Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Objects
2.2. Research Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.E. Obesity and Gestational Diabetes Mellitus Pathways for Programming in Mouse, Monkey, and Man-Where Do We Go Next? The 2014 Norbert Freinkel Award Lecture. Diabetes Care 2015, 38, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Bornstein, S.R.; Abu-Asab, M.; Glasow, A.; Path, G.; Hauner, H.; Tsokos, M.; Chrousos, G.P.; Scherbaum, W.A. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes 2000, 49, 532–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamshad, M.; Aoki, V.T.; Adkison, M.G.; Warren, W.S.; Bartness, T.J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998, 275, R291–R299. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Shapiro, A.L.; Brinton, J.T.; Glueck, D.H.; Martinez, M.; Kohn, M.; Harrod, C.; Friedman, J.E.; Dabelea, D. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: The healthy start study. J. Clin. Endocrinol. Metab. 2015, 100, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Ghio, A.; Bertolotto, A.; Resi, V.; Volpe, L.; Di Cianni, G. Triglyceride metabolism in pregnancy. Adv. Clin. Chem. 2011, 55, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, K.K.; Spracklen, C.N.; Smith, C.J.; Robinson, J.G.; Saftlas, A.F. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG 2015, 122, 643–651. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Wei, Y.; Su, R.; Feng, H.; Hadar, E.; Hod, M.; Yang, H. The associations between early pregnancy lipid profiles and pregnancy outcomes. J. Perinatol. 2017, 37, 127–133. [Google Scholar] [CrossRef]
- Jin, W.Y.; Lin, S.L.; Hou, R.L.; Chen, X.Y.; Han, T.; Jin, Y.; Tang, L.; Zhu, Z.W.; Zhao, Z.Y. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: A population-based study from China. BMC Pregnancy Childb. 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M. Williams Obstetrics, 24th ed.; Mc-Graw-Hill: New York, NY, USA, 2014; p. 1291. [Google Scholar]
- The growth reference standards and curves of birth weight, length and head circumference of newborns of different gestational ages in China. Chin. J. Pediatr. 2020, 58, 738–746. [CrossRef]
- Feitosa, A.C.R.; Barreto, L.T.; da Silva, I.M.; da Silva, F.F.; Feitosa, G.S. Impact of the Use of Different Diagnostic Criteria in the Prevalence of Dyslipidemia in Pregnant Women. Arq. Bras. Cardiol. 2017, 109, 30–38. [Google Scholar] [CrossRef]
