google Scholar] [CrossRef]
- Han, X.; Bao, X.; Lou, Q.; Xie, X.; Zhang, M.; Zhou, S.; Guo, H.; Jiang, G.; Shi, Q. Nicotinamide Riboside Exerts Protective Effect against Aging-Induced NAFLD-like Hepatic Dysfunction in Mice. PeerJ 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.S.; Jun, W.; Yang, S.J. Nicotinamide Riboside Ameliorates Hepatic Metaflammation by Modulating NLRP3 Inflammasome in a Rodent Model of Type 2 Diabetes. J. Med. Food 2015, 18, 1207–1213. [Google Scholar] [CrossRef]
- Poniachik, J.; Csendes, A.; Díaz, J.C.; Rojas, J.; Burdiles, P.; Maluenda, F.; Smok, G.; Rodrigo, R.; Videla, L.A. Increased Production of IL-1α and TNF-α in Lipopolysaccharide-Stimulated Blood from Obese Patients with Non-Alcoholic Fatty Liver Disease. Cytokine 2006, 33, 252–257. [Google Scholar] [CrossRef]
- Ge, C.X.; Yu, R.; Xu, M.X.; Li, P.Q.; Fan, C.Y.; Li, J.M.; Kong, L.D. Betaine Prevented Fructose-Induced NAFLD by Regulating LXRα/PPARα Pathway and Alleviating ER Stress in Rats. Eur. J. Pharmacol. 2016, 770, 154–164. [Google Scholar] [CrossRef]
- Salic, K.; Gart, E.; Seidel, F.; Verschuren, L.; Caspers, M.; van Duyvenvoorde, W.; Wong, K.E.; Keijer, J.; Bobeldijk-Pastorova, I.; Wielinga, P.Y.; et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 2019, 20, 4359. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Chen, Y.C.; Chen, I.S.; Hsieh, S.C.; Chen, S.S.; Chiu, C.H. Curcumin Protects against Thioacetamide-Induced Hepatic Fibrosis by Attenuating the Inflammatory Response and Inducing Apoptosis of Damaged Hepatocytes. J. Nutr. Biochem. 2012, 23, 1352–1366. [Google Scholar] [CrossRef]
- Varela-Rey, M.; Martínez-López, N.; Fernández-Ramos, D.; Embade, N.; Calvisi, D.F.; Woodhoo, A.; Rodríguez, J.; Fraga, M.F.; Julve, J.; Rodríguez-Millán, E.; et al. Fatty Liver and Fibrosis in Glycine N-Methyltransferase Knockout Mice Is Prevented by Nicotinamide. Hepatology 2010, 52, 105–114. [Google Scholar] [CrossRef]
- Werge, M.P.; McCann, A.; Galsgaard, E.D.; Holst, D.; Bugge, A.; Albrechtsen, N.J.W.; Gluud, L.L. The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 1081. [Google Scholar] [CrossRef]
- Deminice, R.; Da Silva, R.P.; Lamarre, S.G.; Kelly, K.B.; Jacobs, R.L.; Brosnan, M.E.; Brosnan, J.T. Betaine Supplementation Prevents Fatty Liver Induced by a High-Fat Diet: Effects on One-Carbon Metabolism. Amino Acids 2015, 47, 839–846. [Google Scholar] [CrossRef]
- Keinicke, H.; Sun, G.; Mentzel, C.M.J.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. Fgf21 Regulates Hepatic Metabolic Pathways to Improve Steatosis and Inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T. Research Perspectives on the Regulation and Physiological Functions of FGF21 and Its Association with NAFLD. Front. Endocrinol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Rusli, F.; Deelen, J.; Andriyani, E.; Boekschoten, M.V.; Lute, C.; Van Den Akker, E.B.; Müller, M.; Beekman, M.; Steegenga, W.T. Fibroblast Growth Factor 21 Reflects Liver Fat Accumulation and Dysregulation of Signalling Pathways in the Liver of C57BL/6J Mice. Sci. Rep. 2016, 6, 30484. [Google Scholar] [CrossRef] [PubMed]
