Alcohol Use and the Risk of Communicable Diseases
Abstract
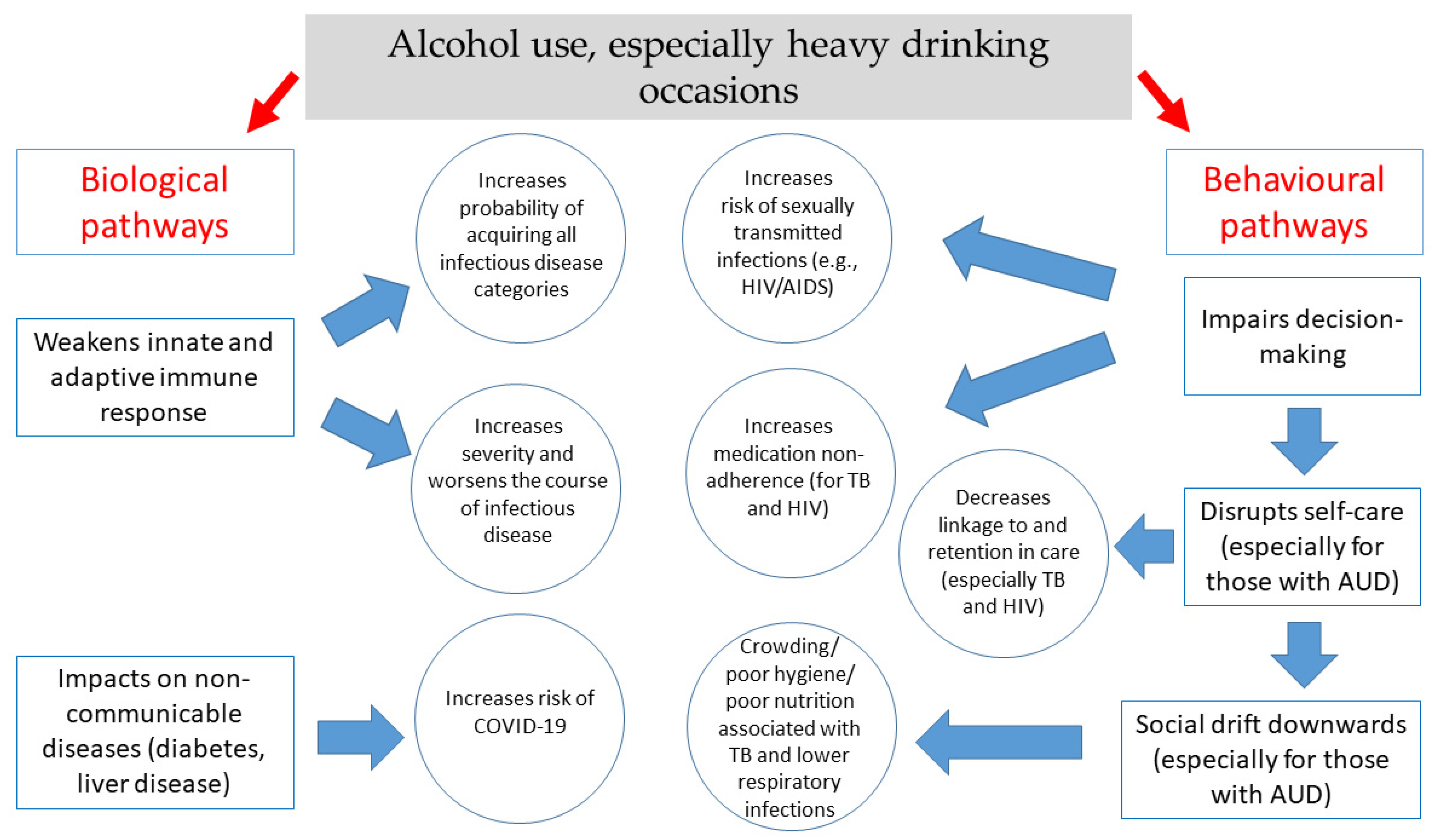
1. Introduction
2. Alcohol and the Risk of Human Immunodeficiency Virus (HIV) and Acquired Immune Deficiency Syndrome (AIDS)
2.1. Alcohol and HIV Acquisition/Transmission
2.1.1. Behavioral Mechanisms
2.1.2. Biological Mechanisms
2.2. Alcohol Use and HIV Disease Progression
2.2.1. Behavioral Mechanisms
2.2.2. Biological Mechanisms
2.3. Addressing the Intersection of Alcohol Use and HIV
3. Alcohol Use and the Risk of Tuberculosis
3.1. Behavioral Mechanisms
3.2. Biological Mechanisms
3.3. Addressing the Intersection of Alcohol Use and TB
4. Alcohol Use and the Risk of Lower Respiratory Infections (Pneumonia)
5. Alcohol Use and the Risk of COVID-19
5.1. Behavioral Pathways
5.2. Biological Pathways
- (1)
- COVID-19 travels from the upper respiratory tract (highest transmission risk) to the lower respiratory tract (highest disease risk), causing pneumonia;
- (2)
- COVID-19 initiates innate and adaptive immune responses that are often maladaptive, leading to ineffective pathogen eradication combined with inflammation that causes host tissue damage;
- (3)
- Damage is concentrated not at the alveolus (i.e., the interface of air–blood oxygen exchange), as is typical of pneumonia, but instead at epithelial cells (i.e., cells lining the lower respiratory tract) and endothelial cells (i.e., cells lining blood vessels);
- (4)
- Endothelial damage occurs not only in the lungs but also systematically, leading to vasculitis (i.e., damaged small blood vessels) and thrombosis (i.e., blood clots), potentially causing multi-organ failure.
