Neuropeptide Y Reduces Nasal Epithelial T2R Bitter Taste Receptor–Stimulated Nitric Oxide Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sinonasal Epithelial Air–Liquid Interface (ALI) Cultures
2.3. Measurement of Ciliary Beat Frequency
2.4. Calcium and NO Imaging
2.5. Immunofluorescence
2.6. Statistical Analyses
3. Results
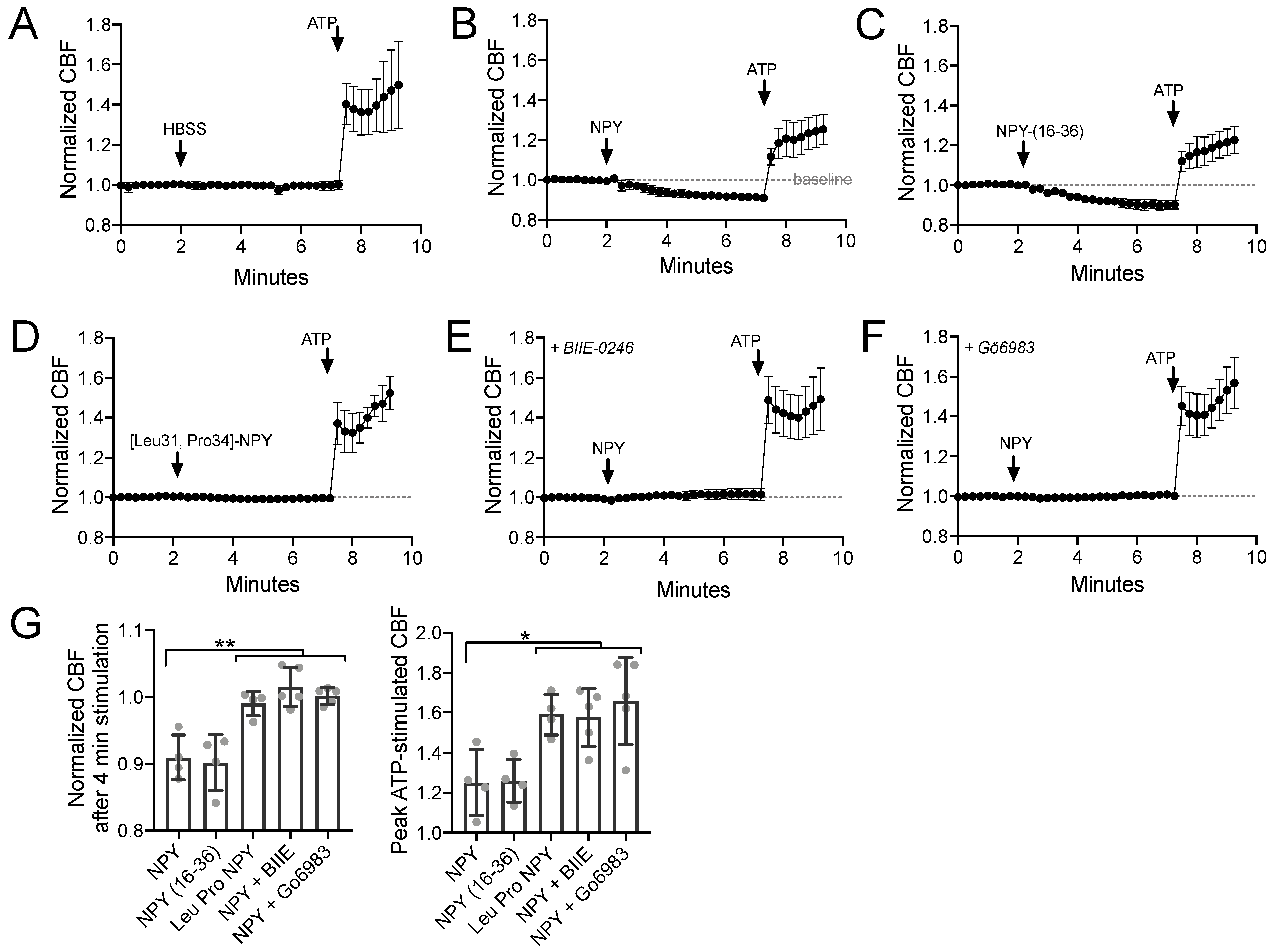
3.1. NPY Reduces CBF through NPY2R Activation of PKC
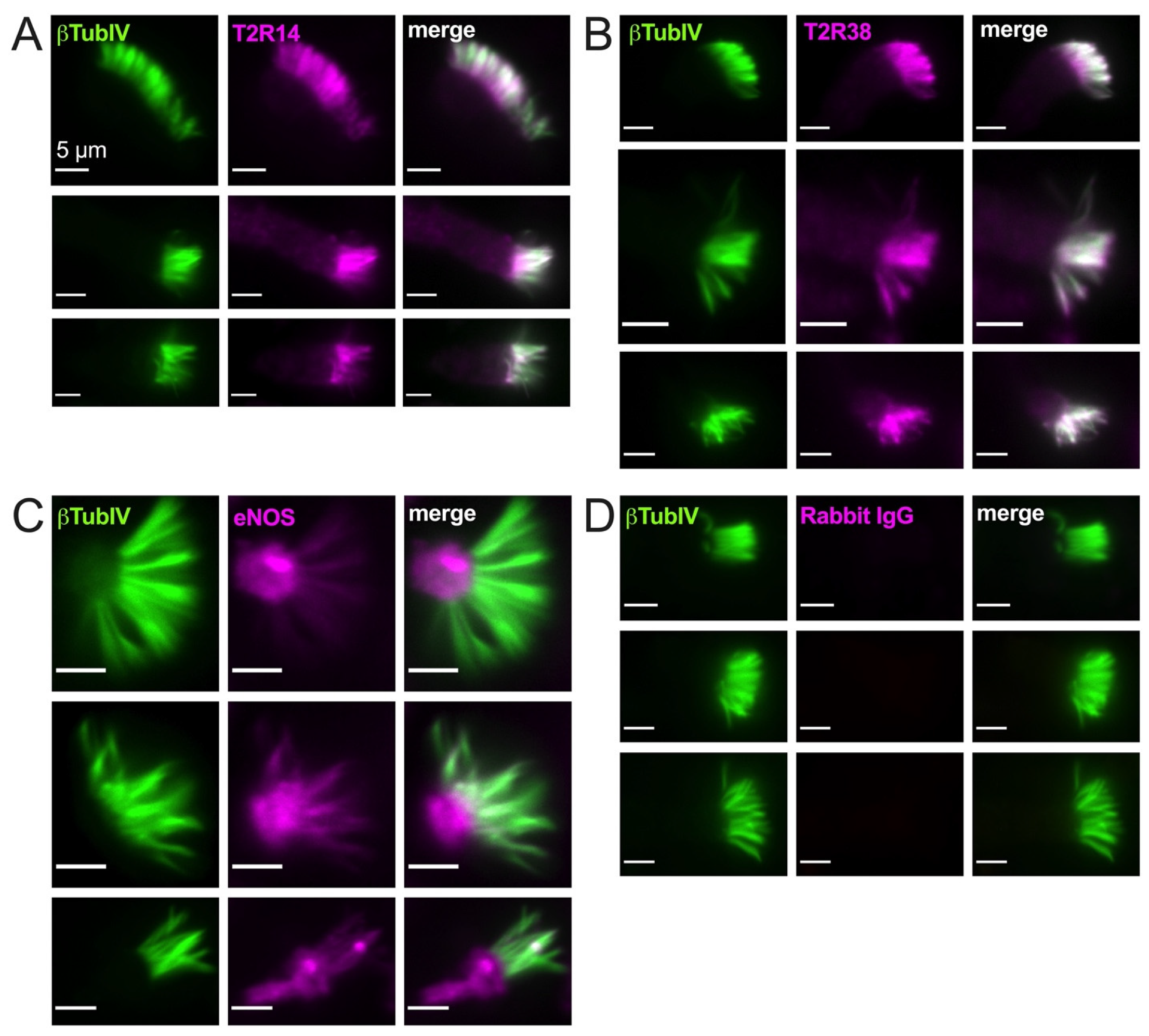
3.2. T2R38, T2R14, and eNOS Have Different Patterns of Cilia Localization
3.3. NPY Attenuates CBF Response to T2R14 Agonists via NPY2R Receptors
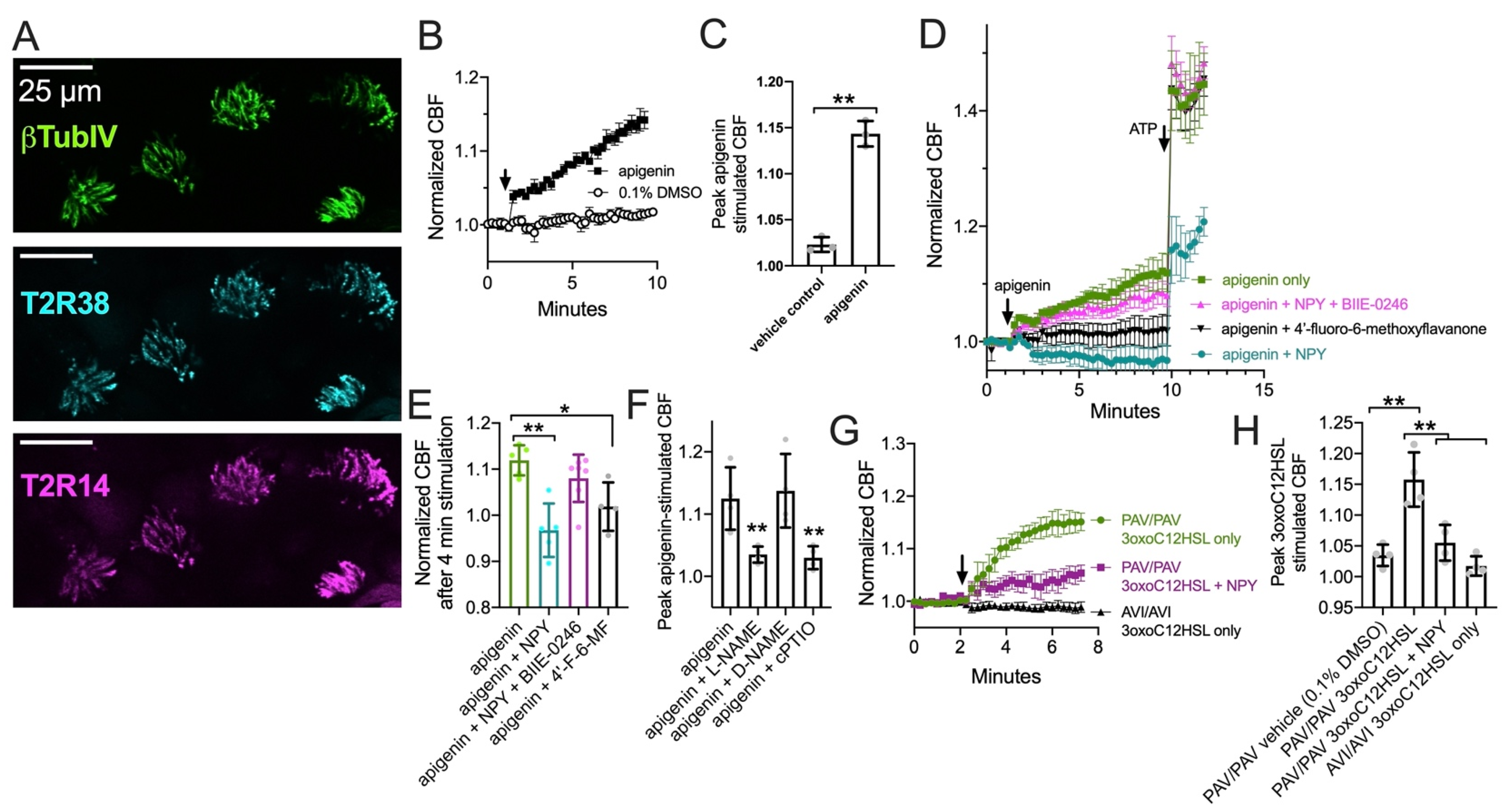
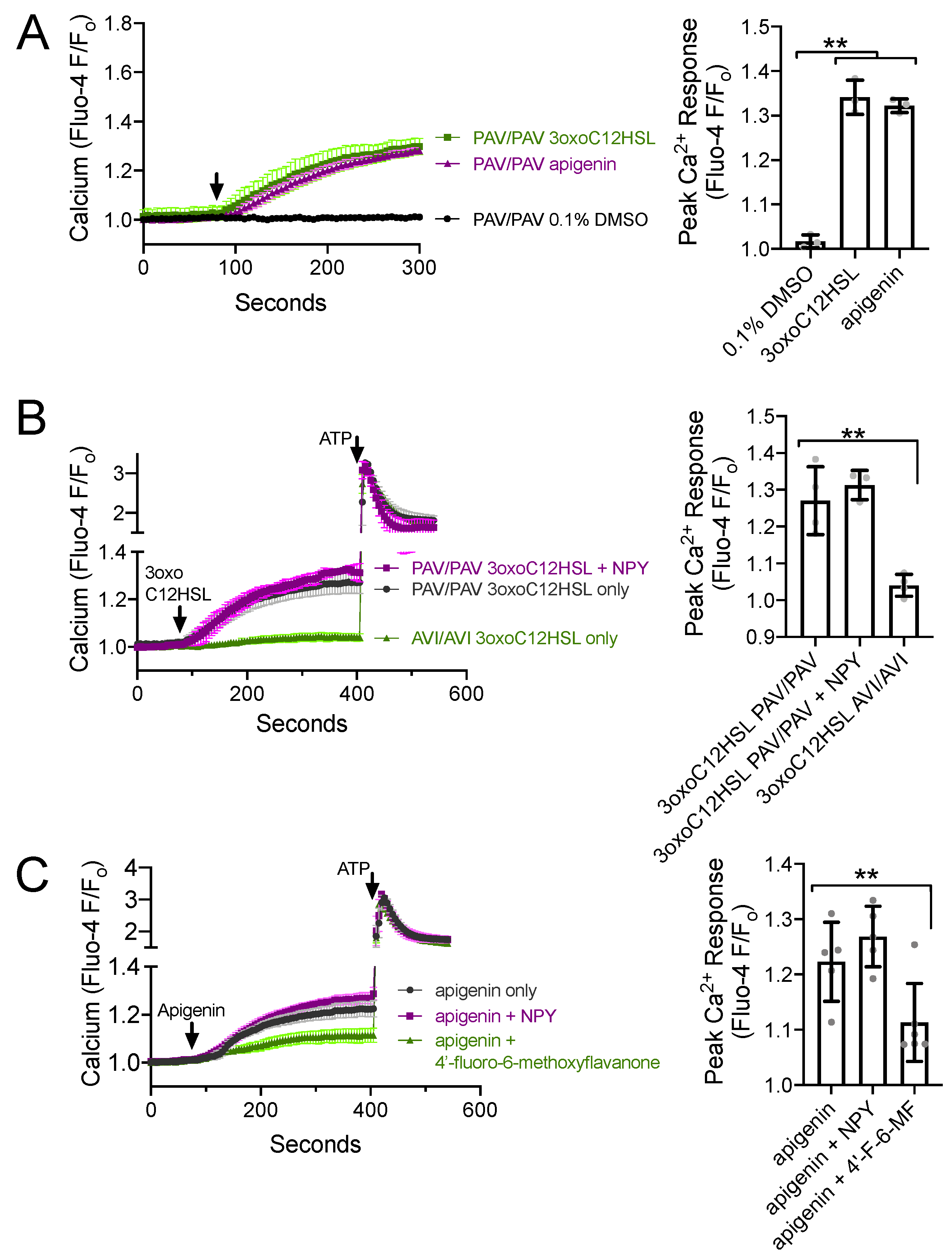
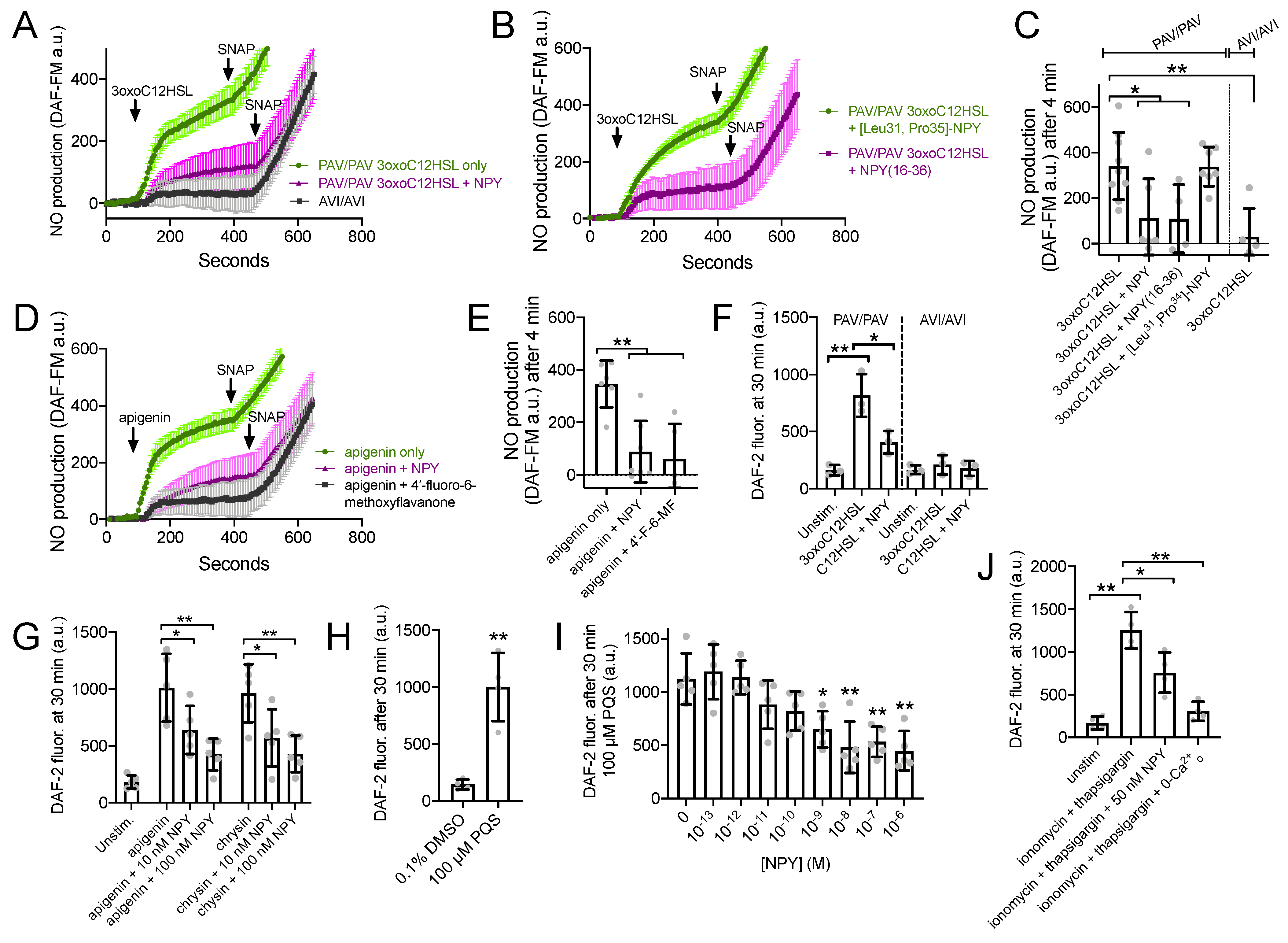
3.4. NPY Does Not Alter T2R-Induced Calcium Elevation but Does Reduce T2R-Stimulated NO Production
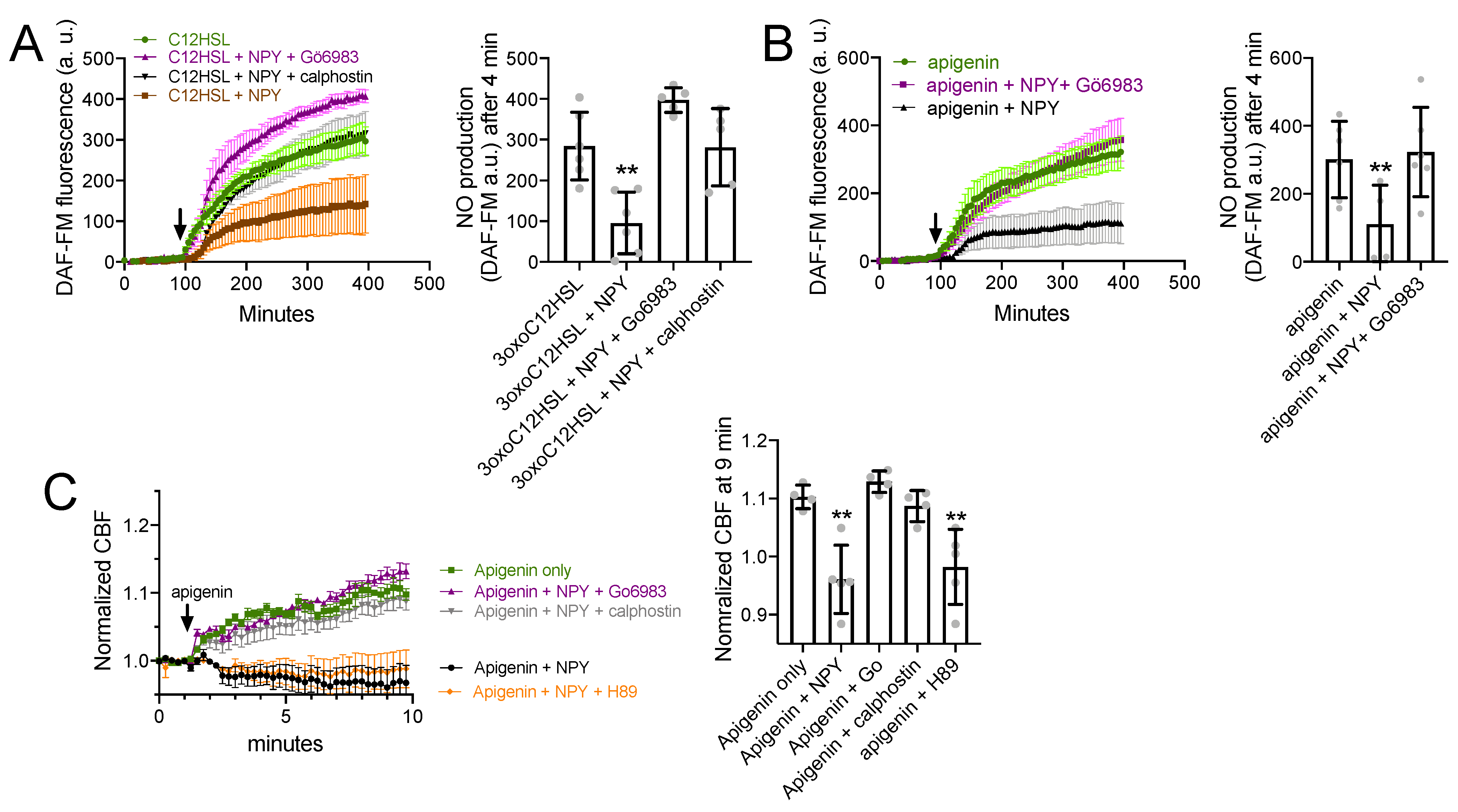
3.5. NPY Effects on T2R-Mediated NO and CBF Responses Are Dependent on PKC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, D.; Prince, A. Innate Immunity in the Respiratory Epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.A. Sinonasal Mucociliary Clearance in Health and Disease. Ann. Otol. Rhinol. Laryngol. 2006, 196, 20–26. [Google Scholar] [CrossRef]
- Waterer, G.W. Airway Defense Mechanisms. Clin. Chest Med. 2012, 33, 199–209. [Google Scholar] [CrossRef]
- Carey, R.M.; Lee, R.J. Taste Receptors in Upper Airway Innate Immunity. Nutrients 2019, 11, 2017. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, N. Incremental Health Care Utilization and Expenditures for Chronic Rhinosinusitis in the United States. Ann. Otol. Rhinol. Laryngol. 2011, 120, 423–427. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Grebner, J.; Martinson, N.G. Recurrent Acute Rhinosinusitis: Epidemiology and Health Care Cost Burden. Otolaryngol. Head Neck Surg. 2012, 146, 307–312. [Google Scholar] [CrossRef]
- Blackwell, D.L.; Collins, J.G.; Coles, R. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 1997. Vital Health Stat. 2002, 205, 1–109. [Google Scholar]
- Ly, N.; McCaig, L.F. National Hospital Ambulatory Medical Care Survey: 2000 Outpatient Department Summary. Adv. Data Vital Health Stat. 2002, 327, 1–27. [Google Scholar]
- Ray, N.F.; Baraniuk, J.N.; Thamer, M.; Rinehart, C.S.; Gergen, P.J.; Kaliner, M.; Josephs, S.; Pung, Y.H. Healthcare Expenditures for Sinusitis in 1996: Contributions of Asthma, Rhinitis, and Other Airway Disorders. J. Allergy Clin. Immunol. 1999, 103, 408–414. [Google Scholar] [CrossRef]
- Cherry, D.K.; Woodwell, D.A. National Ambulatory Medical Care Survey: 2000 summary. Adv. Data Vital Health Stat. 2002, 328, 1–32. [Google Scholar]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 23, 3. [Google Scholar]
- Bhattacharyya, N.; Kepnes, L.J. Assessment of Trends in Antimicrobial Resistance in Chronic Rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2008, 117, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Genoway, K.A.; Philpott, C.M.; Javer, A.R. Pathogen Yield and Antimicrobial Resistance Patterns of Chronic Rhinosinusitis Patients Presenting to a Tertiary Rhinology Centre. J. Otolaryngol. Head Neck Surg. 2011, 40, 232–237. [Google Scholar] [PubMed]
- Kingdom, T.T.; Swain, R.E., Jr. The Microbiology and Antimicrobial Resistance Patterns in Chronic Rhinosinusitis. Am. J. Otolaryngol. 2004, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Casey, G. Antibiotics and the Rise of Superbugs. Kai Tiaki Nurs. N. Z. 2012, 18, 20–24. [Google Scholar]