- Wang, X.X.; Guan, Q.B.; Zhao, J.J.; Yang, F.F.; Yuan, Z.S.; Yin, Y.C.; Fang, R.; Liu, L.W.; Zuo, C.T.; Gao, L. Association of maternal serum lipids at late gestation with the risk of neonatal macrosomia in women without diabetes mellitus. Lipids Health Dis. 2018, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Martinez, A.D.; Ortega, R.P.; Opazo, R.B.; Moreno-Moreno, P.; Puerta, M.J.M.; Galvez-Moreno, M.A. Hyperlipidemia during gestational diabetes and its relation with maternal and offspring complications. Nutr. Hosp. 2018, 35, 698–706. [Google Scholar] [CrossRef]
- Vrijkotte, T.G.; Krukziener, N.; Hutten, B.A.; Vollebregt, K.C.; van Eijsden, M.; Twickler, M.B. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J. Clin. Endocrinol. Metab. 2012, 97, 3917–3925. [Google Scholar] [CrossRef]
- Yu, M.R.; Wang, W.T.; Wang, H. The Late-Gestational Triglyceride to High-Density Lipoprotein Cholesterol Ratio Is Associated with Neonatal Macrosomia in Women without Diabetes Mellitus. Int. J. Endocrinol. 2020, 2020, 7250287. [Google Scholar] [CrossRef] [PubMed]
- Hegelund, E.R.; Wimmelmann, C.L.; Strizzi, J.M.; Folker, A.P.; Mortensen, E.L.; Flensborg-Madsen, T. Birth weight and quality of life in midlife: A 50-year follow-up study of 2079 individuals in Denmark. Qual. Life Res. 2020, 29, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Eide, M.G.; Oyen, N.; Skjaerven, R.; Nilsen, S.T.; Bjerkedal, T.; Tell, G.S. Size at birth and gestational age as predictors of adult height and weight. Epidemiology 2005, 16, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, S.F.; Mu, M.; Sheng, J. Birth weight and overweight/obesity in adults: A meta-analysis. Eur. J. Pediatr. 2012, 171, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zeng, L.; Wang, D.; Li, C.; Liu, Y.; Yan, H.; Xiao, Y. Effects of birth weight on body composition and overweight/obesity at early school age. Clin. Nutr. 2020, 39, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauguel-de Mouzon, S.; Lepercq, J.; Catalano, P. The known and unknown of leptin in pregnancy. Am. J. Obs. Gynecol. 2006, 194, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Marginean, C.; Marginean, C.O.; Iancu, M.; Melit, L.E.; Tripon, F.; Banescu, C. The FTO rs9939609 and LEPR rs1137101 mothers-newborns gene polymorphisms and maternal fat mass index effects on anthropometric characteristics in newborns: A cross-sectional study on mothers-newborns gene polymorphisms-The FTO-LEPR Study (STROBE-compliant article). Medicine 2016, 95, e5551. [Google Scholar] [CrossRef] [PubMed]