5.3. Association with Alcohol Use or Heavy Alcohol Use/AUDs
6. Interventions for Preventing Transmission and Improving Treatment Outcomes of Alcohol-Attributable Diseases
6.1. Reducing the Incidence of Communicable Diseases
6.2. Improving Treatment Outcomes
6.3. Alcohol Control Measures
7. Discussion/Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rush, B. An Inquiry into the Effects of Ardent Spirits upon the Human Body and Mind: With an Account of the Means of Preventing, and of the Remedies for Curing Them, 6th ed.; Cornelius Davis: New York, NY, USA, 1811. (originally published 1785). [Google Scholar]
- Murray, C.J.L.; Lopez, A. Quantifying the burden of disease and injury attributable to ten major risk factors. In The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020; Murray, C.J.L., Lopez, A.D., Eds.; Harvard School of Public Health on Behalf of the World Health Organization and the World Bank: Boston, MA, USA, 1996; pp. 295–324. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Rehm, J.; Baliunas, D.; Borges, G.L.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Gmel, G.E., Sr.; Gmel, G.; Hasan, O.S.M.; Imtiaz, S.; Popova, S.; Probst, C.; Roerecke, M.; Room, R.; Samokhvalov, A.V.; et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017, 112, 968–1001. [Google Scholar] [CrossRef] [PubMed]
- Lönnroth, K.; Williams, B.G.; Stadlin, S.; Jaramillo, E.; Dye, C. Alcohol use as a risk factor for tuberculosis: A systematic review. BMC Public Health 2008, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Simbayi, L.C.; Kaufman, M.; Cain, D.; Jooste, S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: Systematic review of empirical findings. Prev. Sci. 2007, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.W.; Scott-Sheldon, L.A.; Carey, K.B.; Johnson, B.T.; Carey, M.P. Alcohol and sexual risk reduction interventions among people living in Russia: A systematic review and meta-analysis. AIDS Behav. 2014, 18, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Scott-Sheldon, L.A.; Walstrom, P.; Carey, K.B.; Johnson, B.T.; Carey, M.P. Alcohol use and sexual risk behaviors among individuals infected with HIV: A systematic review and meta-analysis 2012 to early 2013. Curr. HIV/AIDS Rep. 2013, 10, 314–323. [Google Scholar] [CrossRef]
- Okoro, U.J.; Carey, K.B.; Johnson, B.T.; Carey, M.P.; Scott-Sheldon, L.A.J. Alcohol consumption, risky sexual behaviors, and HIV in Nigeria: A meta-analytic review. Curr. Drug. Res. Rev. 2019, 11, 92–110. [Google Scholar] [CrossRef]
- Shuper, P.A.; Joharchi, N.; Irving, H.; Rehm, J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: Review and meta-analysis. AIDS Behav. 2009, 13, 1021–1036. [Google Scholar] [CrossRef]
- Przybyla, S.M.; Krawiec, G.; Godleski, S.A.; Crane, C.A. Meta-Analysis of alcohol and serodiscordant condomless sex among people living with HIV. Arch. Sex. Behav. 2018, 47, 1351–1366. [Google Scholar] [CrossRef]
- Fisher, J.C.; Bang, H.; Kapiga, S.H. The association between HIV infection and alcohol use: A systematic review and meta-analysis of African studies. Sex. Transm. Dis. 2007, 34, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, D.; Rehm, J.; Irving, H.; Shuper, P. Alcohol consumption and risk of incident human immunodeficiency virus infection: A meta-analysis. Int. J. Public Health 2010, 55, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Scott-Sheldon, L.A.; Carey, K.B.; Cunningham, K.; Johnson, B.T.; Carey, M.P. Alcohol use predicts sexual decision-making: A systematic review and meta-analysis of the experimental literature. AIDS Behav. 2016, 20 (Suppl. 1), S19–S39. [Google Scholar] [CrossRef]
- Rehm, J.; Probst, C.; Shield, K.D.; Shuper, P.A. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul. Health Metr. 2017, 15, 4. [Google Scholar] [CrossRef]
- Braithwaite, R.S.; McGinnis, K.A.; Conigliaro, J.; Maisto, S.A.; Crystal, S.; Day, N.; Cook, R.L.; Gordon, A.; Bridges, M.W.; Seiler, J.F.; et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol. Clin. Exp. Res. 2005, 29, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Rosof, E.; Mustanski, B. The temporal relationship between alcohol consumption and HIV-medication adherence: A multilevel model of direct and moderating effects. Health Psychol. 2008, 27, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Schensul, J.J.; Ha, T.; Schensul, S.; Sarna, A.; Bryant, K. Identifying the intersection of alcohol, adherence and sex in HIV positive men on ART treatment in India using an adapted timeline followback procedure. AIDS Behav. 2017, 21, 228–242. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral load and transmissibility of HIV infection: Undetectable equals untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Happel, K.I.; Nelson, S. Alcohol, immunosuppression, and the lung. Proc. Am. Thorac. Soc. 2005, 2, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Alcohol’s contribution to compromised immunity. Alcohol. Health Res. World 1997, 21, 30. [Google Scholar]
- Rehm, J.; Samokhvalov, A.V.; Neuman, M.G.; Room, R.; Parry, C.; Lönnroth, K.; Patra, J.; Poznyak, V.; Popova, S. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; Clark, D.B. Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sex. Transm. Dis. 2005, 32, 156–164. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet; UNAIDS: Geneva, Switzerland, 2021. [Google Scholar]
- UNAIDS. Seizing the Moment: Tackling Entrenched Inequalities to End Epidemics; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2020. [Google Scholar]
- Steele, C.M.; Josephs, R.A. Alcohol myopia: Its prized and dangerous effects. Am. Psychol. 1990, 45, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Shuper, P.A.; Neuman, M.; Kanteres, F.; Baliunas, D.; Joharchi, N.; Rehm, J. Causal considerations on alcohol and HIV/AIDS: A systematic review. Alcohol Alcohol. 2010, 45, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Shield, K.D.; Joharchi, N.; Shuper, P. Alcohol consumption and the intention to engage in unprotected sex: Systematic review and meta-analysis of experimental studies. Addiction 2012, 107, 51–59. [Google Scholar] [CrossRef]
- Shuper, P.A.; Joharchi, N.; Monti, P.M.; Loutfy, M.; Rehm, J. Acute alcohol consumption directly increases HIV transmission risk: A randomized controlled experiment. J. Acquir. Immune Defic. Syndr. 2017, 76, 493–500. [Google Scholar] [CrossRef]
- Shuper, P.A.; Joharchi, N.; Rehm, J. Protocol for a controlled experiment to identify the causal role of acute alcohol consumption in condomless sex among HIV-positive MSM: Study procedures, ethical considerations, and implications for HIV prevention. AIDS Behav. 2016, 20 (Suppl. 1), S173–S184. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapia, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, C.S.; Stoner, S.A.; Pantalone, D.W.; Simoni, J.M. Alcohol use and antiretroviral adherence: Review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2009, 52, 180–202. [Google Scholar] [CrossRef] [PubMed]
