Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
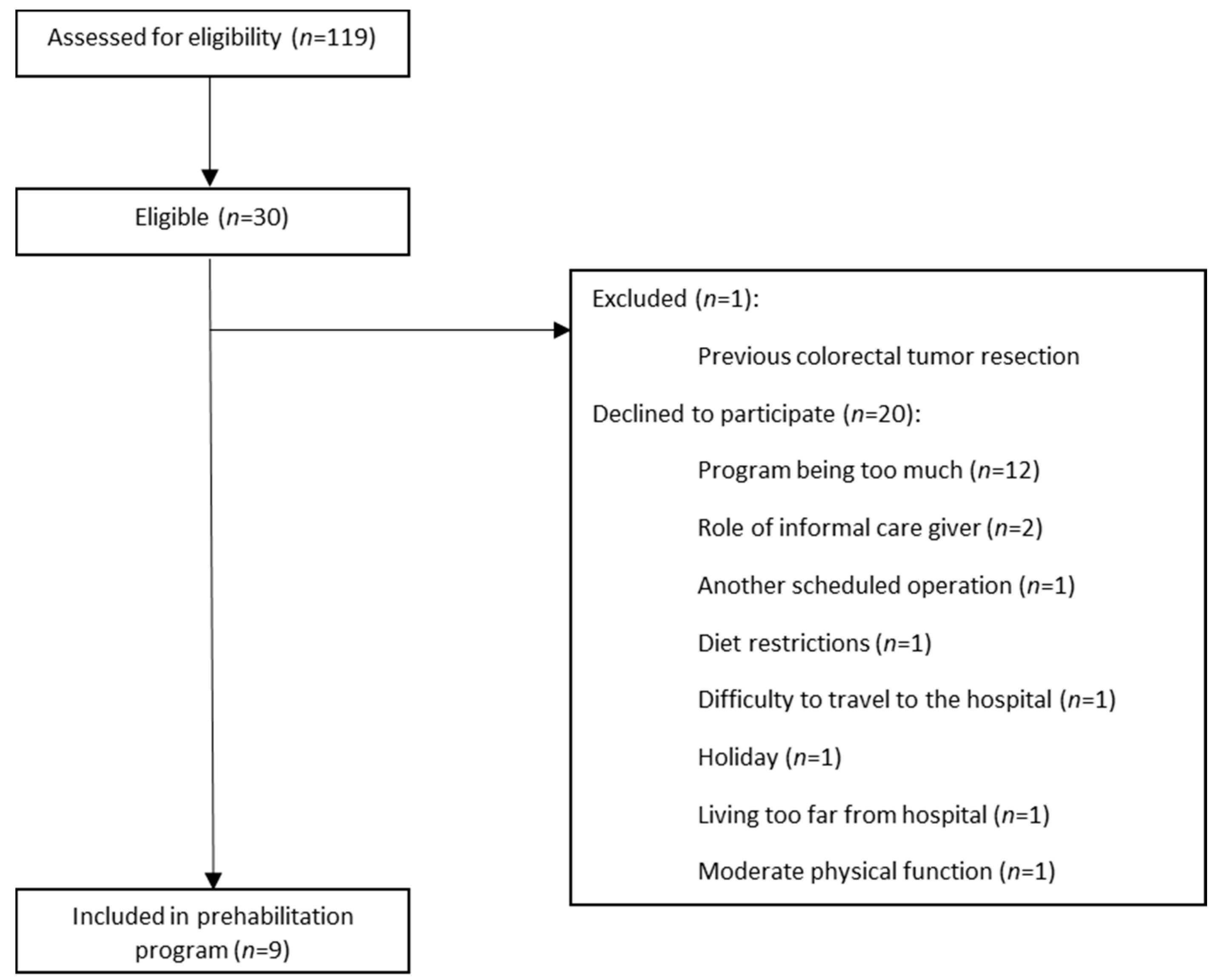
2.2. Study Participants
2.3. Baseline Assessment
2.4. Exercise Intervention
2.5. Nutritional Intervention
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Adherence Prehabilitation
3.2. Organizational Feasibility
3.3. Acceptability of the Interventions
3.4. Physical Outcome Measures
3.5. Surgical Outcomes
4. Discussion
Acceptability of Interventions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B



Appendix C
Section of the Menu
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Multigrain bread | 2 | 35 | 70 |
| Butter | 2 | 5 | 10 |
| Chicken breast | 2 | 15 | 30 |
| Mature cheese | 1 | 20 | 20 |
| Boiled egg | 1 | 50 | 50 |
| Yogurt with muesli | 1 | 170 | 170 |
| Banana | 1 | 80 | 80 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Cream puff with stuffing: | 5 | 3 | 15 |
| Curd | 1 | 40 | 40 |
| Cream | 1 | 15 | 15 |
| Mascarpone | 1 | 20 | 20 |
| Powdered sugar | 1 | 10 | 10 |
| Passion fruit | 1 | 15 | 15 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Sandwich: | 2 | 35 | 70 |
| Herb cheese | 2 | 15 | 30 |
| Smoked meat | 2 | 15 | 30 |
| Baked onions | 1 | 10 | 10 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Smoked chicken salad with dressing | |||
| Salad: | |||
| Smoked chicken breast | 1 | 35 | 35 |
| Red pepper | 1 | 15 | 15 |
| Cucumber | 1 | 5 | 5 |
| Apple | 1 | 9 | 9 |
| Raisins | 1 | 4 | 4 |
| Shallot | 1 | 2 | 2 |
| Little yam lettuce | 1 | 1 | 1 |
| Dressing: | |||
| Mayonnaise | 1 | 20 | 20 |
| Crème fraiche | 1 | 10 | 10 |
| Lemon juice | 1 | 3.5 | 3.5 |
| Parsley | 1 | 0.5 | 0.5 |
| Chives | 1 | 0.05 | 0.05 |
| Pepper | 1 | 0.05 | 0.05 |
| Avocado | 1 | 60 | 60 |
| Quail egg | 3 | 10 | 30 |
| Wheat bread | 1 | 35 | 35 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Red curry soup | 1 | 200 | 200 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Grilled salmon | 1 | 80 | 80 |
| Remoulade sauce: | |||
| Mayonnaise | 1 | 20 | 20 |
| Yogurt | 1 | 20 | 20 |
| Mustard | 1 | 2 | 2 |
| Capers | 1 | 5 | 5 |
| Shallot | 1 | 3 | 3 |
| Pepper | 1 | 0.0001 | 0.0001 |
| Mashed potatoes with parmesan | 2 | 150 | 300 |
| Peas | 1 | 150 | 150 |
| Cottage cheese dessert | 1 | 125 | 125 |
| Raspberry | 1 | 25 | 25 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Chicken meatball: | |||
| Chicken breast | 2 | 14.5 | 29 |
| Chicken thigh | 2 | 27 | 54 |
| Water | 2 | 2.5 | 5 |
| Chicken seasoning | 2 | 1.5 | 3 |
| Bread-crumbs | 2 | 1.5 | 3 |
| Egg | 2 | 3 | 6 |
| Satay sauce | 1 | 50 | 50 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Whole grain bread | 2 | 35 | 70 |
| Butter | 2 | 5 | 10 |
| Smoked meat | 1 | 25 | 25 |
| Mature cheese | 1 | 20 | 20 |
| Tomato | 1 | 70 | 70 |