- Hawser, S. Surveillance Programmes and Antibiotic Resistance: Worldwide and Regional Monitoring of Antibiotic Resistance Trends. Handb. Exp. Pharmacol. 2012, 211, 31–43. [Google Scholar] [CrossRef]
- Foucault, C.; Brouqui, P. How to Fight Antimicrobial Resistance. FEMS Immunol. Med. Microbiol. 2007, 49, 173–183. [Google Scholar] [CrossRef]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Domin, M.A. Highly Virulent Pathogens-A Post Antibiotic Era? Br. J. Theatre Nurs. 1998, 8, 14–18. [Google Scholar] [CrossRef]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-Resistant Gram-Negative Bacteria: The Dawn of the Post-Antibiotic Era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 Taste Receptor Polymorphisms Underlie Susceptibility to Upper Respiratory Infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [Green Version]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; et al. Flavones Modulate Respiratory Epithelial Innate Immunity: Anti-Inflammatory Effects and Activation of the T2R14 Receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef] [Green Version]
- Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; Jiang, P.; Lee, R.J. Activation of Airway Epithelial Bitter Taste Receptors by Pseudomonas aeruginosa Quinolones Modulates Calcium, Cyclic-AMP, and Nitric Oxide Signaling. J. Biol. Chem. 2018, 293, 9824–9840. [Google Scholar] [CrossRef] [Green Version]
- Stout, S.L.; Wyatt, T.A.; Adams, J.J.; Sisson, J.H. Nitric Oxide-Dependent Cilia Regulatory Enzyme Localization in Bovine Bronchial Epithelial Cells. J. Histochem. Cytochem. 2007, 55, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Sisson, J.H.; Pavlik, J.A.; Wyatt, T.A. Alcohol Stimulates Ciliary Motility of Isolated Airway Axonemes through a Nitric Oxide, Cyclase, and Cyclic Nucleotide-Dependent Kinase Mechanism. Alcohol. Clin. Exp. Res. 2009, 33, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Sisson, J.H.; May, K.; Wyatt, T.A. Nitric Oxide-Dependent Ethanol Stimulation of Ciliary Motility Is Linked to cAMP-Dependent Protein Kinase (PKA) Activation in Bovine Bronchial Epithelium. Alcohol. Clin. Exp. Res. 1999, 23, 1528–1533. [Google Scholar] [CrossRef]
- Simet, S.M.; Pavlik, J.A.; Sisson, J.H. Proteomic Analysis of Bovine Axonemes Exposed to Acute Alcohol: Role of Endothelial Nitric Oxide Synthase and Heat Shock Protein 90 in Cilia Stimulation. Alcohol. Clin. Exp. Res. 2013, 37, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Chen, B.; Redding, K.M.; Margolskee, R.F.; Cohen, N.A. Mouse Nasal Epithelial Innate Immune Responses to Pseudomonas aeruginosa Quorum-Sensing Molecules Require Taste Signaling Components. Innate Immun. 2014, 20, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Cohen, N.A. The Emerging Role of the Bitter Taste Receptor T2R38 in Upper Respiratory Infection and Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2013, 27, 283–286. [Google Scholar] [CrossRef]
- Gaida, M.M.; Dapunt, U.; Hänsch, G.M. Sensing Developing Biofilms: The Bitter Receptor T2R38 on Myeloid Cells. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.T.T.; Herz, C.; Ruf, P.; Stetter, R.; Lamy, E. Human T2R38 Bitter Taste Receptor Expression in Resting and Activated Lymphocytes. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Maurer, S.; Wabnitz, G.H.; Kahle, N.A.; Stegmaier, S.; Prior, B.; Giese, T.; Gaida, M.M.; Samstag, Y.; Hänsch, G.M. Tasting Pseudomonas aeruginosa Biofilms: Human Neutrophils Express the Bitter Receptor T2R38 as Sensor for the Quorum Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine Lactone. Front. Immunol. 2015, 6, 369. [Google Scholar] [CrossRef] [Green Version]