- Mǎrginean, C.O.; Mǎrginean, C.; Meliţ, L.E. New Insights Regarding Genetic Aspects of Childhood Obesity: A Minireview. Front. Pediatr. 2018, 6, 271. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Arata, N.; Tomotaki, A.; Yamamoto-Hanada, K.; Mezawa, H.; Konishi, M.; Ishitsuka, K.; Saito-Abe, M.; Sato, M.; Nishizato, M.; et al. Time course of metabolic status in pregnant women: The Japan Environment and Children’s Study. J. Diabetes Investig. 2020, 11, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Liu, X.H.; Chen, Y.; He, B.W.; Cheng, W.W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: A prospective longitudinal cohort study. BMJ Open 2016, 6, e013509. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.L.; Lin, J.; Yan, J.Y.; Lin, X.Q.; Han, Q.; Zhang, H.L. Prepregnancy body mass indexes are associated with perinatal outcomes in females with preeclampsia. Exp. Med. 2020, 20, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obs. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, K.W.; Tinius, R.A.; Cade, W.T.; Steele, E.M.; Cahill, A.G.; Parra, D.C. Relationships between consumption of ultra-processed foods, gestational weight gain and neonatal outcomes in a sample of US pregnant women. PeerJ 2017, 5, e4091. [Google Scholar] [CrossRef] [PubMed]
- Petherick, E.S.; Goran, M.I.; Wright, J. Relationship between artificially sweetened and sugar-sweetened cola beverage consumption during pregnancy and preterm delivery in a multi-ethnic cohort: Analysis of the Born in Bradford cohort study. Eur. J. Clin. Nutr. 2014, 68, 404–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, M.Y.; Baik, S.H.; Woo, J.T.; Kwon, Y.J.; Daily, J.W.; Park, Y.M.; Yang, J.H.; Kim, S.H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 2013, 67, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Clark, E.; Isler, C.; Strickland, D.; McMillan, A.G.; Fang, X.; Kuehn, D.; Ravisankar, S.; Strom, C.; May, L.E. Influence of aerobic exercise on maternal lipid levels and offspring morphometrics. Int. J. Obes. 2019, 43, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Koyac, U.; Jasper, E.A.; Smith, C.J.; Baer, R.J.; Bedell, B.; Donovan, B.M.; Weathers, N.; Zmrzljak, U.P.; Jeffiffe-Pawlowski, L.L.; Rozman, D.; et al. The Association of Polymorphisms in Circadian Clock and Lipid Metabolism Genes With 2nd Trimester Lipid Levels and Preterm Birth. Front. Genet. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2017, 91, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.J.; Ravi, S.; Loomba, R.; Gray, M.E. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 2020, 26, 383–400. [Google Scholar] [CrossRef]