| Omelet | 1 | 90 | 90 |
| Description | Portion | Product weight (gram) | Total weight (gram) |
|---|---|---|---|
| Brownie chocolate/hazelnut | 1 | 125 | 125 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Chicken and bean burger: | |||
| Minced chicken | 1 | 55 | 55 |
| Bread-crumbs | 1 | 4 | 4 |
| Egg | 1 | 3 | 3 |
| Lemon juice | 1 | 1 | 1 |
| Mascarpone | 1 | 1.8 | 1.8 |
| Coconut milk | 1 | 2 | 2 |
| Coriander | 1 | 0.2 | 0.2 |
| Parsley | 1 | 1 | 1 |
| Kidney beans | 1 | 8 | 8 |
| Shallot | 1 | 3 | 3 |
| Salt | 1 | 1 | 1 |
| Brioche bun with pickle: | |||
| Brioche bun | 1 | 35 | 35 |
| Pickle | 1 | 15 | 15 |
| Yogurt dip | |||
| Low-fat yogurt | 1 | 20 | 20 |
| Crème fraiche | 1 | 15 | 15 |
| Parsley | 1 | 1 | 1 |
| Garlic clove powder | 1 | 0.05 | 0.05 |
| Lime juice | 1 | 1 | 1 |
| Salt | 1 | 0.5 | 0.5 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Red pepper soup | 1 | 200 | 200 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Macaroni | 1 | 145 | 145 |
| Healthy bolognaise | 1 | 145 | 145 |
| Parmesan | 1 | 10 | 10 |
| Cucumber salad | 1 | 50 | 50 |
| Skyr with forest fruits: | |||
| Skyr | 1 | 125 | 125 |
| Forest fruits | |||
| Raspberry | 1 | 8 | 8 |
| Blueberry | 1 | 9 | 9 |
| Strawberry | 1 | 8 | 8 |
| Description | Portion | Product Weight (gram) | Total Weight (gram) |
|---|---|---|---|
| Tortilla with hummus dip | |||
| Tortilla | 1 | 25 | 25 |
| Hummus | 1 | 60 | 60 |
References
- Govaert, J.A.; Fiocco, M.; van Dijk, W.A.; Scheffer, A.C.; de Graaf, E.J.R.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M. Costs of complications after colorectal cancer surgery in the Netherlands: Building the business case for hospitals. Eur. J. Surg. Oncol. 2015, 41, 1059–1067. [Google Scholar] [CrossRef]
- Tevis, S.E.; Kennedy, G.D. Postoperative Complications: Looking Forward to a Safer Future. Clin. Colon Rectal Surg. 2016, 29, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Ripollés-Melchor, J.; Ramírez-Rodríguez, J.M.; Casans-Francés, R.; Aldecoa, C.; Abad-Motos, A.; Logroño-Egea, M.; García-Erce, J.A.; Camps-Cervantes, Á.; Ferrando-Ortolá, C.; Suarez de la Rica, A.; et al. For the POWER Study Investigators Group for the Spanish Perioperative Audit and Research Network (REDGERM) Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery: The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg. 2019, 154, 725–736. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Salimzadeh, H.; Delavari, A.; Ansari, R.; Merat, S.; Fitzmaurice, C.; Abegaz, K.H.; Akinyemiju, T.; Alikhani, M.; Alvis-Guzman, N.; et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Nederlandse Kankerregistratie (NKR), Integraal Kankercentrum Nederland (IKNL). Available online: https://iknl.nl/kankersoorten/darmkanker (accessed on 7 March 2021).