- Shuper, P.A.; Joharchi, N.; Bogoch, I.I.; Loutfy, M.; Crouzat, F.; El-Helou, P.; Knox, D.C.; Woodward, K.; Rehm, J. Alcohol consumption, substance use, and depression in relation to HIV Pre-Exposure Prophylaxis (PrEP) nonadherence among gay, bisexual, and other men-who-have-sex-with-men. BMC Public Health 2020, 20, 1782. [Google Scholar] [CrossRef] [PubMed]
- Haberer, J.E.; Baeten, J.M.; Campbell, J.; Wangisi, J.; Katabira, E.; Ronald, A.; Tumwesigye, E.; Psaros, C.; Safren, S.A.; Ware, N.C.; et al. Adherence to antiretroviral prophylaxis for HIV prevention: A substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013, 10, e1001511. [Google Scholar] [CrossRef] [PubMed]
- Mugo, P.M.; Sanders, E.J.; Mutua, G.; van der Elst, E.; Anzala, O.; Barin, B.; Bangsberg, D.R.; Priddy, F.H.; Haberer, J.E. Understanding adherence to daily and intermittent regimens of oral HIV pre-exposure prophylaxis among men who have sex with men in Kenya. AIDS Behav. 2015, 19, 794–801. [Google Scholar] [CrossRef] [PubMed]
- van der Elst, E.M.; Mbogua, J.; Operario, D.; Mutua, G.; Kuo, C.; Mugo, P.; Kanungi, J.; Singh, S.; Haberer, J.; Priddy, F.; et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: Qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav. 2013, 17, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.H.; Szabo, G.; Thomson, A.W. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009, 30, 13–22. [Google Scholar] [CrossRef]
- McClain, C.J.; Shedlofsky, S.; Barve, S.; Hill, D.B. Cytokines and alcoholic liver disease. Alcohol. Health Res. World 1997, 21, 317–320. [Google Scholar]
- Szabo, G.; Mandrekar, P. A recent perspective on alcohol, immunity, and host defense. Alcohol. Clin. Exp. Res. 2009, 33, 220–232. [Google Scholar] [CrossRef]
- Friedman, H.; Newton, C.; Klein, T.W. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 2003, 16, 209–219. [Google Scholar] [CrossRef]
- Friedman, H.; Pross, S.; Klein, T.W. Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 2006, 47, 330–342. [Google Scholar] [CrossRef]
- Goral, J.; Choudhry, M.A.; Kovacs, E.J. Acute ethanol exposure inhibits macrophage IL-6 production: Role of p38 and ERK1/2 MAPK. J. Leukoc. Biol. 2004, 75, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Goral, J.; Kovacs, E.J. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 2005, 174, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Pruett, S.B.; Schwab, C.; Zheng, Q.; Fan, R. Suppression of innate immunity by acute ethanol administration: A global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J. Immunol. 2004, 173, 2715–2724. [Google Scholar] [CrossRef]
- Brodie, C.; Domenico, J.; Gelfand, E.W. Ethanol inhibits early events in T-lymphocyte activation. Clin. Immunol. Immunopathol. 1994, 70, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, C.J.; Douglas, S.D.; Metzger, D.S.; O’Brien, C.P.; Li, Y.; Wang, Y.J.; Wang, X.; Ho, W.Z. Alcohol suppresses IL-2-induced CC chemokine production by natural killer cells. Alcohol. Clin. Exp. Res. 2005, 29, 1559–1567. [Google Scholar] [CrossRef]
- Shellito, J.E.; Olariu, R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol. Clin. Exp. Res. 1998, 22, 658–663. [Google Scholar] [CrossRef]
- Cook, R.T. Alcohol abuse, alcoholism, and damage to the immune system—A review. Alcohol. Clin. Exp. Res. 1998, 22, 1927–1942. [Google Scholar]
- Szabo, G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999, 34, 830–841. [Google Scholar] [CrossRef]
- Neuman, M.G. Cytokines—central factors in alcoholic liver disease. Alcohol Res. Health 2003, 27, 307–316. [Google Scholar]
- Mathew, T.A.; Yanov, S.A.; Mazitov, R.; Mishustin, S.P.; Strelis, A.K.; Yanova, G.V.; Golubchikova, V.T.; Taran, D.V.; Golubkov, A.; Shields, A.L.; et al. Integration of alcohol use disorders identification and management in the tuberculosis programme in Tomsk Oblast, Russia. Eur. J. Public Health 2009, 19, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Sha, K.; Esguerra, R.; Zakhari, S.; Winkler, R.E.; Hilzenrat, N.; Wyse, J.; Cooper, C.L.; Seth, D.; Gorrell, M.D.; et al. Inflammation and repair in viral hepatitis C. Dig. Dis. Sci. 2008, 53, 1468–1487. [Google Scholar] [CrossRef]
- Voiculescu, M.; Winkler, R.E.; Moscovici, M.; Neuman, M.G. Chemotherapies and targeted therapies in advanced hepatocellular carcinoma: From laboratory to clinic. J. Gastrointestin Liver Dis. 2008, 17, 315–322. [Google Scholar] [PubMed]
- Alcohol-Induced hepatic fibrosis: Mechanisms. Proceedings of a satellite symposium. Hilton Head Island, South Carolina, USA. June 1998. Alcohol. Clin. Exp. Res. 1999, 23, 901–954.
- Theall, K.P.; Amedee, A.; Clark, R.A.; Dumestre, J.; Kissinger, P. Alcohol consumption and HIV-1 vaginal RNA shedding among women. J. Stud. Alcohol. Drugs 2008, 69, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.S.; Hitti, J.; Bukusi, E.A.; Mwachari, C.; Muliro, A.; Nguti, R.; Gausman, R.; Jensen, S.; Patton, D.; Lockhart, D.; et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 2007, 21, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Rebbapragada, A.; Howe, K.; Wachihi, C.; Pettengell, C.; Sunderji, S.; Huibner, S.; Ball, T.B.; Plummer, F.A.; Jaoko, W.; Kaul, R. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J. Acquir. Immune Defic. Syndr. 2008, 49, 520–522. [Google Scholar] [CrossRef]
- Pandrea, I.; Happel, K.I.; Amedee, A.M.; Bagby, G.J.; Nelson, S. Alcohol’s role in HIV transmission and disease progression. Alcohol Res. Health 2010, 33, 203–218. [Google Scholar]
- Azar, M.M.; Springer, S.A.; Meyer, J.P.; Altice, F.L. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol. Depend. 2010, 112, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Vagenas, P.; Azar, M.M.; Copenhaver, M.M.; Springer, S.A.; Molina, P.E.; Altice, F.L. The impact of alcohol use and related disorders on the HIV continuum of care: A systematic review. Curr. HIV/AIDS Rep. 2015, 12, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.W.; Lundgren, L.; Umez-Eronini, A.; Ritter, G.A. Alcohol use and HIV testing in a national sample of women. AIDS Behav. 2016, 20 (Suppl. 1), S84–S96. [Google Scholar] [CrossRef]
- Maughan-Brown, B.; Harrison, A.; Galarraga, O.; Kuo, C.; Smith, P.; Bekker, L.G.; Lurie, M.N. Factors affecting linkage to HIV care and ART initiation following referral for ART by a mobile health clinic in South Africa: Evidence from a multimethod study. J. Behav. Med. 2019, 42, 883–897. [Google Scholar] [CrossRef]