- Sandau, M.M.; Goodman, J.R.; Thomas, A.; Rucker, J.B.; Rawson, N.E. A Functional Comparison of the Domestic Cat Bitter Receptors Tas2r38 and Tas2r43 with Their Human Orthologs. BMC Neurosci. 2015, 16, 33. [Google Scholar] [CrossRef] [Green Version]
- Lossow, K.; Hübner, S.; Roudnitzky, N.; Slack, J.P.; Pollastro, F.; Behrens, M.; Meyerhof, W. Comprehensive Analysis of Mouse Bitter Taste Receptors Reveals Different Molecular Receptive Ranges for Orthologous Receptors in Mice and Humans. J. Biol. Chem. 2016, 291, 15358–15377. [Google Scholar] [CrossRef] [Green Version]
- Freund, J.; Lee, R.J. Taste Receptors in the Upper Airway. World J. Otorhinolaryngol.-Head Neck Surg. 2018, 4, 67–76. [Google Scholar] [CrossRef]
- Carey, R.M.; Workman, A.D.; Yan, C.; Chen, B.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Lee, R.; Cohen, N.A. Sinonasal T2R-Mediated Nitric Oxide Production in Response to Bacillus Cereus. Am. J. Rhinol. Allergy 2017, 31, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Adappa, N.D.; Truesdale, C.M.; Ba, A.D.W.; Rn, L.D.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Cowart, B.J.; Cohen, N.A. Correlation of T2R38 Taste Phenotype and In Vitro Biofilm Formation from Nonpolypoid Chronic Rhinosinusitis Patients. Int. Forum Allergy Rhinol. 2016, 6, 783–791. [Google Scholar] [CrossRef]
- Rom, D.; Christensen, J.; Alvarado, R.; Sacks, R.; Harvey, R. The Impact of Bitter Taste Receptor Genetics on Culturable Bacteria in Chronic Rhinosinusitis. Rhinol. J. 2017, 55, 90–94. [Google Scholar] [CrossRef]
- Cantone, E.; Negri, R.; Roscetto, E.; Grassia, R.; Catania, M.R.; Capasso, P.; Maffei, M.; Soriano, A.A.; Leone, C.A.; Iengo, M.; et al. In Vivo Biofilm Formation, Gram-Negative Infections and TAS2R38 Polymorphisms in CRSw NP Patients. Laryngoscope 2018, 128, 339–345. [Google Scholar] [CrossRef]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Rn, L.D.; Lysenko, A.; Reed, D.; Scott, T.; Zhao, N.; Owens, D.; et al. The Bitter Taste Receptor T2R38 Is an Independent Risk Factor for Chronic Rhinosinusitis Requiring Sinus Surgery. Int. Forum Allergy Rhinol. 2013, 4, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adappa, N.D.; Bs, T.J.H.; Palmer, J.N.; Kennedy, D.W.; Rn, L.D.; Lysenko, A.; Reed, D.R.; Lee, R.J.; Cohen, N.A. Genetics of the Taste Receptor T2R38 Correlates with Chronic Rhinosinusitis Necessitating Surgical Intervention. Int. Forum Allergy Rhinol. 2013, 3, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Msc, L.M.E.; Filali-Mouhim, A.; Boisvert, P.; Boulet, L.-P.; Bossé, Y.; Desrosiers, M. Genetic Variations in Taste Receptors Are Associated with Chronic Rhinosinusitis: A Replication Study. Int. Forum Allergy Rhinol. 2014, 4, 200–206. [Google Scholar] [CrossRef]
- Dżaman, K.; Zagor, M.; Sarnowska, E.; Krzeski, A.; Kantor, I. The Correlation of TAS2R38 Gene Variants with Higher Risk for Chronic Rhinosinusitis in Polish Patients. Otolaryngol. Polska 2016, 70, 13–18. [Google Scholar] [CrossRef]
- Adappa, N.D.; Ba, D.F.; Palmer, J.N.; Kennedy, D.W.; Rn, L.D.; Bs, S.A.M.; Owens, D.; Bs, C.M.; Lysenko, A.; Lee, R.; et al. TAS2R38genotype Predicts Surgical Outcome in Nonpolypoid Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 6, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wine, J.J. Parasympathetic Control of Airway Submucosal Glands: Central Reflexes and the Airway Intrinsic Nervous System. Auton. Neurosci. 2007, 133, 35–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, J.S.; Anggard, A.; Hokfelt, T.; O’Hare, M.M.; Fahrenkrug, J.; Lundberg, J.M. Neuropeptide Y: Presence in Sympathet-Ic and Parasympathetic Innervation of the Nasal Mucosa. Cell Tissue Res. 1990, 259, 119–128. [Google Scholar] [CrossRef]