- Soenen, S.; Westerterp-Plantenga, M.S. Proteins and satiety: Implications for weight management. Curr. Opin. Clin. Nutr. 2008, 11, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Hochstenbach-Waelen, A.; Westerterp-Plantenga, M.S. Efficacy of alpha-Lactalbumin and Milk Protein on Weight Loss and Body Composition During Energy Restriction. Obesity 2011, 19, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Adank, M.C.; Benschop, L.; Peterbroers, K.R.; Smak Gregoor, A.M.; Kors, A.W.; Mulder, M.T.; Schalekamp-Timmermans, S.; Roeters Van Lennep, J.E.; Steegers, E.A.P. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am. J. Obs. Gynecol. 2019, 221, 150.e1–150.e13. [Google Scholar] [CrossRef] [Green Version]
| Items | N | M ± SD/% |
|---|---|---|
| Pregnant women | ||
| Age (y) | 338 | 29.31 ± 3.99 |
| Height (cm) | 338 | 162.30 ± 5.33 |
| Weight before pregnancy(kg) | 338 | 58.31 ± 9.99 |
| Antenatal weight (kg) | 338 | 74.75 ± 10.54 |
| Newborns | ||
| Birth weight (kg) | 329 | 3.38 ± 0.41 |
| Birth length (cm) | 329 | 50.58 ± 1.57 |
| Six months | ||
| Weight (kg) | 315 | 8.75 ± 1.07 |
| Height (cm) | 315 | 69.34 ± 2.77 |
| One year old | ||
| Weight (kg) | 280 | 10.36 ± 1.15 |
| Height (cm) | 280 | 77.10 ± 2.70 |
| Items | N | Abnormal | Detection Rate |
|---|---|---|---|
| TC (mmol/L) | 295 | 21 | 7.1% |
| TG (mmol/L) | 295 | 69 | 23.4% |
| HDL-c (mmol/L) | 295 | 5 | 1.7% |
| LDL-c (mmol/L) | 295 | 19 | 6.4% |
| One of the above was abnormal | 295 | 93 | 31.5% |
| Self-report | 291 | 15 | 5.2% |
| Items | LGA (n = 71) | Appropriate for Gestational Age (n = 230) | Test Value | p Value |
|---|---|---|---|---|
| TC(mmol/L) | 6.04(5.39, 7.01) | 6.01(5.35, 6.86) | 0.166 | 0.868 |
| TG(mmol/L) | 3.90(2.94, 4.68) | 3.26(2.69, 4.07) | 2.668 | 0.008 |
| HDL(mmol/L) | 1.87(1.66, 2.11) | 1.90(1.65, 2.24) | 0.587 | 0.558 |
| LDL(mmol/L) | 3.07(2.42, 3.72) | 3.13(2.52, 3.95) | 0.933 | 0.351 |
| TC/HDL | 3.19(2.79, 3.57) | 3.07(2.77, 3.59) | 0.493 | 0.622 |
| TG/HDL | 2.08(1.59, 2.68) | 1.74(1.35, 2.20) | 2.746 | 0.006 |
| LDL/HDL | 1.65(1.23, 1.94) | 1.62(1.33, 2.05) | 0.686 | 0.493 |
| Weight (six months, kg) | 9.00(8.50, 9.60) | 8.50(8.00, 9.50) | 2.973 | 0.003 |
| Height (six months, cm) | 70.00(69.00, 72.00) | 69.00(67.50, 70.50) | 3.743 | <0.001 |
| Weight (one year old, kg) | 10.50(10.00, 11.50) | 10.00(9.50, 11.00) | 3.128 | 0.002 |
| Height (one year old, cm) | 78.50(76.00, 80.00) | 76.50(75.00, 78.50) | 3.739 | <0.001 |
| Items | Weight (Six Months, kg) | Height (Six Months, cm) | Weight (One Year Old, kg) | Height (One Year Old, cm) |