- Monroe, A.K.; Lau, B.; Mugavero, M.J.; Mathews, W.C.; Mayer, K.H.; Napravnik, S.; Hutton, H.E.; Kim, H.S.; Jabour, S.; Moore, R.D.; et al. Heavy alcohol use is associated with worse retention in HIV care. J. Acquir. Immune Defic. Syndr. 2016, 73, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; Zhou, Z.; Kelso-Chichetto, N.E.; Janelle, J.; Morano, J.P.; Somboonwit, C. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: An observational study. Addict. Sci. Clin. Pract. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fatch, R.; Bellows, B.; Bagenda, F.; Mulogo, E.; Weiser, S.; Hahn, J.A. Alcohol consumption as a barrier to prior HIV testing in a population-based study in rural Uganda. AIDS Behav. 2013, 17, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.; Banas, E.; Kalichman, M.; Mathews, C. Stigmatisation of alcohol use among people receiving antiretroviral therapy for HIV infection, Cape Town, South Africa. Glob. Public Health 2020, 15, 1040–1049. [Google Scholar] [CrossRef]
- Britton, M.K.; Porges, E.C.; Bryant, V.; Cohen, R.A. Neuroimaging and Cognitive Evidence for Combined HIV-Alcohol Effects on the Central Nervous System: A Review. Alcohol. Clin. Exp. Res. 2021, 45, 290–306. [Google Scholar] [CrossRef]
- Heinz, A.J.; Fogler, K.A.; Newcomb, M.E.; Trafton, J.A.; Bonn-Miller, M.O. Problematic alcohol use among individuals with HIV: Relations with everyday memory functioning and HIV symptom severity. AIDS Behav. 2014, 18, 1302–1314. [Google Scholar] [CrossRef]
- El-Krab, R.; Kalichman, S.C. Alcohol-antiretroviral therapy interactive toxicity beliefs and intentional medication nonadherence: Review of research with implications for interventions. AIDS Behav. 2021, 25, 1–14. [Google Scholar] [CrossRef]
- Bagasra, O.; Kajdacsy-Balla, A.; Lischner, H.W.; Pomerantz, R.J. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J. Infect. Dis. 1993, 167, 789–797. [Google Scholar] [CrossRef]
- Bagasra, O.; Bachman, S.E.; Jew, L.; Tawadros, R.; Cater, J.; Boden, G.; Ryan, I.; Pomerantz, R.J. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: Potential immunopathogenic mechanisms. J. Infect. Dis. 1996, 173, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zha, J.; Gowans, R.E.; Camargo, P.; Nishitani, J.; McQuirter, J.L.; Cole, S.W.; Zack, J.A.; Liu, X. Alcohol enhances HIV type 1 infection in normal human oral keratinocytes by up-regulating cell-surface CXCR4 coreceptor. AIDS Res. Hum. Retrovir. 2004, 20, 513–519. [Google Scholar] [CrossRef]
- Liu, X.; Zha, J.; Nishitani, J.; Chen, H.; Zack, J.A. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology 2003, 307, 37–44. [Google Scholar] [CrossRef][Green Version]
- Crum, R.M.; Galai, N.; Cohn, S.; Celentano, D.D.; Vlahov, D. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: A prospective analysis. Alcohol. Clin. Exp. Res. 1996, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Rafie, C.; Lai, S.; Sales, S.; Page, J.B.; Campa, A. Alcohol use accelerates HIV disease progression. AIDS Res. Hum. Retrovir. 2010, 26, 511–518. [Google Scholar] [CrossRef]
- Samet, J.H.; Cheng, D.M.; Libman, H.; Nunes, D.P.; Alperen, J.K.; Saitz, R. Alcohol consumption and HIV disease progression. J. Acquir. Immune Defic. Syndr. 2007, 46, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, S.B.; Fatch, R.; Patts, G.; Winter, M.; Lloyd-Travaglini, C.; Emenyonu, N.; Muyindike, W.; Kekibiina, A.; Blokhina, E.; Gnatienko, N. Alcohol types and HIV disease progression among HIV-infected drinkers not yet on antiretroviral therapy in Russia and Uganda. AIDS Behav. 2017, 21, 204–215. [Google Scholar] [CrossRef]
- Miguez, M.J.; Shor-Posner, G.; Morales, G.; Rodriguez, A.; Burbano, X. HIV treatment in drug abusers: Impact of alcohol use. Addict. Biol. 2003, 8, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.H.; Horton, N.J.; Traphagen, E.T.; Lyon, S.M.; Freedberg, K.A. Alcohol consumption and HIV disease progression: Are they related? Alcohol. Clin. Exp. Res. 2003, 27, 862–867. [Google Scholar] [CrossRef]
- McDowell, J.A.; Chittick, G.E.; Stevens, C.P.; Edwards, K.D.; Stein, D.S. Pharmacokinetic interaction of abacavir (1592U89) and ethanol in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 2000, 44, 1686–1690. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J. Biol. Chem. 1970, 245, 2505–2512. [Google Scholar] [CrossRef]
- Neuman, M.G.; Monteiro, M.; Rehm, J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Subst. Use Misuse 2006, 41, 1395–1463. [Google Scholar] [CrossRef]
- Neuman, M.G.; Schneider, M.; Nanau, R.M.; Parry, C. HIV-antiretroviral therapy induced liver, gastrointestinal, and pancreatic injury. Int. J. Hepatol. 2012, 2012, 760706. [Google Scholar] [CrossRef]
- Hahn, J.A.; Samet, J.H. Alcohol and HIV disease progression: Weighing the evidence. Curr. HIV/AIDS Rep. 2010, 7, 226–233. [Google Scholar] [CrossRef]
- Scott-Sheldon, L.A.J.; Carey, K.B.; Johnson, B.T.; Carey, M.P. Behavioral interventions targeting alcohol use among people living with HIV/AIDS: A systematic review and meta-analysis. AIDS Behav. 2017, 21, 126–143. [Google Scholar] [CrossRef]
- Farhadian, N.; Moradi, S.; Zamanian, M.H.; Farnia, V.; Rezaeian, S.; Farhadian, M.; Shahlaei, M. Effectiveness of naltrexone treatment for alcohol use disorders in HIV: A systematic review. Subst. Abuse Treat. Prev. Policy 2020, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.L.; Taylor, S.L.; Elliott, M.N.; Ringel, J.S.; Kanouse, D.E.; Beckman, R. Off-premise alcohol sales policies, drinking, and sexual risk among people living with HIV. Am. J. Public Health 2010, 100, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.A.; Wu, S.Y.; Farley, T.A. Structural interventions to prevent HIV/sexually transmitted disease: Are they cost-effective for women in the southern United States? Sex. Transm. Dis. 2006, 33, S46–S49. [Google Scholar] [CrossRef] [PubMed]
- Shuper, P.A. The role of alcohol-related behavioral research in the design of HIV secondary prevention interventions in the era of antiretroviral therapy: Targeted research priorities moving forward. AIDS Behav. 2021, 25, 1–16. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Peltzer, K.; Davids, A.; Njuho, P. Alcohol use and problem drinking in South Africa: Findings from a national population-based survey. Afr. J. Psychiatry 2011, 14, 30–37. [Google Scholar] [CrossRef]
- Imtiaz, S.; Shield, K.D.; Roerecke, M.; Samokhvalov, A.V.; Lönnroth, K.; Rehm, J. Alcohol consumption as a risk factor for tuberculosis: Meta-analyses and burden of disease. Eur. Respir. J. 2017, 50, 1700216. [Google Scholar] [CrossRef]
- Francisco, J.; Oliveira, O.; Felgueiras, Ó.; Gaio, A.R.; Duarte, R. How much is too much alcohol in tuberculosis? Eur. Respir. J. 2017, 49, 1601468. [Google Scholar] [CrossRef] [PubMed]
- Cords, O.; Martinez, L.; Warren, J.L.; O’Marr, J.M.; Walter, K.S.; Cohen, T.; Zheng, J.; Ko, A.I.; Croda, J.; Andrews, J.R. Incidence and prevalence of tuberculosis in incarcerated populations: A systematic review and meta-analysis. Lancet Public Health 2021, 6, e300–e308. [Google Scholar] [CrossRef]