- Sheppard, M.N.; Polak, J.M.; Allen, J.M.; Bloom, S.R. Neuropeptide Tyrosine (NPY): A Newly Discovered Peptide Is Present in the Mammalian Respiratory Tract. Thorax 1984, 39, 326–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanez, P.; Springall, D.; Vignola, A.M.; Moradoghi-Hattvani, A.; Polak, J.M.; Godard, P.; Bousquet, J. Bronchial Mucosal Immunoreactivity of Sensory Neuropeptides in Severe Airway Diseases. Am. J. Respir. Crit. Care Med. 1998, 158, 985–990. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, Y.; Hirano, M. Precise Localization of VIP-, NPY-, and TH-Immunoreactivities of Cat Laryngeal Glands. Brain Res. Bull. 1995, 36, 219–224. [Google Scholar] [CrossRef]
- Bowden, J.J.; Gibbins, I.L. Vasoactive Intestinal Peptide and Neuropeptide Y Coexist in Non-Noradrenergic Sympathetic Neu-Rons to Guinea Pig Trachea. J. Auton. Nerv. Syst. 1992, 38, 1–19. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Kaliner, M.A. Neuropeptides and Nasal Secretion. J. Allergy Clin. Immunol. 1990, 86, 620–627. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Lundgren, J.; Okayama, M.; Mullol, J.; Merida, M.; Shelhamer, J.H.; Kaliner, M.A. Vasoactive Intestinal Peptide in Human Nasal Mucosa. J. Clin. Investig. 1990, 86, 825–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, R.D.; Shannon, W.A., Jr.; Said, S.I. Localization of VIP-Immunoreactive Nerves in Airways and Pulmonary Vessels of Dogs, Cats, and Human Subjects. Cell Tissue Res. 1981, 220, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, J.C.; Dolci, J.E. Neuropeptide Immunofluorescence in Human Nasal Mucosa: Assessment of the Technique for Vas-Oactive Intestinal Peptide (VIP). Braz. J. Otorhinolaryngol. 2005, 71, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Uddman, R.; Sundler, F. Neuropeptides in the Airways: A Review. Am. Rev. Respir. Dis. 1987, 136, 3–8. [Google Scholar] [CrossRef]
- Uddman, R.; Alumets, J.; Densert, O.; Hakanson, R.; Sundler, F. Occurrence and Distribution of VIP Nerves in the Nasal Mu-Cosa and Tracheobronchial Wall. Acta Otolaryngol. 1978, 86, 443–448. [Google Scholar] [CrossRef]
- Luts, A.; Uddman, R.; Alm, R.; Basterra, J.; Sundler, F. Peptide-Containing Nerve Fibers in Human Airways: Distribution and Coexistence Pattern. Int. Arch. Allergy Immunol. 1993, 101, 52–60. [Google Scholar] [CrossRef]
- Uddman, R.; Sundler, F.; Emson, P. Occurrence and Distribution of Neuropeptide-Y-Immunoreactive Nerves in the Respiratory Tract and Middle Ear. Cell Tissue Res. 1984, 237, 321–327. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Folkerts, G.; Peiser, C.; Chung, K.F.; Fischer, A. Neuropeptide Y (NPY). Pulm. Pharmacol. Ther. 2004, 17, 173–180. [Google Scholar] [CrossRef]
- Lin, J.K.; Wang, H.-W.; Su, W.Y. Noradrenergic Innervation of Nasal Polyps and Polypoid Mucosae. Auris Nasus Larynx 1996, 23, 121–126. [Google Scholar] [CrossRef]
- Fang, S.-Y.; Shen, C.-L.; Ohyama, M. Presence of Neuropeptides in Human Nasal Polyps. Acta Oto-Laryngol. 1994, 114, 324–328. [Google Scholar] [CrossRef]
- Heppt, W.; Dinh, Q.T.; Cryer, A.; Zweng, M.; Noga, O.; Peiser, C.; Melvan, M.; Witt, C.; Fischer, A.; Groneberg, D.A. Pheno-Typic Alteration of Neuropeptide-Containing Nerve Fibres in Seasonal Intermittent Allergic Rhinitis. Clin. Exp. Allergy 2004, 34, 1105–1110. [Google Scholar] [CrossRef]