|---|---|---|---|---|
| rs (n = 229) | rs (n = 229) | rs (n = 229) | rs (n = 229) | |
| TC (mmol/L) | 0.003 | 0.008 | −0.015 | −0.025 |
| p value | 0.966 | 0.901 | 0.815 | 0.701 |
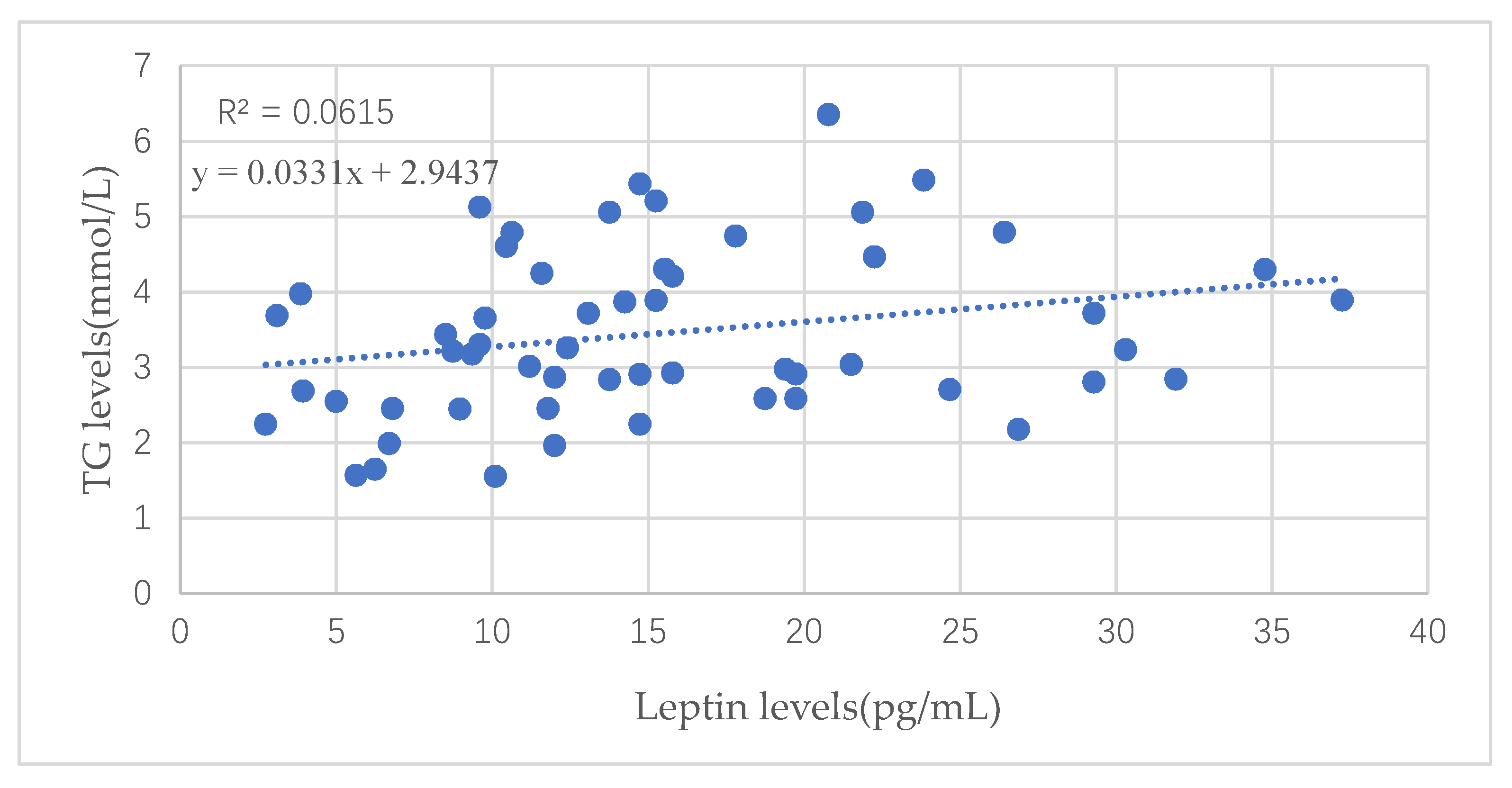
| TG (mmol/L) | 0.073 | 0.014 | 0.018 | 0.001 |
| p value | 0.272 | 0.835 | 0.780 | 0.990 |
| HDL (mmol/L) | −0.157 | −0.144 | −0.053 | −0.023 |
| p value | 0.017 | 0.029 | 0.421 | 0.733 |
| LDL (mmol/L) | 0.013 | 0.038 | 0.006 | 0.005 |
| p value | 0.845 | 0.571 | 0.926 | 0.942 |
| TC/HDL | 0.100 | 0.102 | 0.017 | −0.005 |
| p value | 0.131 | 0.123 | 0.799 | 0.937 |
| TG/HDL | 0.077 | 0.025 | 0.010 | −0.004 |
| p value | 0.247 | 0.704 | 0.884 | 0.957 |
| LDL/HDL | 0.092 | 0.124 | 0.041 | 0.031 |
| p value | 0.162 | 0.061 | 0.537 | 0.644 |
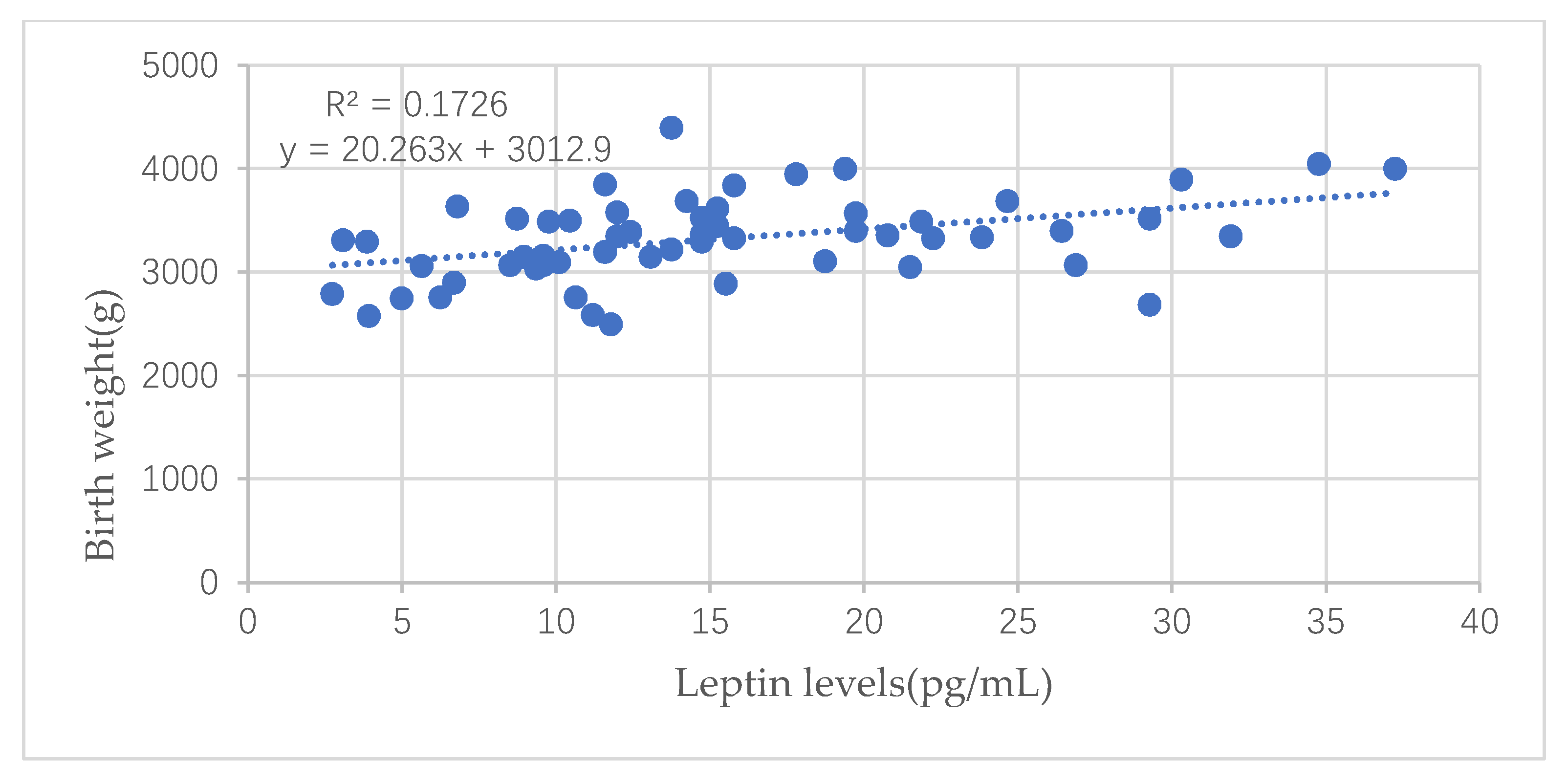
| Birth weight (kg) | 0.322 | 0.344 | 0.290 | 0.316 |
| p value(n = 251) | <0.001 | <0.001 | <0.001 | <0.001 |
| Items | Control Group (n = 202) | Case Group (n = 93) | Test Value | p Value | |
|---|---|---|---|---|---|
| Age | ≤24 | 13(6.4) | 8(8.6) | 2.388 | 0.496 |
| 25–29 | 109(54.0) | 43(46.2) | |||
| 30–35 | 59(29.2) | 34(36.6) | |||
| >35 | 18(8.9) | 7(7.5) | |||
| Education | high school or below | 44(21.8) | 25(26.9) | 0.820 | 0.664 |
| Undergraduate | 140(69.3) | 61(65.6) | |||
| Master degree or above | 13(6.4) | 6(6.5) | |||
| Family monthly income (yuan) | >3000 | 178(88.1) | 83(89.2) | 0.570 | 0.450 |
| ≤3000 | 12(5.9) | 8(8.6) | |||
| Family financial status | Well | 11(5.4) | 10(10.8) | 2.662 | 0.264 |
| Normal | 156(77.2) | 69(74.2) | |||
| Bad | 31(15.3) | 13(14.0) | |||
| BMI before pregnancy | Thin | 33(16.3) | 14(15.1) | 0.708 | 0.871 |
| Normal weight | 117(57.9) | 54(58.1) | |||
| Overweight | 34(16.8) | 16(17.2) | |||
| Obesity | 12(5.9) | 8(8.6) | |||
| Weight gain(kg) | Insufficient | 50(24.8) | 19(20.4) | 1.045 | 0.593 |
| Suitable | 79(39.1) | 37(39.8) | |||
| Excessive | 67(33.2) | 36(38.7) | |||
| Passive smoking before pregnancy | Yes | 93(46.0) | 39(41.9) | 0.425 | 0.514 |
| No | 105(52.0) | 52(55.9) | |||
| Passive smoking after pregnancy | Yes | 66(32.7) | 26(28.0) | 0.788 | 0.375 |
| No | 127(62.9) | 64(68.8) | |||
| Family history of diabetes | Yes | 25(12.4) | 14(15.1) | 0.340 | 0.560 |
| No | 174(86.1) | 79(84.9) | |||
| Family history of hypertension | Yes | 26(12.9) | 15(16.1) | 0.470 | 0.493 |
| No | 172(85.1) | 78(83.9) | |||
| Mood during pregnancy | Well | 78(38.6) | 32(34.4) | 0.417 | 0.812 |
| Better | 92(45.5) | 45(48.4) | |||
| Normal | 30(14.9) | 13(14.0) |