- Friedman, L.N.; Sullivan, G.M.; Bevilaqua, R.P.; Loscos, R. Tuberculosis screening in alcoholics and drug addicts. Am. Rev. Respir. Dis. 1987, 136, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Ragan, E.J.; Kleinman, M.B.; Sweigart, B.; Gnatienko, N.; Parry, C.D.; Horsburgh, C.R.; LaValley, M.P.; Myers, B.; Jacobson, K.R. The impact of alcohol use on tuberculosis treatment outcomes: A systematic review and meta-analysis. Int. J. Tuberc. Lung. Dis. 2020, 24, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.T.; Arroyo, L.H.; Alves, Y.M.; Alves, L.S.; Berra, T.Z.; Crispim, J.A.; Alves, J.D.; Ramos, D.A.C.; Alonso, J.B.; de Assis, I.S.; et al. Survival time among patients who were diagnosed with tuberculosis, the precocious deaths and associated factors in southern Brazil. Trop. Med. Health 2021, 49, 31. [Google Scholar] [CrossRef] [PubMed]
- Workie, M.G.; Aycheh, M.W.; Birhanu, M.Y.; Tsegaye, T.B. Treatment interruption among drug-susceptible pulmonary tuberculosis patients in Southern Ethiopia. Patient Prefer. Adherence 2021, 15, 1143–1151. [Google Scholar] [CrossRef]
- Arora, U.; Garg, P.; Agarwal, S.; Nischal, N.; Wig, N. Complexities in the treatment of coinfection with HIV, hepatitis B, hepatitis C, and tuberculosis. Lancet Infect. Dis. 2021, 21, e182. [Google Scholar] [CrossRef]
- Thomas, B.E.; Thiruvengadam, K.; Kadam, D.; Ovung, S.; Sivakumar, S.; Bala Yogendra Shivakumar, S.V.; Paradkar, M.; Gupte, N.; Suryavanshi, N. Smoking, alcohol use disorder and tuberculosis treatment outcomes: A dual co-morbidity burden that cannot be ignored. PLoS ONE 2019, 14, e0220507. [Google Scholar] [CrossRef]
- Kamarulzaman, A.; Reid, S.E.; Schwitters, A.; Wiessing, L.; El-Bassel, N.; Dolan, K.; Moazen, B.; Wirtz, A.L.; Verster, A.; Altice, F.L. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet 2016, 388, 1115–1126. [Google Scholar] [CrossRef]
- Zelnick, J.R.; Daftary, A.; Hwang, C.; Labar, A.S.; Boodhram, R.; Maharaj, B.; Wolf, A.K.; Mondal, S.; Amico, K.R.; Orrell, C.; et al. Electronic dose monitoring identifies a high-risk subpopulation in the treatment of drug-resistant tuberculosis and HIV. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Bouton, T.C.; Ragan, E.J.; White, L.F.; McIlleron, H.; Theron, D.; Parry, C.D.H.; Horsburgh, C.R.; Warren, R.M.; Jacobson, K.R. Impact of alcohol consumption on tuberculosis treatment outcomes: A prospective longitudinal cohort study protocol. BMC Infect. Dis. 2018, 18, 488. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, G.M.; Morojele, N.; Siddiqi, K.; Mdege, N.D.; Tumbo, J.; Omole, O.; Pitso, L.; Bachmann, M.O.; Ayo-Yusuf, O.A. Addressing tobacco smoking and drinking to improve TB treatment outcomes, in South Africa: A feasibility study of the ProLife program. Transl. Behav. Med. 2020, 10, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, A.S.; Louwagie, G.M.; Mdege, N.D.; Morojele, N.; Tumbo, J.; Omole, O.B.; Bachmann, M.O.; Kanaan, M.; Turner, A.; Parrott, S.; et al. Improving TB outcomes by modifying life-style behaviours through a brief motivational intervention followed by short text messages (ProLife): Study protocol for a randomised controlled trial. Trials 2019, 20, 457. [Google Scholar] [CrossRef]
- Myers, B.; Parry, C.D.H.; Morojele, N.K.; Nkosi, S.; Shuper, P.A.; Kekwaletswe, C.T.; Sorsdahl, K.R. “Moving forward with life”: Acceptability of a brief alcohol reduction intervention for people receiving antiretroviral therapy in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 5706. [Google Scholar] [CrossRef]
- Magidson, J.F.; Satinsky, E.N.; Luberto, C.M.; Myers, B.; Funes, C.J.; Vanderkruik, R.; Andersen, L.S. “Cooling of the mind”: Assessing the relevance of mindfulness training among people living with HIV using alcohol and other substances in South Africa. Soc. Sci. Med. 2020, 266, 113424. [Google Scholar] [CrossRef]
- Greenfield, S.F.; Shields, A.; Connery, H.S.; Livchits, V.; Yanov, S.A.; Lastimoso, C.S.; Strelis, A.K.; Mishustin, S.P.; Fitzmaurice, G.; Mathew, T.A.; et al. Integrated management of physician-delivered alcohol care for tuberculosis patients: Design and implementation. Alcohol. Clin. Exp. Res. 2010, 34, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; de Wit, Z.; Cirota, J.; Orrell, C.; Myers, B.; Decker, S.; Stein, D.J.; Sorsdahl, K.; Dawson, R. Brief psychotherapy administered by non-specialised health workers to address risky substance use in patients with multidrug-resistant tuberculosis: A feasibility and acceptability study. Pilot Feasibility Stud. 2021, 7, 28. [Google Scholar] [CrossRef]
- Peltzer, K.; Naidoo, P.; Louw, J.; Matseke, G.; Zuma, K.; Mchunu, G.; Tutshana, B.; Mabaso, M. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: Results from a cluster randomized controlled trial. BMC Public Health 2013, 13, 699. [Google Scholar] [CrossRef]
- Aiwale, A.S.; Patel, U.A.; Barvaliya, M.J.; Jha, P.R.; Tripathi, C. Isoniazid induced convulsions at therapeutic dose in an alcoholic and smoker patient. Curr. Drug Saf. 2015, 10, 94–95. [Google Scholar] [CrossRef]
- Cross, F.S.; Long, M.W.; Banner, A.S.; Snider, D.E., Jr. Rifampin-isoniazid therapy of alcoholic and nonalcoholic tuberculous patients in a U.S. Public Health Service Cooperative Therapy Trial. Am. Rev. Respir. Dis. 1980, 122, 349–353. [Google Scholar] [CrossRef]
- Karamanakos, P.N.; Pappas, P.; Boumba, V.; Vougiouklakis, T.; Marselos, M. The alcohol intolerance produced by isoniazid is not due to a disulfiram-like reaction despite aldehyde dehydrogenase inhibition. Pharmacology 2016, 98, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pettit, A.C.; Bethel, J.; Hirsch-Moverman, Y.; Colson, P.W.; Sterling, T.R. Female sex and discontinuation of isoniazid due to adverse effects during the treatment of latent tuberculosis. J. Infect. 2013, 67, 424–432. [Google Scholar] [CrossRef]
- Freiman, J.M.; Fatch, R.; Cheng, D.; Emenyonu, N.; Ngabirano, C.; Geadas, C.; Adong, J.; Muyindike, W.R.; Linas, B.P.; Jacobson, K.R.; et al. Prevalence of elevated liver transaminases and their relationship with alcohol use in people living with HIV on anti-retroviral therapy in Uganda. PLoS ONE 2021, 16, e0250368. [Google Scholar] [CrossRef] [PubMed]
- Freiman, J.M.; Jacobson, K.R.; Muyindike, W.R.; Horsburgh, C.R.; Ellner, J.J.; Hahn, J.A.; Linas, B.P. Isoniazid preventive therapy for people with HIV who are heavy alcohol drinkers in high TB/HIV-burden countries: A risk-benefit analysis. J. Acquir. Immune Defic. Syndr. 2018, 77, 405–412. [Google Scholar] [CrossRef]
- McClintock, A.H.; Eastment, M.; McKinney, C.M.; Pitney, C.L.; Narita, M.; Park, D.R.; Dhanireddy, S.; Molnar, A. Treatment completion for latent tuberculosis infection: A retrospective cohort study comparing 9 months of isoniazid, 4 months of rifampin and 3 months of isoniazid and rifapentine. BMC Infect. Dis. 2017, 17, 146. [Google Scholar] [CrossRef]