- Poos, M.J.J.C. (RIVM), van der Wilk, E.A. red. (RIVM). Levensverwachting, Volksgezondheidenzorg.info. 2020. Available online: https://www.volksgezondheidenzorg.info/onderwerp/levensverwachting/cijfers-context/trends#node-prognose-levensverwachting (accessed on 2 March 2021).
- Vogel, J.D.; Eskicioglu, C.; Weiser, M.R.; Feingold, D.L.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis. Colon Rectum 2017, 60, 999–1017. [Google Scholar] [CrossRef]
- Audisio, R.A.; Pope, D.; Ramesh, H.S.J.; Gennari, R.; van Leeuwen, B.L.; West, C.; Corsini, G.; Maffezzini, M.; Hoekstra, H.J.; Mobarak, D.; et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help A SIOG surgical task force prospective study. Crit. Rev. Oncol. Hematol. 2008, 65, 156–163. [Google Scholar] [CrossRef]
- Nederlandse Vereniging voor Klinische Geriatrie. Comprehensive Geriatric Assessment. Nederlandse Vereniging voor Klinische Geriatrie. 2010. Available online: file:///H:/Downloads/Comprehensive_geriatric_assessment_CGA_.pdf (accessed on 12 April 2021).
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Matteo, C.; Chumlea, W.C.; Wolfram, D.; Jonathan, E.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, J.S.L.; Harari, D.; Dhesi, J.K. Frailty in the older surgical patient: A review. Age Ageing 2012, 41, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milder, D.A.; Pillinger, N.L.; Kam, P.C.A. The role of prehabilitation in frail surgical patients: A systematic review. Acta Anaesthesiol. Scandinavica 2018, 62, 1356–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carli, F.; Zavorsky, G. Optimizing functional exercise capacity in the elderly surgical population. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.; Stein, B.; Charlebois, P.; Feldman, L.; et al. Prehabilitation versus Rehabilitation: A Randomized Control Trial in Patients Undergoing Colorectal Resection for Cancer. Anesthesiology (Philadelphia) 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Gillis, C.; Fiore, J.F.; Liberman, A.S.; Charlebois, P.; Stein, B.; Bousquet-Dion, G.; Feldman, L.S.; Carli, F.; et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery 2016, 160, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Minnella, E.M.; Bousquet-Dion, G.; Awasthi, R.; Scheede-Bergdahl, C.; Carli, F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: A five-year research experience. Acta Oncol. 