- Fischer, A.; Wussow, A.; Cryer, A.; Schmeck, B.; Noga, O.; Zweng, M.; Peiser, C.; Dinh, Q.T.; Heppt, W.; Groneberg, D. Neuronal Plasticity in Persistent Perennial Allergic Rhinitis. J. Occup. Environ. Med. 2005, 47, 20–25. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Heppt, W.; Cryer, A.; Wussow, A.; Peiser, C.; Zweng, M.; Dinh, Q.T.; Witt, C.; Fischer, A. Toxic Rhinitis-Induced Changes of Human Nasal Mucosa Innervation. Toxicol. Pathol. 2003, 31, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, H.; Villiger, P.M.; von Kempis, J.; Lotz, M. Neuropeptide Y Is an Inducible Gene in the Human Immune System. J. Neuroimmunol. 1994, 51, 53–61. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hoshizaki, M.; Ichida, Y.; Lex, D.; Kuroda, E.; Ishii, K.J.; Magi, S.; Okada, M.; Takao, H.; Gandou, M.; et al. Pulmonary Phagocyte-Derived NPY Controls the Pathology of Severe Influenza Virus Infection. Nat. Microbiol. 2018, 4, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Morris, D.; Oatmen, K.E.; Wang, T.; Del Proposto, J.; Mergian, T.; Cho, K.W.; Lumeng, C.N. Neuropeptide Y Is Produced by Adipose Tissue Macrophages and Regulates Obesity-Induced Inflammation. PLoS ONE 2013, 8, e57929. [Google Scholar] [CrossRef]
- Shimizu, M.; Shigeri, Y.; Tatsu, Y.; Yoshikawa, S.; Yumoto, N. Enhancement of Antimicrobial Activity of Neuropeptide Y by N-Terminal Truncation. Antimicrob. Agents Chemother. 1998, 42, 2745–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.J.; Burnell, K.K.; Brogden, K.A. Antimicrobial Activity of Substance P and Neuropeptide Y against Laboratory Strains of Bacteria and Oral Microorganisms. J. Neuroimmunol. 2006, 177, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Nowak, J.; Lundy, F.T. Direct and Indirect Antimicrobial Activities of Neuropeptides and their Therapeutic Potential. Curr. Protein Pept. Sci. 2012, 13, 723–738. [Google Scholar] [CrossRef] [Green Version]
- Dimitrijević, M.; Stanojević, S. The Intriguing Mission of Neuropeptide Y in the Immune System. Amino Acids 2011, 45, 41–53. [Google Scholar] [CrossRef]
- Bedoui, S.; von Hörsten, S.; Gebhardt, T. A Role for Neuropeptide Y (NPY) in Phagocytosis: Implications for Innate and Adaptive Immunity. Peptides 2007, 28, 373–376. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Wahbi, A.-H.; Nordlind, K. Neuropeptides Modulate a Murine Monocyte/Macrophage Cell Line Capacity for Phagocytosis and Killing Ofleishmania Majorparasites. Immunopharmacol. Immunotoxicol. 2001, 23, 397–409. [Google Scholar] [CrossRef]
- McMahon, D.B.; Carey, R.M.; Kohanski, M.A.; Tong, C.C.; Papagiannopoulos, P.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Neuropeptide Regulation of Secretion and Inflammation in Human Airway Gland Serous Cells. Eur. Respir. J. 2020, 55, 1901386. [Google Scholar] [CrossRef]
- Widdicombe, J.H.; Wine, J.J. Airway Gland Structure and Function. Physiol. Rev. 2015, 95, 1241–1319. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Chen, B.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Cohen, N.A. Vasoactive Intestinal Peptide Regulates Sinonasal Mucociliary Clearance and Synergizes with Histamine in Stimulating Sinona-Sal Fluid Secretion. FASEB J. 2013, 27, 5094–5103. [Google Scholar] [CrossRef]
- Lee, R.; Workman, A.D.; Carey, R.M.; Chen, B.; Rosen, P.L.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Cohen, N.A. Fungal Aflatoxins Reduce Respiratory Mucosal Ciliary Function. Sci. Rep. 2016, 6, 33221. [Google Scholar] [CrossRef]