- Mukherjee, T.I.; Hirsch-Moverman, Y.; Saito, S.; Gadisa, T.; Melaku, Z.; Howard, A.A. Determinants of alcohol use among people living with HIV initiating isoniazid preventive therapy in Ethiopia. Drug Alcohol. Depend. 2019, 204, 107465. [Google Scholar] [CrossRef] [PubMed]
- Lodi, S.; Emenyonu, N.I.; Marson, K.; Kwarisiima, D.; Fatch, R.; McDonell, M.G.; Cheng, D.M.; Thirumurthy, H.; Gandhi, M.; Camlin, C.S.; et al. The Drinkers’ Intervention to Prevent Tuberculosis (DIPT) trial among heavy drinkers living with HIV in Uganda: Study protocol of a 2 × 2 factorial trial. Trials 2021, 22, 355. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Saha, B. Alcohol’s effect on host defense. Alcohol Res. 2015, 37, 159–170. [Google Scholar] [PubMed]
- Yeligar, S.M.; Chen, M.M.; Kovacs, E.J.; Sisson, J.H.; Burnham, E.L.; Brown, L.A. Alcohol and lung injury and immunity. Alcohol 2016, 55, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015, 37, 185–197. [Google Scholar] [PubMed]
- Haiqing, C.; Chen, L.; Yin, C.; Liao, Y.; Meng, X.; Lu, C.; Tang, S.; Li, X.; Wang, X. The effect of micro-nutrients on malnutrition, immunity and therapeutic effect in patients with pulmonary tuberculosis: A systematic review and meta-analysis of randomised controlled trials. Tuberculosis 2020, 125, 101994. [Google Scholar] [CrossRef]
- Sinha, P.; Lönnroth, K.; Bhargava, A.; Heysell, S.K.; Sarkar, S.; Salgame, P.; Rudgard, W.; Boccia, D.; Van Aartsen, D.; Hochberg, N.S. Food for thought: Addressing undernutrition to end tuberculosis. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Enoh, J.E.; Cho, F.N.; Manfo, F.P.; Ako, S.E.; Akum, E.A. Abnormal levels of liver enzymes and hepatotoxicity in HIV-positive, TB, and HIV/TB-coinfected patients on treatment in Fako division, southwest region of Cameroon. Biomed. Res. Int. 2020, 2020, 9631731. [Google Scholar] [CrossRef]
- Melikyan, N.; Huerga, H.; Atshemyan, H.; Kirakosyan, O.; Sargsyants, N.; Aydinyan, T.; Saribekyan, N.; Khachatryan, N.; Oganezova, I.; Falcao, J.; et al. Concomitant treatment of chronic hepatitis C with direct-acting antivirals and multidrug-resistant tuberculosis is effective and safe. Open Forum. Infect. Dis. 2021, 8, ofaa653. [Google Scholar] [CrossRef]
- Yew, W.W.; Leung, C.C. Antituberculosis drugs and hepatotoxicity. Respirology (Carlton Vic.) 2006, 11, 699–707. [Google Scholar] [CrossRef]
- Alghamdi, W.A.; Al-Shaer, M.H.; Kipiani, M.; Barbakadze, K.; Mikiashvili, L.; Kempker, R.R.; Peloquin, C.A. Pharmacokinetics of bedaquiline, delamanid and clofazimine in patients with multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 2021, 76, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef]
- Global Health Data Exchange (GHDx). GBD Results Tool for the Global Burden of Disease 2019 Study. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 12 April 2020).
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Rehm, J. Societal development and the alcohol—Attributable burden of disease. Addiction 2021, 116, 2326–2338. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Roerecke, M.; Behrendt, S.; Rehm, J. Socioeconomic differences in alcohol-attributable mortality compared with all-cause mortality: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Kilian, C.; Sanchez, S.; Lange, S.; Rehm, J. The role of alcohol use and drinking patterns in socioeconomic inequalities in mortality: A systematic review. Lancet Public Health 2020, 5, e324–e332. [Google Scholar] [CrossRef]
- Babor, T.F.; Casswell, S.; Graham, K.; Huckle, T.; Livingston, M.; Österberg, E.; Rehm, J.; Room, R.; Rossow, I.; Sornpaisarn, B. Alcohol: No Ordinary Commodity. Research and Public Policy, 3rd ed.; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Bhatty, M.; Pruett, S.B.; Swiatlo, E.; Nanduri, B. Alcohol abuse and Streptococcus pneumoniae infections: Consideration of virulence factors and impaired immune responses. Alcohol 2011, 45, 523–539. [Google Scholar] [CrossRef]
- Mehta, A.J.; Guidot, D.M. Alcohol and the lung. Alcohol Res. 2017, 38, 243–254. [Google Scholar]
- Simet, S.M.; Sisson, J.H. Alcohol’s effects on lung health and immunity. Alcohol Res. Curr. Rev. 2015, 37, 199. [Google Scholar]
- Gamble, L.; Mason, C.; Nelson, S. The effects of alcohol on immunity and bacterial infection in the lung. Med. Mal. Infect. 2006, 36, 72–77. [Google Scholar] [CrossRef]
- Heermans, E.H. Booze and blood: The effects of acute and chronic alcohol abuse on the hematopoietic system. Clin. Lab. Sci. 1998, 11, 229–232. [Google Scholar]
- Nelson, S.; Zhang, P.; Bagby, G.J.; Happel, K.I.; Raasch, C.E. Alcohol abuse, immunosuppression, and pulmonary infection. Curr. Drug Abuse Rev. 2008, 1, 56–67. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P.; Catalano, D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1β, TNF-α, and IL-6 production by acute ethanol treatment. J. Leukoc. Biol. 1995, 58, 342–350. [Google Scholar] [CrossRef]
- Samokhvalov, A.; Irving, H.; Rehm, J. Alcohol consumption as a risk factor for pneumonia: A systematic review and meta-analysis. Epidemiol. Infect. 2010, 138, 1789–1795. [Google Scholar] [CrossRef]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of pneumonia: A systematic review and meta-analysis. BMJ Open 2018, 8, e022344. [Google Scholar] [CrossRef]
- Rehm, J.; Anderson, P.; Gual, A.; Kraus, L.; Marmet, S.; Nutt, D.; Room, R.; Samokhvalov, A.; Scafato, E.; Shield, K. The tangible common denominator of substance use disorders: A reply to commentaries to Rehm et al. (2013a). Alcohol Alcohol. 2014, 49, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Thiébaut, S.P.; Baillot, S.; Mallet, V.; Rehm, J. Alcohol use disorders and associated chronic disease: A national retrospective cohort study from France. BMC Public Health 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Solá, J.; Junqué, A.; Estruch, R.; Monforte, R.; Torres, A.; Urbano-Márquez, A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch. Intern. Med. 1995, 155, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Koivula, I.; Sten, M.; Makela, P.H. Risk factors for pneumonia in the elderly. Am. J. Med. 1994, 96, 313–320. [Google Scholar] [CrossRef]
- Luján, M.; Gallego, M.; Belmonte, Y.; Fontanals, D.; Valles, J.; Lisboa, T.; Rello, J. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur. Respir. J. 2010, 36, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Trevejo-Nunez, G.; Kolls, J.K.; De Wit, M. Alcohol use as a risk factor in infections and healing: A clinician’s perspective. Alcohol Res. 2015, 37, 177. [Google Scholar]
- Gupta, N.M.; Deshpande, A.; Rothberg, M.B. Pneumonia and alcohol use disorder: Implications for treatment. Cleve Clin. J. Med. 2020, 87, 493–500. [Google Scholar] [CrossRef] [PubMed]
- WHO Director General. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 July 2021).
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 10 July 2021).