2017, 56, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.; Hackney, R.; Lamb, P.; Wigmore, S.; Christopher Deans, D.; Skipworth, R. Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-analysis. World J. Surg. 2019, 43, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; McCourt, O.; Edbrooke, L.; Granger, C.L.; Ismail, H.; Riedel, B.; Denehy, L. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front Surg. 2021, 8, 628848. [Google Scholar] [CrossRef]
- Bousquet-Dion, G.; Awasthi, R.; Loiselle, S.; Minnella, E.M.; Agnihotram, R.V.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncol. 2018, 57, 849–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, S.L.; Lee, M.J.; George, J.; Kerr, K.; Moug, S.; Wilson, T.R.; Brown, S.R.; Wyld, L. Prehabilitation in elective abdominal cancer surgery in older patients: Systematic review and meta-analysis. BJS Open 2020, 4, 1022–1041. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Jen, T.; Mookerji, N.; Patel, A.; Lalu, M.M. Interventions to improve the outcomes of frail people having surgery: A systematic review. PLoS ONE 2017, 12, e0190071. [Google Scholar] [CrossRef]
- Carli, F.; Charlebois, P.; Stein, B.; Feldman, L.; Zavorsky, G.; Kim, D.J.; Scott, S.; Mayo, N.E. Randomized clinical trial of prehabilitation in colorectal surgery. Br. J. Surg. 2010, 97, 1187–1197. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs. Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef]
- Looijaard, S.M.L.M.; Slee-Valentijn, M.; Otten, R.H.J.; Maier, A.B. Physical and Nutritional Prehabilitation in Older Patients With Colorectal Carcinoma: A Systematic Review. J. Geriatr. Phys. Ther. 2018, 41, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.R.J.; Argillander, T.E.; Van Den Heuvel, B.; Buskens, C.J.; Van Duijvendijk, P.; Winkels, R.M.; Kalf, A.; Van Der Zaag, E.S.; Wassenaar, E.B.; Bemelman, W.A.; et al. Oral Nutrition as a Form of Pre-Operative Enhancement in Patients Undergoing Surgery for Colorectal Cancer: A Systematic Review. Surg. Infect. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-analysis. Gastroenterology 2018, 155, 391–410. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.W.; Wierdsma, N.J.; van Bokhorst–de van der Schueren, M.A.E. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ©). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef]
- Steverink, N.; Slaets, J.; Schuurmans, H.; Lis, M. Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). Gerontologist 2001, 41, 236–237. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Freyd, M. The Graphic Rating Scale. J. Educ. Psychol. 1923, 14, 83–102. [Google Scholar] [CrossRef]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Clin. Otolaryngol. 2012, 37, 390–392. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Tanaka, H. Exercise Prescription for the Elderly: Current Recommendations. Sports Med. 2001, 31, 809–818. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surgery 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- van Rooijen, S.; Molenaar, C.; Schep, G.; van Lieshout, R.; Beijer, S.; Dubbers, R.; Rademakers, N.; Papen-Botterhuis, N.; van Kempen, S.; Carli, F.; et al. Making Patients Fit for Surgery: Introducing a Four Pillar Multimodal Prehabilitation Program in Colorectal Cancer. Am. J. Phys. Med. Rehabil. 2019, 98, 888–896. [Google Scholar] [CrossRef]
- Karlsson, E.; Farahnak, P.; Franzen, E.; Nygren-Bonnier, M.; Dronkers, J.; van Meeteren, N.; Rydwik, E. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery–A randomized controlled design. PLoS ONE 2019, 14, e0219158. [Google Scholar] [CrossRef] [PubMed]
- Northgraves, M.J.; Arunachalam, L.; Madden, L.A.; Marshall, P.; Hartley, J.E.; MacFie, J.; Vince, R.V. Feasibility of a novel exercise prehabilitation programme in patients scheduled for elective colorectal surgery: A feasibility randomised controlled trial. Supportive Care Cancer 2020, 28, 