- Wong, L.B.; Park, C.L.; Yeates, D.B. Neuropeptide Y Inhibits Ciliary Beat Frequency in Human Ciliated Cells via nPKC, Independently of PKA. Am. J. Physiol. Content 1998, 275, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cervin, A.; Lindberg, S.; Mercke, U. The Effect of Neuropeptide Y on Mucociliary Activity in the Rabbit Maxillary Sinus. Acta Oto-Laryngol. 1991, 111, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Cervin, A. Neuropeptide Y 16–36 Inhibits Mucociliary Activity but Does Not Affect Blood Flow in the Rabbit Maxillary Sinus In Vivo. Regul. Pept. 1992, 39, 237–246. [Google Scholar] [CrossRef]
- Lindberg, S.; Hybbinette, J.-C.; Mercke, U. Effects of Neuropeptides on Mucociliary Activity. Ann. Otol. Rhinol. Laryngol. 1986, 95, 94–100. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Dimmeler, S.; Kemp, B.E.; Busse, R. Phosphorylation of Thr 495 Regulates Ca2+ /Calmodulin-Dependent Endothelial Nitric Oxide Synthase Activity. Circ. Res. 2001, 88, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Michell, B.J.; Chen, Z.-P.; Tiganis, T.; Stapleton, D.; Katsis, F.; Power, D.A.; Sim, A.T.; Kemp, B.E. Coordinated Control of Endothelial Nitric-oxide Synthase Phosphorylation by Protein Kinase C and the cAMP-dependent Protein Kinase. J. Biol. Chem. 2001, 276, 17625–17628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Kumar, S.; Yu, Y.; Aggarwal, S.; Gross, C.; Wang, Y.; Chakraborty, T.; Verin, A.D.; Catravas, J.D.; Lucas, R.; et al. PKC-Dependent Phosphorylation of eNOS at T495 Regulates eNOS Coupling and Endothelial Barrier Function in Response to G+ -Toxins. PLoS ONE 2014, 9, e99823. [Google Scholar] [CrossRef] [Green Version]
- Carey, R.M.; Freund, J.; Hariri, B.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Polarization of Protease-Activated Receptor 2 (PAR-2) Signaling Is Altered during Airway Epithelial Remodeling and Deciliation. J. Biol. Chem. 2020, 295, 6721–6740. [Google Scholar] [CrossRef] [Green Version]
- McMahon, D.B.; Workman, A.D.; Kohanski, M.A.; Carey, R.M.; Freund, J.R.; Hariri, B.M.; Chen, B.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; et al. Protease-Activated Receptor 2 Activates Airway Apical Membrane Chloride Permeability and Increases Ciliary Beating. FASEB J. 2017, 32, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Chem. Biol. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salathe, M. Regulation of Mammalian Ciliary Beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef]
- German, Z.; Chambliss, K.L.; Pace, M.C.; Arnet, U.A.; Lowenstein, C.J.; Shaul, P.W. Molecular Basis of Cell-Specific Endotheli-Al Nitric-Oxide Synthase Expression in Airway Epithelium. J. Biol. Chem. 2000, 275, 8183–8189. [Google Scholar] [CrossRef] [Green Version]
- Roland, W.S.U.; Gouka, R.J.; Gruppen, H.; Driesse, M.; Van Buren, L.; Smit, G.; Vincken, J.-P. 6-Methoxyflavanones as Bitter Taste Receptor Blockers for hTAS2R39. PLoS ONE 2014, 9, e94451. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Singh, N.; De Jesus, V.C.; Duan, K.; Chelikani, P. Characterization of the Binding Sites for Bacterial Acyl Homoserine Lactones (AHLs) on Human Bitter Taste Receptors (T2Rs). ACS Infect. Dis. 2018, 4, 1146–1156. [Google Scholar] [CrossRef]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.-K.; Drayna, D.; Meyerhof, W.; Bufe, B.; et al. The Molecular Basis of Individual Differences in Phenylthiocarbamide and Propylthiouracil Bitterness Perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerald, C.; Walker, M.W.; Vaysse, P.J.-J.; He, C.; Branchek, T.A.; Weinshank, R.L. Expression Cloning and Pharmacological Characterization of a Human Hippocampal Neuropeptide Y/Peptide YY Y2 Receptor Subtype. J. Biol. Chem. 1995, 270, 26758–26761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laugwitz, J.M.; Haeri, H.H.; Kaiser, A.; Krug, U.; Hinderberger, D.; Beck-Sickinger, A.G.; Schmidt, P. Probing the Y2 Receptor on Transmembrane, Intra- and Extra-Cellular Sites for EPR Measurements. Molecules 2020, 25, 4143. [Google Scholar] [CrossRef]