- Islam, N.; Shkolnikov, V.M.; Acosta, R.J.; Klimkin, I.; Kawachi, I.; Irizarry, R.A.; Alicandro, G.; Khunti, K.; Yates, T.; Jdanov, D.A.; et al. Excess deaths associated with covid-19 pandemic in 2020: Age and sex disaggregated time series analysis in 29 high income countries. BMJ 2021, 373, n1137. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Alzahrani, M.A.; Alsharif, N.A.; Alrobaie, N.S.; Alsaab, H.O.; Uddin, M.N. The possible relationship between the abuse of tobacco, opioid, or alcohol with COVID-19. Healthcare 2020, 9, 2. [Google Scholar] [CrossRef]
- Kendler, K.S.; Ohlsson, H.; Karriker-Jaffe, K.J.; Sundquist, J.; Sundquist, K. Social and economic consequences of alcohol use disorder: A longitudinal cohort and co-relative analysis. Psychol. Med. 2017, 47, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Buu, A.; Mansour, M.; Wang, J.; Refior, S.K.; Fitzgerald, H.E.; Zucker, R.A. Alcoholism effects on social migration and neighborhood effects on alcoholism over the course of 12 years. Alcohol. Clin. Exp. Res. 2007, 31, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Samuels-Kalow, M.E.; Dorner, S.; Cash, R.E.; Dutta, S.; White, B.; Ciccolo, G.E.; Brown, D.F.M.; Camargo, C.A., Jr. Neighborhood disadvantage measures and COVID-19 cases in Boston, 2020. Public Health Rep. 2021, 136, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, L.; Fairbairn, C.E.; Sayette, M.A.; Bosch, N. Alcohol narrows physical distance between strangers. Proc. Natl. Acad. Sci. USA 2021, 118, e2101937118. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Bailey, K.L.; Samuelson, D.R.; Wyatt, T.A. Alcohol use disorder: A pre-existing condition for COVID-19? Alcohol 2021, 90, 11–17. [Google Scholar] [CrossRef]
- Vasudeva, A.; Patel, T.K. Alcohol consumption: An important epidemiological factor in COVID-19? J. Glob. Health 2020, 10, 020335. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, H.; Hodgkinson, C.; Montero, A.; Goldman, D.; Chang, S.L. Network meta-analysis on the mechanisms underlying alcohol augmentation of COVID-19 pathologies. Alcohol. Clin. Exp. Res. 2021, 45, 675–688. [Google Scholar] [CrossRef]
- Wendt, F.R.; De Lillo, A.; Pathak, G.A.; De Angelis, F.; Polimanti, R. Host genetic liability for severe COVID-19 overlaps with alcohol drinking behavior and diabetic outcomes and in over 1 million participants. medRxiv 2020, 12. [Google Scholar] [CrossRef]
- Benzano, D.; Ornell, F.; Schuch, J.B.; Pechansky, F.; Sordi, A.O.; von Diemen, L.; Kessler, F.H.P. Clinical vulnerability for severity and mortality by COVID-19 among users of alcohol and other substances. Psychiatry Res. 2021, 300, 113915. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Z.; Poulsen, K.L.; Wu, X.; Miyata, T.; Dasarathy, S.; Rotroff, D.M.; Nagy, L.E. Alcohol consumption is associated with poor prognosis in obese patients with COVID-19: A Mendelian randomization study using UK Biobank. Nutrients 2021, 13, 1592. [Google Scholar] [CrossRef] [PubMed]
- Kushner, T.; Cafardi, J. Chronic liver disease and COVID-19: Alcohol use disorder/alcohol-associated liver disease, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis. Clin. Liver Dis. 2020, 15, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Curtis, B.; Mandrekar, P.; Singal, A.K.; Verna, E.C.; Fix, O.K. Alcohol-associated liver disease before and after COVID-19: An overview and call for ongoing investigation. Hepatol. Commun. 2021, 5, 1616–1621. [Google Scholar] [CrossRef]
- Cava, E.; Neri, B.; Carbonelli, M.G.; Riso, S.; Carbone, S. Obesity pandemic during COVID-19 outbreak: Narrative review and future considerations. Clin. Nutr. 2021, 40, 1637–1643. [Google Scholar] [CrossRef]
- Bilal, B.; Saleem, F.; Fatima, S.S. Alcohol consumption and obesity: The hidden scare with COVID-19 severity. Med. Hypotheses 2020, 144, 110272. [Google Scholar] [CrossRef]
- Alberca, R.W.; Rigato, P.O.; Ramos YÁ, L.; Teixeira, F.M.E.; Branco, A.C.C.; Fernandes, I.G.; Pietrobon, A.J.; Duarte, A.; Aoki, V.; Orfali, R.L.; et al. Clinical characteristics and survival analysis in frequent alcohol consumers with COVID-19. Front. Nutr. 2021, 8, 689296. [Google Scholar] [CrossRef]
- Saurabh, S.; Verma, M.K.; Gautam, V.; Kumar, N.; Jain, V.; Goel, A.D.; Gupta, M.K.; Sharma, P.P.; Bhardwaj, P.; Singh, K.; et al. Tobacco, alcohol use and other risk factors for developing symptomatic COVID-19 vs asymptomatic SARS-CoV-2 infection: A case-control study from western Rajasthan, India. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 820–831. [Google Scholar] [CrossRef]
- Varela Rodríguez, C.; Arias Horcajadas, F.; Martín-Arriscado Arroba, C.; Combarro Ripoll, C.; Juanes Gonzalez, A.; Esperesate Pajares, M.; Rodrigo Holgado, I.; Cadenas Manceñido, Á.; Sánchez Rodríguez, L.; Baselga Penalva, B.; et al. COVID-19-related neuropsychiatric symptoms in patients with alcohol abuse conditions during the SARS-CoV-2 pandemic: A retrospective cohort study using real world data from electronic health records of a tertiary hospital. Front. Neurol. 2021, 12, 630566. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Kaelber, D.C.; Xu, R.; Volkow, N.D. COVID-19 risk and outcomes in patients with substance use disorders: Analyses from electronic health records in the United States. Mol. Psychiatry 2021, 26, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Tao, L.; Chen, Z.; Tian, Z.; Guo, X.; Allen-Gipson, D.S.; Tan, R.; Li, R.; Chai, L.; Ai, F.; et al. Influence of cigarettes and alcohol on the severity and death of COVID-19: A multicenter retrospective study in Wuhan, China. Front. Physiol. 2020, 11, 588553. [Google Scholar] [CrossRef]
- Lv, L.; Zhou, Y.W.; Yao, R. Alcohol consumption and COVID-19 severity: A propensity score matched study in China. Signa Vitae 2021, 17, 112–120. [Google Scholar] [CrossRef]
- Pro, G.; Gilbert, P.A.; Baldwin, J.A.; Brown, C.C.; Young, S.; Zaller, N. Multilevel modeling of county-level excessive alcohol use, rurality, and COVID-19 case fatality rates in the US. PLoS ONE 2021, 16, e0253466. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Khodadadi, I. Alcohol misuse may increase the severity of COVID-19 infections. Disaster. Med. Public Health Prep. 2020, 18, 1–2. [Google Scholar] [CrossRef]
- Saengow, U.; Assanangkornchai, S.; Casswell, S. Alcohol: A probable risk factor of COVID-19 severity. Addiction 2021, 116, 204–220. [Google Scholar] [CrossRef]