| Items | Control Group (n = 202) | Case Group (n = 93) | Test Value | p Value | |
|---|---|---|---|---|---|
| Staple food (rice/steamed bun/noodles, etc.) | ≥1 time/day | 159(78.7) | 73(78.5) | 0.020 | 0.887 |
| <1 time/day | 41(20.3) | 18(19.4) | |||
| Coarse grain | ≥1 time/day | 25(12.4) | 15(16.1) | 0.749 | 0.387 |
| <1 time/day | 172(85.1) | 76(81.7) | |||
| Vegetables | ≥1 time/day | 166(82.2) | 81(87.1) | 0.523 | 0.469 |
| <1 time/day | 32(16.3) | 12(12.9) | |||
| Fruits | ≥1 time/day | 173(85.6) | 84(90.3) | 0.393 | 0.531 |
| <1 time/day | 24(11.9) | 9(9.7) | |||
| Lean meat (livestock, poultry) | ≥1 time/day | 91(45.0) | 40(43.0) | 0.189 | 0.664 |
| <1 time/day | 108(53.5) | 53(57.0) | |||
| Eggs | ≥1 time/day | 102(50.5) | 42(45.2) | 1.022 | 0.312 |
| <1 time/day | 96(47.5) | 51(54.8) | |||
| Soy products | ≥1 time/day | 30(14.9) | 15(16.1) | 0.074 | 0.785 |
| <1 time/day | 167(82.7) | 76(81.7) | |||
| Milk products | ≥1 time/day | 102(50.5) | 51(54.8) | 0.513 | 0.474 |
| <1 time/day | 96(47.5) | 40(43.0) | |||
| Seafoods | ≤1 time/week | 136(67.3) | 68(73.1) | 0.748 | 0.688 |
| 2–3 times/week | 42(20.8) | 17(18.3) | |||
| ≥4 times/week | 19(9.4) | 7(7.5) | |||
| Liver | ≤1 time/week | 161(79.7) | 81(87.1) | 1.570 | 0.456 |
| 2–3 times/week | 24(11.9) | 9(9.7) | |||
| ≥4 times/week | 12(5.9) | 3(3.2) |
| Items | Control Group (n = 202) | Case Group (n = 93) | Test Value | p Value | |
|---|---|---|---|---|---|
| Sausages and cooked meat products | barely | 158(78.2) | 70(75.3) | 0.689 | 0.708 |
| occasionally | 31(15.3) | 18(19.4) | |||
| regularly | 8(4.0) | 4(4.3) | |||
| Canned food (pork, beef, fish, mutton, etc.) | barely | 196(97.0) | 91(97.8) | * | 0.554 |
| occasionally | 3(1.5) | 0(0.0) | |||
| Barbecue | barely | 194(96.0) | 90(96.8) | 2.314 * | 0.436 |
| occasionally | 2(1.0) | 3(3.2) | |||
| regularly | 1(0.5) | 0(0.0) | |||
| Fried food | barely | 188(93.1) | 86(92.5) | 0.231 * | 1.000 |
| occasionally | 8(4.0) | 4(4.3) | |||
| regularly | 4(2.0) | 2(2.2) | |||
| Nuts (walnuts, peanuts, melon seeds, etc.) | barely | 77(38.1) | 39(41.9) | 0.757 | 0.685 |
| occasionally | 50(24.8) | 19(20.4) | |||
| regularly | 72(35.6) | 34(36.6) | |||
| Sweets (ice cream, candies, biscuits, pastries, etc.) | barely | 132(65.3) | 55(59.1) | 3.031 | 0.220 |
| occasionally | 34(16.8) | 24(25.8) | |||
| regularly | 33(16.3) | 14(15.1) | |||
| Puffed food (shrimp crackers, potato chips, etc.) | barely | 182(90.1) | 82(88.2) | 1.327 | 0.515 |
| occasionally | 12(5.9) | 5(5.4) | |||
| regularly | 4(2.0) | 4(4.3) | |||