- Vernia, S.; Cavanagh-Kyros, J.; Garcia-Haro, L.; Sabio, G.; Barrett, T.; Jung, D.Y.; Kim, J.K.; Xu, J.; Shulha, H.P.; Garber, M.; et al. The PPARα-FGF21 Hormone Axis Contributes to Metabolic Regulation by the Hepatic JNK Signaling Pathway. Cell Metab. 2014, 20, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Martinez-Guino, L.; Goldfine, A.B.; Ribas-Aulinas, F.; De Nigris, V.; Ribó, S.; Gonzalez-Franquesa, A.; Garcia-Roves, P.M.; Li, E.; Dreyfuss, J.M.; et al. Dietary Betaine Supplementation Increases Fgf21 Levels to Improve Glucose Homeostasis and Reduce Hepatic Lipid Accumulation in Mice. Diabetes 2016, 65, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Ni, Y.; Feng, X.; Zhao, Z.; Wang, P.; Sun, J.; Yu, H.; Yan, Z.; Liu, D.; et al. TRPV1 Activation Prevents Nonalcoholic Fatty Liver through UCP2 Upregulation in Mice. Pflug. Arch. Eur. J. Physiol. 2012, 463, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Tamborra, R.; Rollo, T.; Capitanio, N.; Romano, A.D.; Sastre, J.; Vendemiale, G.; Altomare, E. Uncoupling Protein-2 (UCP2) Induces Mitochondrial Proton Leak and Increases Susceptibility of Non-Alcoholic Steatohepatitis (NASH) Liver to Ischaemia-Reperfusion Injury. Gut 2008, 57, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Chavin, K.D.; Yang, S.Q.; Lin, H.Z.; Chatham, J.; Chacko, V.P.; Hock, J.B.; Walajtys-Rode, E.; Rashid, A.; Chen, C.H.; Huang, C.C.; et al. Obesity Induces Expression of Uncoupling Protein-2 in Hepatocytes and Promotes Liver ATP Depletion. J. Biol. Chem. 1999, 274, 5692–5700. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Villani, R.; Tamborra, R.; Blonda, M.; Iannelli, G.; di Bello, G.; Facciorusso, A.; Poli, G.; Iuliano, L.; Avolio, C.; et al. Synergistic Interaction of Fatty Acids and Oxysterols Impairs Mitochondrial Function and Limits Liver Adaptation during Nafld Progression. Redox Biol. 2018, 15, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.H.; Messiha, B.A.S.; Abdel-Latif, H.A.T. Protective Effect of Ursodeoxycholic Acid, Resveratrol, and N-Acetylcysteine on Nonalcoholic Fatty Liver Disease in Rats. Pharm. Biol. 2016, 54, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Nicco, C.; Van Nhieu, J.T.; Borderie, D.; Chéreau, C.; Conti, F.; Jaffray, P.; Soubrane, O.; Calmus, Y.; Weill, B.; et al. Pivotal Role of Superoxide Anion and Beneficial Effect of Antioxidant Molecules in Murine Steatohepatitis. Hepatology 2004, 39, 1277–1285. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; Nandgiri, G.; Sandoval, B.C.; Caudill, M.A.; Bottiglieri, T.; French, S.W.; Morgan, T.R. Betaine Improves Nonalcoholic Fatty Liver and Associated Hepatic Insulin Resistance: A Potential Mechanism for Hepatoprotection by Betaine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1068–G1077. [Google Scholar] [CrossRef]
- Jwa, H.; Choi, Y.; Park, U.H.; Um, S.J.; Yoon, S.K.; Park, T. Piperine, an LXRα Antagonist, Protects against Hepatic Steatosis and Improves Insulin Signaling in Mice Fed a High-Fat Diet. Biochem. Pharmacol. 2012, 84, 1501–1510. [Google Scholar] [CrossRef]
- Hagiwara, A.; Cornu, M.; Cybulski, N.; Polak, P.; Betz, C.; Trapani, F.; Terracciano, L.; Heim, M.H.; Rüegg, M.A.; Hall, M.N. Hepatic MTORC2 Activates Glycolysis and Lipogenesis through Akt, Glucokinase, and SREBP1c. Cell Metab. 2012, 15, 725–738. [Google Scholar] [CrossRef]
- Iizuka, K.; Horikawa, Y. ChREBP: A Glucose-Activated Transcription Factor Involved in the Development of Metabolic Syndrome. Endocr. J. 2008, 55, 617–624. [Google Scholar] [CrossRef]