3197–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurdo, M.E.T.; Roberts, H.; Parker, S.; Wyatt, N.; May, H.; Goodman, C.; Jackson, S.; Gladman, J.; O’Mahony, S.; Ali, K.; et al. Improving recruitment of older people to research through good practice. Age Ageing 2011, 40, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Dronkers, J.; Lamberts, H.; Reutelingsperger, I.; Naber, R.; Dronkers-Landman, C.; Veldman, A.; van Meeteren, N. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: A randomized controlled pilot study. Clin. Rehabil. 2010, 24, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Picorelli, A.M.A.; Pereira, L.S.M.; Pereira, D.S.; Felício, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Nederlandse Zorgautoriteit. Toezichtkader Zorgplicht Zorgverzekeraar Zvw-TH/BR-018. 2014. Available online: https://puc.overheid.nl/doc/PUC_21832_22/1 (accessed on 20 May 2021).
- Dekker, J.W. Indicatorenset. DCRA Verslagjaar. Zorginzicht.nl: Zorginstituut Nederland. 2020. Available online: https://www.zorginzicht.nl/binaries/content/assets/zorginzicht/kwaliteitsinstrumenten/Indicatorgids+Colorectaal+carcinoom+%28DCRA%29+verslagjaar+2020.pdf (accessed on 20 May 2021).
- Molenaar, C.J.L.; Janssen, L.; van der Peet, D.L.; Winter, D.C.; Roumen, R.M.H.; Slooter, G.D. Conflicting Guidelines: A Systematic Review on the Proper Interval for Colorectal Cancer Treatment. World J. Surg. 2021, 45, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Darmon, P.; Karsegard, V.L.; Nardo, P.; Dupertuis, Y.M.; Pichard, C. Oral nutritional supplements and taste preferences: 545 days of clinical testing in malnourished in-patients. Clin. Nutr. 2008, 27, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, M.; Shin, S. What is the Minimum Clinically Important Difference in Grip Strength? Clin. Orthop. Relat. Res. 2014, 472, 2536–2541. [Google Scholar] [CrossRef] [Green Version]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-Week Multimodal Prehabilitation Program Improves Perioperative Functional Capability in Patients Undergoing Thoracoscopic Lobectomy for Lung Cancer: A Randomized Controlled Trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh Frans, H.C.; Eijsvogel, M.M.M.; Wymenga, A.N.M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications. Ann. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 9) | |