| Beverages (juice, cola, sprite, etc.) | barely | 173(85.6) | 85(91.4) | 3.202 | 0.202 |
| occasionally | 15(7.4) | 5(5.4) | |||
| regularly | 10(5.0) | 1(1.1) | |||
| Coffee | barely | 194(96.0) | 91(97.8) | 0.994 * | 1.000 |
| occasionally | 1(0.5) | 0(0.0) | |||
| regularly | 2(1.0) | 0(0.0) | |||
| Health products (calcium, zinc, iron, vitamins, etc.) | barely | 36(17.8) | 14(15.1) | 1.902 | 0.386 |
| occasionally | 15(7.4) | 4(4.3) | |||
| regularly | 144(71.3) | 75(80.6) | |||
| Vitamin D supplement | Yes | 158(78.2) | 66(71.0) | 2.082 | 0.149 |
| No | 41(20.3) | 26(28.0) |
| Items | Control Group (n = 202) | Case Group (n = 93) | Test Value | p Value | |
|---|---|---|---|---|---|
| Breakfast | regularly | 185(91.6) | 81(87.1) | 0.708 | 0.400 |
| occasionally | 16(7.9) | 10(10.8) | |||
| Often to eat food before going to bed | Yes | 72(35.6) | 34(36.6) | 0.023 | 0.879 |
| No | 130(64.4) | 59(63.4) | |||
| whether to eat on time | Yes | 175(86.6) | 80(86.0) | 0.020 | 0.887 |
| No | 27(13.4) | 13(14.0) | |||
| Meals per day | 1 time | 8(4.0) | 2(2.2) | 12.738 | 0.005 |
| 2 times | 137(67.8) | 48(51.6) | |||
| 3 times | 51(25.2) | 33(35.5) | |||
| ≥4 times | 6(3.0) | 10(10.8) | |||
| Like to eat lightly flavored food | Yes | 133(65.8) | 73(78.5) | 4.839 | 0.028 |
| No | 69(34.2) | 20(21.5) | |||
| Like to eat fried food | Yes | 24(11.9) | 10(10.8) | 0.080 | 0.778 |
| No | 178(88.1) | 83(89.2) | |||
| Like to eat salty food | Yes | 35(17.3) | 19(20.4) | 0.410 | 0.522 |
| No | 167(82.7) | 74(79.6) | |||
| Like to eat sour food | Yes | 50(24.8) | 23(24.7) | 0.000 | 0.997 |
| No | 152(75.2) | 70(75.3) | |||
| Like to eat sweet food | Yes | 67(33.2) | 28(30.1) | 0.216 | 0.642 |
| No | 135(66.8) | 64(68.8) | |||
| Like to eat spicy food | Yes | 53(26.2) | 15(16.1) | 3.587 | 0.058 |
| No | 148(73.3) | 77(82.8) | |||
| Drink boiled water regularly | Yes | 161(79.7) | 73(78.5) | 0.057 | 0.812 |
| No | 41(20.3) | 20(21.5) | |||
| Drink juice regularly | Yes | 7(3.5) | 5(5.4) | * | 0.527 |
| No | 195(96.5) | 88(94.6) | |||
| Drink sweet drinks regularly | Yes | 1(0.5) | 3(3.2) | * | 0.094 |
| No | 201(99.5) | 90(96.8) | |||
| Drink carbonated beverages regularly | Yes | 3(1.5) | 1(1.1) | * | 1.000 |
| No | 199(98.5) | 92(98.9) | |||
| Drink plastic barreled water regularly | Yes | 33(16.3) | 19(20.4) | 0.735 | 0.391 |
| No | 169(83.7) | 74(79.6) | |||
| Drink plastic bottled water regularly | Yes | 25(12.4) | 10(10.8) | 0.161 | 0.689 |
| No | 177(87.6) | 83(89.2) |
| Items | Control Group (n = 202) | Case Group (n = 93) | Test Value | p Value | |