- Piccone, M.; Littzi, J.; Krupin, T.; Stone, R.A.; Davis, M.; Wax, M.B. Effects of Neuropeptide Y on the Isolated Rabbit Iris Dilator Muscle. Investig. Ophthalmol. Vis. Sci. 1988, 29, 330–332. [Google Scholar]
- Molosh, A.; Sajdyk, T.J.; Truitt, W.A.; Zhu, W.; Oxford, G.S.; Shekhar, A. NPY Y1 Receptors Differentially Modulate GABAA and NMDA Receptors via Divergent Signal-Transduction Pathways to Reduce Excitability of Amygdala Neurons. Neuropsychopharmacol. 2013, 38, 1352–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pack, R.J.; Richardson, P.S.; Smith, I.C.; Webb, S.R. The Functional Significance of the Sympathetic Innervation of Mucous Glands in the Bronchi of Man. J. Physiol. 1988, 403, 211–219. [Google Scholar] [CrossRef]
- Roland, W.S.; Vincken, J.-P.; Gouka, R.J.; Van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.-P. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.B.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2009, 35, 157–170. [Google Scholar] [CrossRef]
- Ba, A.D.W.; Carey, R.M.; Kohanski, M.A.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D.; Cohen, N.A. Relative Susceptibility of Airway Organisms to Antimicrobial Effects of Nitric Oxide. Int. Forum Allergy Rhinol. 2017, 7, 770–776. [Google Scholar] [CrossRef]
- Yu, K.; Mitchell, C.; Xing, Y.; Magliozzo, R.; Bloom, B.; Chan, J. Toxicity of Nitrogen Oxides and Related Oxidants on Mycobacteria: M. tuberculosis is Resistant to Peroxynitrite Anion. Tuber. Lung Dis. 1999, 79, 191–198. [Google Scholar] [CrossRef]
- Marcinkiewicz, J. Nitric Oxide and Antimicrobial Activity of Reactive Oxygen Intermediates. Immunopharmacology 1997, 37, 35–41. [Google Scholar] [CrossRef]
- Fang, F.C. Perspectives Series: Host/Pathogen Interactions. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, S.B.; Traeger, L.; Nguyen, H.K.; Rouillard, K.R.; Fischbach, A.; Zadek, F.; Ichinose, F.; Schoenfisch, M.H.; Carroll, R.W.; Bloch, D.B.; et al. Antimicrobial Effects of Nitric Oxide in Murine Models of Klebsiella Pneumonia. Redox Biol. 2020, 39, 101826. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hines, H.B.; Cheng, R.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric Oxide and Redox Mechanisms in the Immune Response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [Green Version]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.-J.; Mirazimi, A. Dual Effect of Nitric Oxide on SARS-CoV Replication: Viral RNA Production and Palmitoylation of the S Protein Are Affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Järhult, J.D.; Lennerstrand, J.; Lundkvist, A. Mitigation of the Replication of SARS-CoV-2 by Nitric Oxide In Vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef] [PubMed]
- Hedenstierna, G.; Chen, L.; Hedenstierna, M.; Lieberman, R.; Fine, D.H. Nitric Oxide Dosed in Short Bursts at High Concentrations May Protect against COVID 19. Nitric Oxide 2020, 103, 1–3. [Google Scholar] [CrossRef]