- Liu, X.; Cui, J.; Li, Z.; Xu, J.; Wang, J.; Xue, C.; Wang, Y. Comparative Study of DHA-Enriched Phospholipids and EPA-Enriched Phospholipids on Metabolic Disorders in Diet-Induced-Obese C57BL/6J Mice. Eur. J. Lipid Sci. Technol. 2014, 116, 255–265. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wang, Q.; Yang, Y. Crude Extracts from Lycium Barbarum Suppress SREBP-1c Expression and Prevent Diet-Induced Fatty Liver through AMPK Activation. BioMed Res. Int. 2014, 2014, 196198. [Google Scholar] [CrossRef]
- Ono, H.; Shimano, H.; Katagiri, H.; Yahagi, N.; Sakoda, H.; Onishi, Y.; Anai, M.; Ogihara, T.; Fujishiro, M.; Viana, A.Y.I.; et al. Hepatic Akt Activation Induces Marked Hypoglycemia, Hepatomegaly, and Hypertriglyceridemia with Sterol Regulatory Element Binding Protein Involvement. Diabetes 2003, 52, 2905–2913. [Google Scholar] [CrossRef]
- Hu, X.Q.; Wang, Y.M.; Wang, J.F.; Xue, Y.; Li, Z.J.; Nagao, K.; Yanagita, T.; Xue, C.H. Dietary Saponins of Sea Cucumber Alleviate Orotic Acid-Induced Fatty Liver in Rats via PPAR and SREBP-1c Signaling. Lipids Health Dis. 2010, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased Levels of Nuclear SREBP-1c Associated with Fatty Livers in Two Mouse Models of Diabetes Mellitus *. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Zhuo, M.Q.; Li, D.D.; Xu, Y.H.; Wu, K.; Luo, Z. SREBP-1 and LXRA Pathways Mediated Cu-Induced Hepatic Lipid Metabolism in Zebrafish Danio Rerio. Chemosphere 2019, 215, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, T.; Ye, M.; Qiu, Y.; Pei, L.; Jiang, R.; Pang, N.; Huang, Y.; Liang, B.; Ling, W.; et al. Nicotinamide Riboside Attenuates Alcohol Induced Liver Injuries via Activation of SirT1/PGC-1α/Mitochondrial Biosynthesis Pathway. Redox Biol. 2018, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Van Loo, P.L.P.; Kuin, N.; Sommer, R.; Avsaroglu, H.; Pham, T.; Baumans, V. Impact of “living Apart Together” on Postoperative Recovery of Mice Compared with Social and Individual Housing. Lab. Anim. 2007, 41, 441–455. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Wang, H.; Zhao, X.; Li, N.; Zhang, H.; Chen, G.; Liu, Z. Fish Oil Alleviates Circadian Bile Composition Dysregulation in Male Mice with NAFLD. J. Nutr. Biochem. 2019, 69, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; González-Muniesa, P.; Sáinz, N.; Laiglesia, L.M.; Escoté, X.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 Regulates Hepatic FGF21 in Diet-Induced Obese Mice and in Cultured Hepatocytes. Mol. Nutr. Food Res. 2019, 63, e1900358. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada-Vázquez, S.; Colom-Pellicer, M.; Navarro-Masip, È.; Aragonès, G.; Del Bas, J.M.; Caimari, A.; Escoté, X. Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients 2021, 13, 3532. https://doi.org/10.3390/nu13103532
Quesada-Vázquez S, Colom-Pellicer M, Navarro-Masip È, Aragonès G, Del Bas JM, Caimari A, Escoté X. Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients. 2021; 13(10):3532. https://doi.org/10.3390/nu13103532
Chicago/Turabian StyleQuesada-Vázquez, Sergio, Marina Colom-Pellicer, Èlia Navarro-Masip, Gerard Aragonès, Josep M. Del Bas, Antoni Caimari, and Xavier Escoté. 2021. "Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice" Nutrients 13, no. 10: 3532. https://doi.org/10.3390/nu13103532
APA StyleQuesada-Vázquez, S., Colom-Pellicer, M., Navarro-Masip, È., Aragonès, G., Del Bas, J. M., Caimari, A., & Escoté, X. (2021). Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients, 13(10), 3532. https://doi.org/10.3390/nu13103532