|---|---|---|---|---|---|
| Elevator at home | Yes | 106(52.5) | 51(54.8) | 0.016 | 0.899 |
| No | 88(43.6) | 41(44.1) | |||
| Ways to go to work | By walking | 26(12.9) | 15(16.1) | 0.598 | 0.439 |
| By cars | 135(66.8) | 59(63.4) | |||
| TV time per day (h) | <1 | 78(38.6) | 33(35.5) | 2.282 | 0.319 |
| 1–2 | 58(28.7) | 21(22.6) | |||
| >2 | 64(31.7) | 37(39.8) | |||
| Phone time per day (h) | <1 | 8(4.0) | 2(2.2) | 1.877 | 0.598 |
| 1–2 | 32(15.5) | 15(16.1) | |||
| 2–3 | 47(23.3) | 17(18.3) | |||
| >3 | 114(56.4) | 59(63.4) | |||
| Exercise time per day(h) | <0.5 | 52(25.7) | 19(20.4) | 2.069 | 0.355 |
| 0.5–1 | 93(46.0) | 51(54.8) | |||
| >1 | 55(27.2) | 22(23.7) | |||
| Like to exercise before pregnancy | Yes | 56(27.7) | 24(25.8) | 0.055 | 0.814 |
| No | 144(71.3) | 66(71.0) | |||
| Like to exercise after pregnancy | Yes | 68(33.7) | 32(34.4) | 0.027 | 0.869 |
| No | 131(64.9) | 59(63.4) | |||
| Sleeping time(h) | <8 | 21(10.4) | 9(9.7) | 0.088 | 0.767 |
| ≥8 | 171(84.7) | 83(89.2) | |||
| Sleeping quality | Well | 88(43.6) | 35(37.6) | 1.218 | 0.749 |
| Better | 64(31.7) | 33(35.5) | |||
| Normal | 44(21.8) | 23(24.7) | |||
| Bad | 6(3.0) | 2(2.2) |
| Items | β | S.E. | Wald | p Value | OR(95%CI) |
|---|---|---|---|---|---|
| Meals per day | |||||
| 1 time | Ref. | ||||
| 2 times | 0.290 | 0.814 | 0.127 | 0.721 | 1.337(0.271–6.591) |
| 3 times | 0.882 | 0.828 | 1.136 | 0.287 | 2.416(0.477–12.238) |
| ≥4 times | 1.880 | 0.952 | 3.899 | 0.048 | 6.552(1.014–42.338) |
| Like to eat lightly flavored food | |||||
| No | Ref. | ||||
| Yes | 0.635 | 0.300 | 4.486 | 0.034 | 1.887(1.048–3.395) |
| Constant | −1.796 | 0.823 | 4.768 | 0.029 | 0.166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, X.; Wu, D.; Chen, Q.; Xiao, Z.; Wen, D.; Zhai, L.; Jia, L. Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study. Nutrients 2021, 13, 3398. https://doi.org/10.3390/nu13103398
Li C, Li X, Wu D, Chen Q, Xiao Z, Wen D, Zhai L, Jia L. Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study. Nutrients. 2021; 13(10):3398. https://doi.org/10.3390/nu13103398
Chicago/Turabian StyleLi, Chenyang, Xuening Li, Dan Wu, Qi Chen, Zhe Xiao, Deliang Wen, Lingling Zhai, and Lihong Jia. 2021. "Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study" Nutrients 13, no. 10: 3398. https://doi.org/10.3390/nu13103398
APA StyleLi, C., Li, X., Wu, D., Chen, Q., Xiao, Z., Wen, D., Zhai, L., & Jia, L. (2021). Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study. Nutrients, 13(10), 3398. https://doi.org/10.3390/nu13103398