- Hariri, B.M.; Payne, S.J.; Chen, B.; Mansfield, C.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Niv, M.Y.; Lee, R.J. In vitro Effects of Anthocyanidins on Sinonasal Epithelial Nitric Oxide Production and Bacterial Physiology. Am. J. Rhinol. Allergy 2016, 30, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, C.S.; An, S.-Q.; Ryan, R.P. The Changing Face of Asthma and Its Relation with Microbes. Trends Microbiol. 2015, 23, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Lemanske, R.F.; Gern, J.E. Role of Viral Respiratory Infections in Asthma and Asthma Exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Busse, W.W.; Gern, J.E. Asthma and Infections: Is the Risk More Profound Than Previously Thought? J. Allergy Clin. Immunol. 2014, 134, 260–261. [Google Scholar] [CrossRef]
- Leppänen, T.; Tuominen, R.K.; Moilanen, E. Protein Kinase C and its Inhibitors in the Regulation of Inflammation: Inducible Nitric Oxide Synthase as an Example. Basic Clin. Pharmacol. Toxicol. 2013, 114, 37–43. [Google Scholar] [CrossRef]
- Oda, N.; Miyahara, N.; Taniguchi, A.; Morichika, D.; Senoo, S.; Fujii, U.; Itano, J.; Gion, Y.; Kiura, K.; Kanehiro, A.; et al. Requirement for Neuropeptide Y in the Development of Type 2 Responses and Allergen-Induced Airway Hyperresponsiveness and Inflammation. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makinde, T.O.; Steininger, R.; Agrawal, D.K. NPY and NPY Receptors in Airway Structural and Inflammatory Cells in Allergic Asthma. Exp. Mol. Pathol. 2012, 94, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Ho, R.C.-M. An Association between Neuropeptide Y Levels and Leukocyte Subsets in Stress-Exacerbated Asthmatic Mice. Neuropeptides 2015, 57, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Heppt, W.; Peiser, C.; Cryer, A.; Dinh, Q.T.; Zweng, M.; Witt, C.; Fischer, A.; Groneberg, D.A. Innervation of Human Nasal Mucosa in Environmentally Triggered Hyperreflectoric Rhinitis. J. Occup. Environ. Med. 2002, 44, 924–929. [Google Scholar] [CrossRef]
- Fang, S.Y.; Shen, C.L. Neuropeptidergic Innervation of Human Nasal Mucosa in Various Pathological Conditions. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1997, 21, 8–12. [Google Scholar]
- Gopallawa, I.; Freund, J.; Lee, R.J. Bitter Taste Receptors Stimulate Phagocytosis in Human Macrophages through Calcium, Nitric Oxide, and Cyclic-GMP Signaling. Experientia 2020, 78, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Foskett, J.K. Ca2+ Signaling and Fluid Secretion by Secretory Cells of the Airway Epithelium. Cell Calcium 2014, 55, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Uddman, R.; Håkanson, R.; Sundler, F. Immunoreactive Avian Pancreatic Polypeptide Occurs in Nerves of the Mammalian Nasal Mucosa and Eustachian Tube. ORL 1980, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carey, R.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Neuropeptide Y Reduces Nasal Epithelial T2R Bitter Taste Receptor–Stimulated Nitric Oxide Production. Nutrients 2021, 13, 3392. https://doi.org/10.3390/nu13103392
Carey RM, Adappa ND, Palmer JN, Lee RJ. Neuropeptide Y Reduces Nasal Epithelial T2R Bitter Taste Receptor–Stimulated Nitric Oxide Production. Nutrients. 2021; 13(10):3392. https://doi.org/10.3390/nu13103392
Chicago/Turabian StyleCarey, Ryan M., Nithin D. Adappa, James N. Palmer, and Robert J. Lee. 2021. "Neuropeptide Y Reduces Nasal Epithelial T2R Bitter Taste Receptor–Stimulated Nitric Oxide Production" Nutrients 13, no. 10: 3392. https://doi.org/10.3390/nu13103392
APA StyleCarey, R. M., Adappa, N. D., Palmer, J. N., & Lee, R. J. (2021). Neuropeptide Y Reduces Nasal Epithelial T2R Bitter Taste Receptor–Stimulated Nitric Oxide Production. Nutrients, 13(10), 3392. https://doi.org/10.3390/nu13103392