- Testino, G. Are patients with alcohol use disorders at increased risk for COVID-19 infection? Alcohol Alcohol. 2020, 55, 344–346. [Google Scholar] [CrossRef]
- Collin, S.M.; Wurie, F.; Muzyamba, M.C.; de Vries, G.; Lonnroth, K.; Migliori, G.B.; Abubakar, I.; Anderson, S.R.; Zenner, D. Effectiveness of interventions for reducing TB incidence in countries with low TB incidence: A systematic review of reviews. Eur. Respir. Rev. 2019, 28, 180107. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Bolibar, I.; Serra-Prat, M. Risk factors for community-acquired pneumonia in adults: Recommendations for its prevention. Community Acquir. Infect. 2015, 2, 32. [Google Scholar] [CrossRef]
- Pletz, M.W.; Rohde, G.G.; Welte, T.; Kolditz, M.; Ott, S. Advances in the prevention, management, and treatment of community-acquired pneumonia. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Cebey-Lopez, M.; Pardo-Seco, J.; Yuste, J.; Redondo, E.; Vargas, D.A.; Mascaros, E.; Diaz-Maroto, J.L.; Linares-Rufo, M.; Jimeno, I.; et al. Lifestyle and comorbid conditions as risk factors for community-acquired pneumonia in outpatient adults (NEUMO-ES-RISK project). BMJ Open Respir. Res. 2019, 6, e000359. [Google Scholar] [CrossRef]
- Carrasco, M.A.; Esser, M.B.; Sparks, A.; Kaufman, M.R. HIV-alcohol risk reduction interventions in sub-Saharan Africa: A systematic review of the literature and recommendations for a way forward. AIDS Behav. 2016, 20, 484–503. [Google Scholar] [CrossRef] [PubMed]
- Morojele, N.K.; Ranchod, C. Review of interventions to reduce alcohol use-related sexual risk behaviour in Africa. Afr. J. Drug Alcohol. Stud. 2011, 10, 95–106. [Google Scholar]
- Fritz, K.; McFarland, W.; Wyrod, R.; Chasakara, C.; Makumbe, K.; Chirowodza, A.; Mashoko, C.; Kellogg, T.; Woelk, G. Evaluation of a peer network-based sexual risk reduction intervention for men in beer halls in Zimbabwe: Results from a randomized controlled trial. AIDS Behav. 2011, 15, 1732–1744. [Google Scholar] [CrossRef][Green Version]
- Kalichman, S.C.; Simbayi, L.C.; Cain, D.; Carey, K.B.; Carey, M.P.; Eaton, L.; Harel, O.; Mehlomakhulu, V.; Mwaba, K. Randomized community-level HIV prevention intervention trial for men who drink in South African alcohol-serving venues. Eur. J. Public Health 2014, 24, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Morojele, N.K.; Kitleli, N.; Ngako, K.; Kekwaletswe, C.T.; Nkosi, S.; Fritz, K.; Parry, C.D. Feasibility and acceptability of a bar-based sexual risk reduction intervention for bar patrons in Tshwane, South Africa. SAHARA J. 2014, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raviglione, M.; Poznyak, V. Targeting harmful use of alcohol for prevention and treatment of tuberculosis: A call for action. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Livchits, V.; Connery, H.S.; Shields, A.; Yanov, S.; Yanova, G.; Fitzmaurice, G.M.; Nelson, A.K.; Greenfield, S.F.; Tomsk Tuberculosis Alcohol Working Group. Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction 2013, 108, 1387–1396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehta, A.J. Alcoholism and critical illness: A review. World J. Crit. Care Med. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Brown, J.L.; DeMartini, K.S.; Sales, J.M.; Swartzendruber, A.L.; DiClemente, R.J. Interventions to reduce alcohol use among HIV-infected individuals: A review and critique of the literature. Curr. HIV/AIDS Rep. 2013, 10, 356–370. [Google Scholar] [CrossRef]
- Madhombiro, M.; Musekiwa, A.; January, J.; Chingono, A.; Abas, M.; Seedat, S. Psychological interventions for alcohol use disorders in people living with HIV/AIDS: A systematic review. Syst. Rev. 2019, 8, 244. [Google Scholar] [CrossRef]
- Samet, J.H.; Walley, A.Y. Interventions targeting HIV-infected risky drinkers: Drops in the bottle. Alcohol Res. Health 2010, 33, 267–279. [Google Scholar] [PubMed]
- Regenauer, K.S.; Myers, B.; Batchelder, A.W.; Magidson, J.F. “That person stopped being human”: Intersecting HIV and substance use stigma among patients and providers in South Africa. Drug Alcohol. Depend. 2020, 216, 108322. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Katner, H.; Hill, M.; Kalichman, M.O.; Hernandez, D. Alcohol-related intentional antiretroviral nonadherence among people living with HIV: Test of an Interactive Toxicity Beliefs Process Model. J. Int. Assoc. Provid. AIDS Care 2019, 18, 2325958219826612. [Google Scholar] [CrossRef]
- World Health Organization. Tackling NCDs: ‘Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ferreira-Borges, C.; Esser, M.B.; Dias, S.; Babor, T.; Parry, C.D. Alcohol control policies in 46 African countries: Opportunities for improvement. Alcohol Alcohol. 2015, 50, 470–476. [Google Scholar] [CrossRef]
- Gururaj, G.; Gautham, M.S.; Arvind, B.A. Alcohol consumption in India: A rising burden and a fractured response. Drug Alcohol Rev. 2021, 40, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mora, M.E.; Monteiro, M.; Rafful, C.; Samano, I. Comprehensive analysis of alcohol policies in the Latin America and the Caribbean. Drug Alcohol Rev. 2021, 40, 385–401. [Google Scholar] [CrossRef]
- Morojele, N.K.; Dumbili, E.W.; Obot, I.S.; Parry, C.D.H. Alcohol consumption, harms and policy developments in sub-Saharan Africa: The case for stronger national and regional responses. Drug Alcohol Rev. 2021, 40, 402–419. [Google Scholar] [CrossRef] [PubMed]
| Deaths (Thousands) | DALYs (Millions) | |
|---|---|---|
| Tuberculosis | 236.3 (74.6–456.6) | 9.9 (3.2–18.6) |
| HIV/AIDS | 30.4 (22.8–56.7) | 1.7 (1.2–3.1) |
| Lower respiratory infections | 95.2 (48.5–177.6) | 2.3 (1.3–4.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morojele, N.K.; Shenoi, S.V.; Shuper, P.A.; Braithwaite, R.S.; Rehm, J. Alcohol Use and the Risk of Communicable Diseases. Nutrients 2021, 13, 3317. https://doi.org/10.3390/nu13103317
Morojele NK, Shenoi SV, Shuper PA, Braithwaite RS, Rehm J. Alcohol Use and the Risk of Communicable Diseases. Nutrients. 2021; 13(10):3317. https://doi.org/10.3390/nu13103317
Chicago/Turabian StyleMorojele, Neo K., Sheela V. Shenoi, Paul A. Shuper, Ronald Scott Braithwaite, and Jürgen Rehm. 2021. "Alcohol Use and the Risk of Communicable Diseases" Nutrients 13, no. 10: 3317. https://doi.org/10.3390/nu13103317
APA StyleMorojele, N. K., Shenoi, S. V., Shuper, P. A., Braithwaite, R. S., & Rehm, J. (2021). Alcohol Use and the Risk of Communicable Diseases. Nutrients, 13(10), 3317. https://doi.org/10.3390/nu13103317