|---|---|
| Age (years) | 73.0 (70.0–76.0) |
| Gender ratio (M:F) | 5:4 |
| Height (cm) | 169.0 (160.5–178.0) |
| Bodyweight (kg) | 84.0 (73.5–91.4) |
| BMI 1 (kg/m2) | 26.9 (25.0–32.7) |
| Smoking; n (%) | 1 (11.1) |
| Alcohol; n (%) | 1 (11.1) |
| Comorbidity; n (%) | |
| Cardiovascular disease | 7 (77.8) |
| Pulmonary disease | 5 (55.6) |
| History of abdominal surgery | 2 (22.2) |
| History of other malignancy | 2 (22.2) |
| ASA score 2; n (%) | |
| II | 7 (77.8) |
| III | 2 (22.2) |
| GFI 3; n (%) | |
| 0 | 1 (11.1) |
| I | 2 (22.2) |
| II | 1 (11.1) |
| III | 1 (11.1) |
| IV | 2 (22.2) |
| V | 2 (22.2) |
| SNAQ 4; n (%) | |
| 0 | 6 (66.7) |
| I | 2 (22.2) |
| V | 1 (11.1) |
| Diagnosis; n | |
| Malignancy | 8 |
| Liver metastasis at diagnosis | 1 |
| Premalignancy | 1 |
| Adjuvant treatment; n | |
| Neoadjuvant chemoradiation | 2 |
| Adjuvant chemotherapy | 1 |
| Study Population (n = 9) | |
|---|---|
| >80% of the prehabilitation program completed | 6 (66.7) |
| >80% of all 12 training sessions attended; n (%) | 7 (77.8) |
| >80% of all available training sessions attended; n (%) | 9 (100) |
| Individual adherence percentage training sessions | 91.7 (75.0–95.8) |
| Number of attended training sessions | 11.0 (9.0–11.5) |
| Prehabilitation period (days) | 26.0 (22.0–27.0) |
| >80% of the required protein consumed; n (%) | 6 (66.7) |
| >80% of the required calories consumed; n (%) | 9 (100) |
| Individual percentage meals consumed | 95.2 (79.8–97.6) |
| Total protein intake (grams/day) | 113.4 (87.1–114.1) |
| Protein intake (grams/kg bodyweight/day) | 1.2 (1.1–1.5) |
| Total energy intake per day (kcal) | 2417.3 (1898.9–2646.3) |
| Pre-Prehabilitation | Post-Prehabilitation | Difference | p-Value | Z-Value | |
|---|---|---|---|---|---|
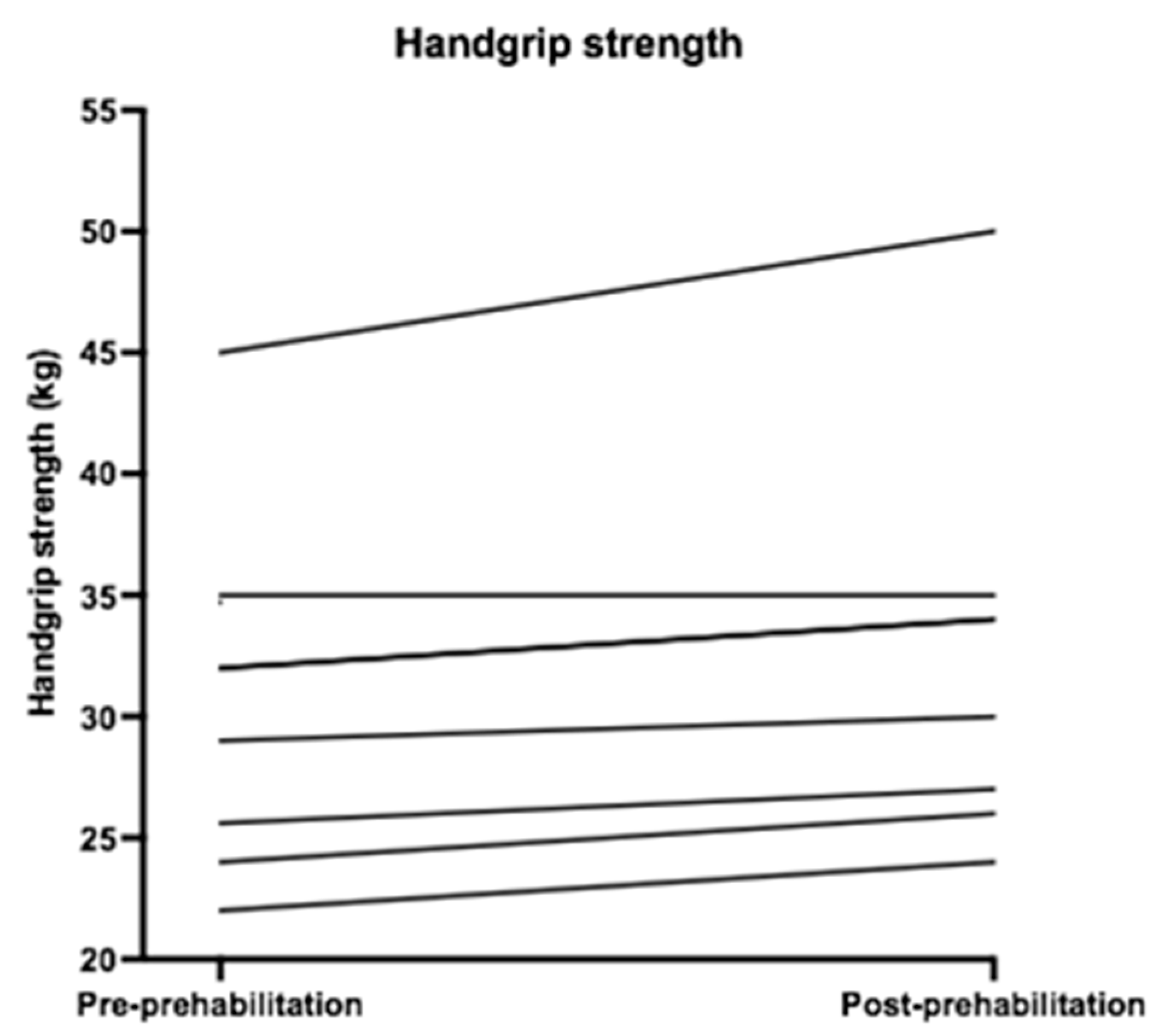
| Handgrip strength | 32.00 (24.80–34.85) | 32.00 (26.25–34.75) | 2 (1.1–2.0) | 0.02 | −2.41 |
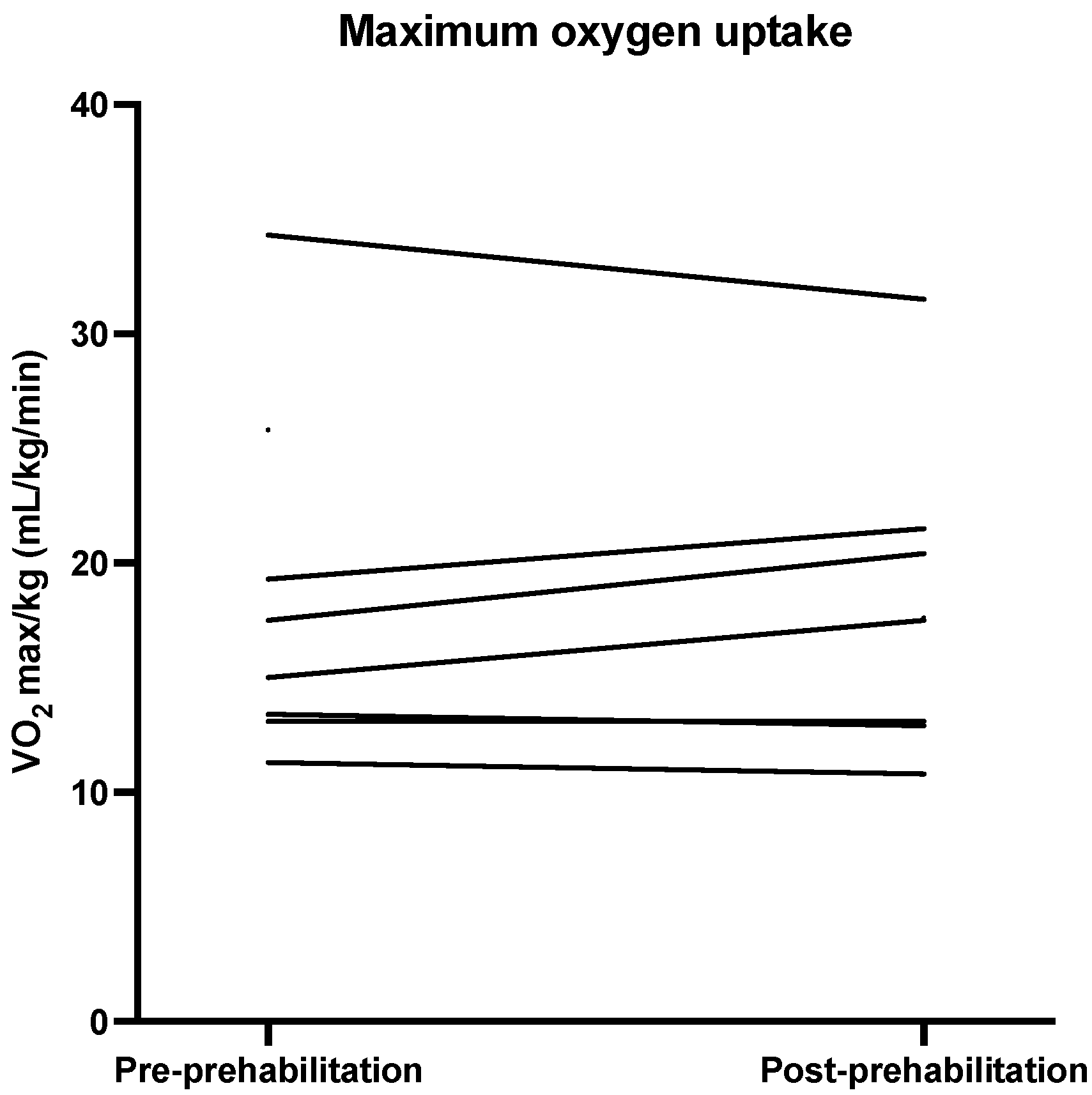
| VO2max 1 mL/min/kg | 16.25 (13.18–24.18) | 17.55 (12.95–21.23) | 0.0 (−0.5–2.5) | 0.60 | −0.53 |
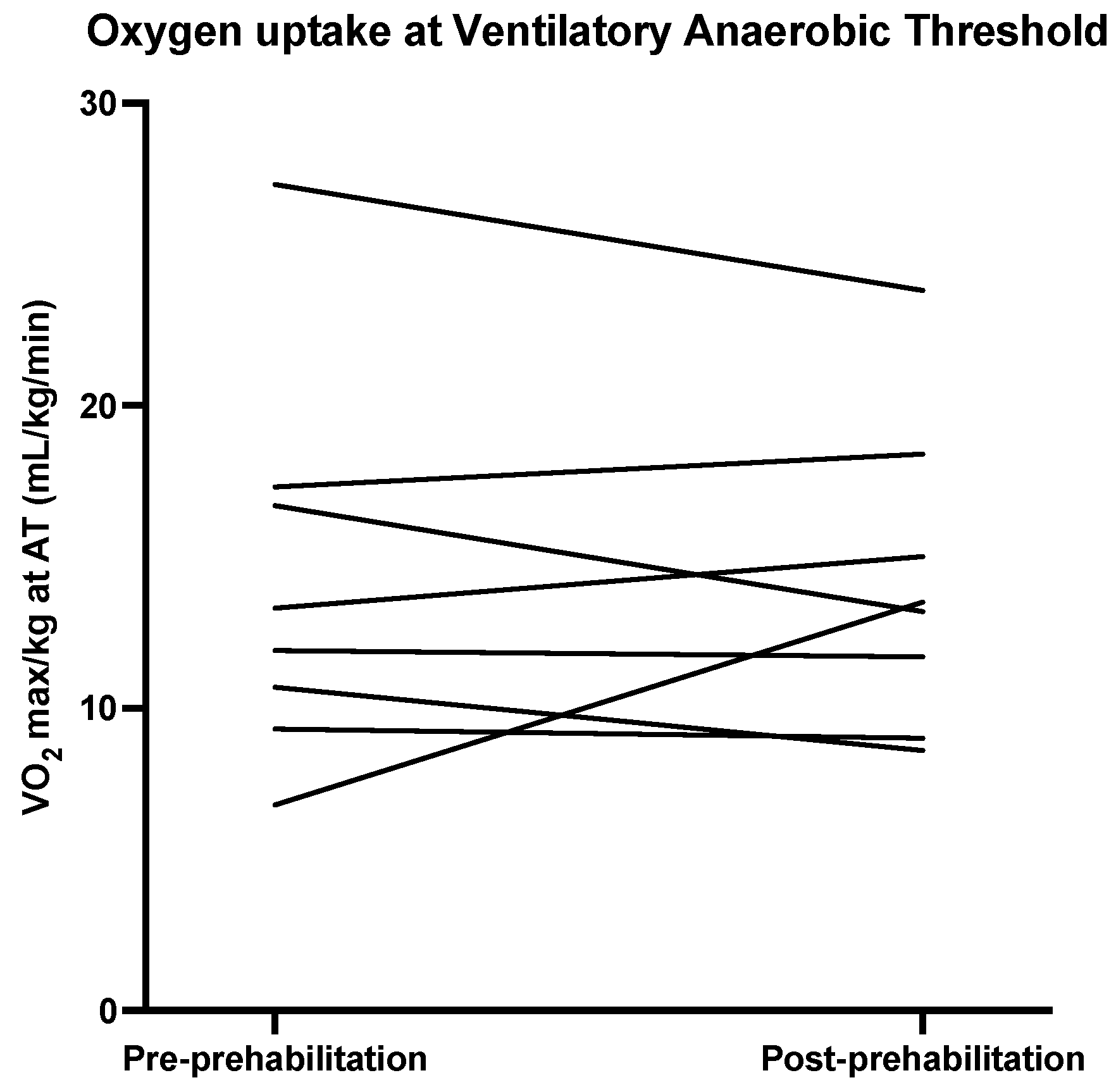
| VO2max at AT 2/kg | 12.60 (9.65–17.15) | 13.55 (9.68–17.55) | −0.25 (−3.15–1.55) | 0.67 | 0.67 |
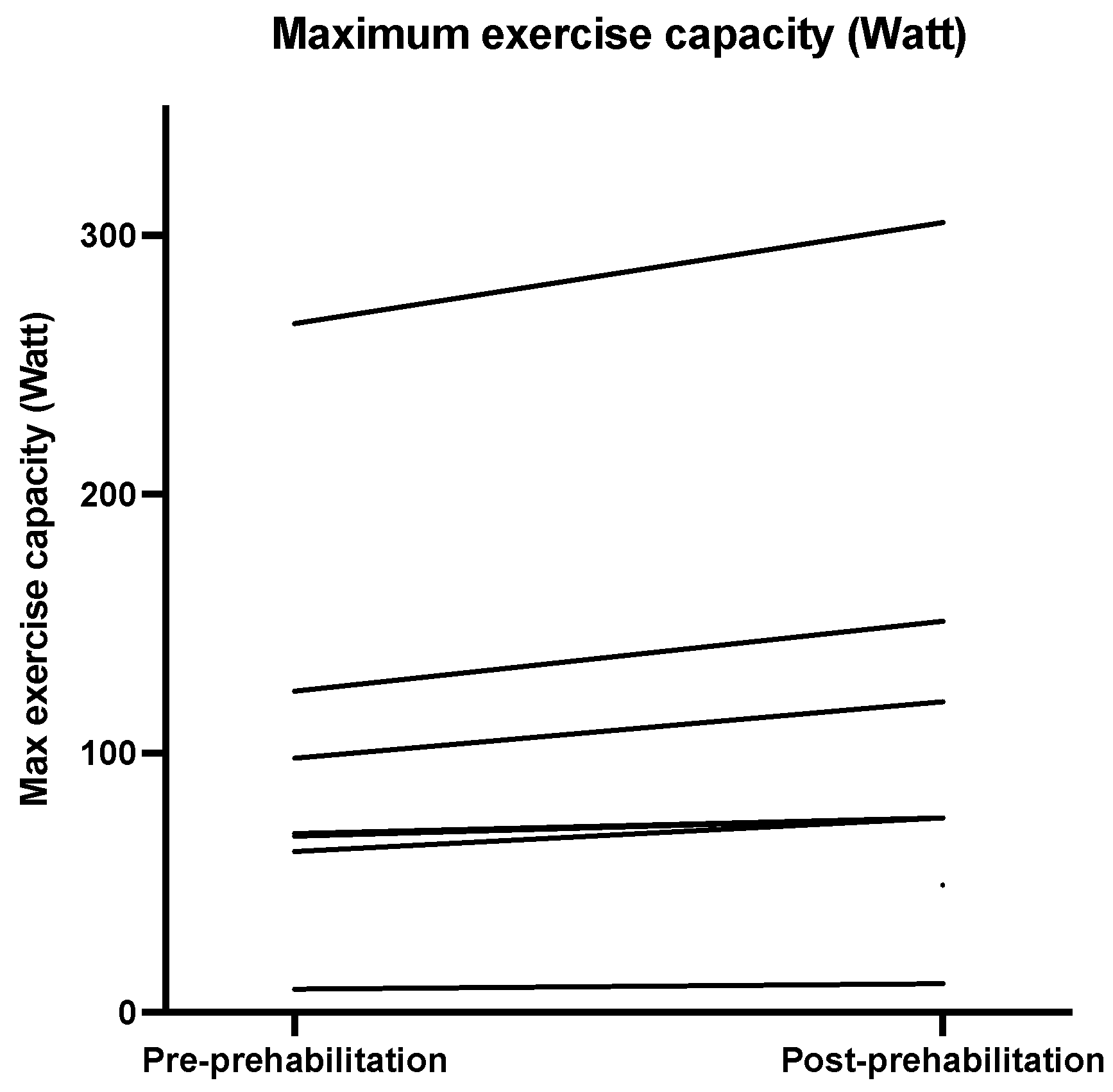
| Maximum exercise capacity (Watt) | 69.00 (62.00–124.00) | 75.00 (55.50–143.25) | 13 (6.0–27.0) | 0.02 | −2.37 |
| FVC 3 (liter) | 2.86 (2.71–4.42) | 3.19 (2.66–4.07) | 0.06 (−0.16–0.32) | 0.48 | −0.70 |
| FEV1 4 (liter) | 2.32 (1.05–3.35) | 2.19 (1.12–3.47) | 0.03 (−0.04–0.12) | 0.57 | −0.56 |
| Study Population (n = 9) | |
|---|---|
| Surgery; n (%) | 6 |
| Left hemicolectomy | 1 (16.7) |
| Right hemicolectomy | 4 (66.7) |
| Sigmoidectomy | 1 (16.7) |
| Re-admission < 30 days | 0 |
| Complication; n (%) | 2/6 (33.3) |
| Microperforation | 1 (16.7) |
| Tachycardia | 1 (16.7) |
| Clavien Dindo, n | |
| II | 1 (Tachycardia) (16.7) |
| IIIb | 1 (Perforation) (16.7) |
| Length of hospital stay (days) | 4 (3.0–8.8) |
| 30-day mortality; n | 0 |
| 1-year mortality; n | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tweed, T.T.T.; Sier, M.A.T.; Van Bodegraven, A.A.; Van Nie, N.C.; Sipers, W.M.W.H.; Boerma, E.-J.G.; Stoot, J.H.M.B. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients 2021, 13, 3493. https://doi.org/10.3390/nu13103493
Tweed TTT, Sier MAT, Van Bodegraven AA, Van Nie NC, Sipers WMWH, Boerma E-JG, Stoot JHMB. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients. 2021; 13(10):3493. https://doi.org/10.3390/nu13103493
Chicago/Turabian StyleTweed, Thaís T. T., Misha A. T. Sier, Ad A. Van Bodegraven, Noémi C. Van Nie, Walther M. W. H. Sipers, Evert-Jan G. Boerma, and Jan H. M. B. Stoot. 2021. "Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program" Nutrients 13, no. 10: 3493. https://doi.org/10.3390/nu13103493
APA StyleTweed, T. T. T., Sier, M. A. T., Van Bodegraven, A. A., Van Nie, N. C., Sipers, W. M. W. H., Boerma, E.-J. G., & Stoot, J. H. M. B. (2021). Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients, 13(10), 3493. https://doi.org/10.3390/nu13103493







