Development and Validation of a Short Food Frequency Questionnaire to Measure Dietary Intake of a Selection of Immune-Modulating Nutrients in Patients with Established Peripheral Arterial Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Food Frequency Questionnaire Development
2.2.1. Selecting Food Items
2.2.2. Piloting the FFQ
2.2.3. FFQ Question Design
2.3. Reference Method
2.4. Administration of FFQ and Reference Method
2.5. Databases
2.6. Statistical Analysis
2.7. Clinical Significance
3. Results
3.1. Population Characteristics
3.2. Agreement between FFQ and Diet History
3.3. Nutritional Adequacy of Diet
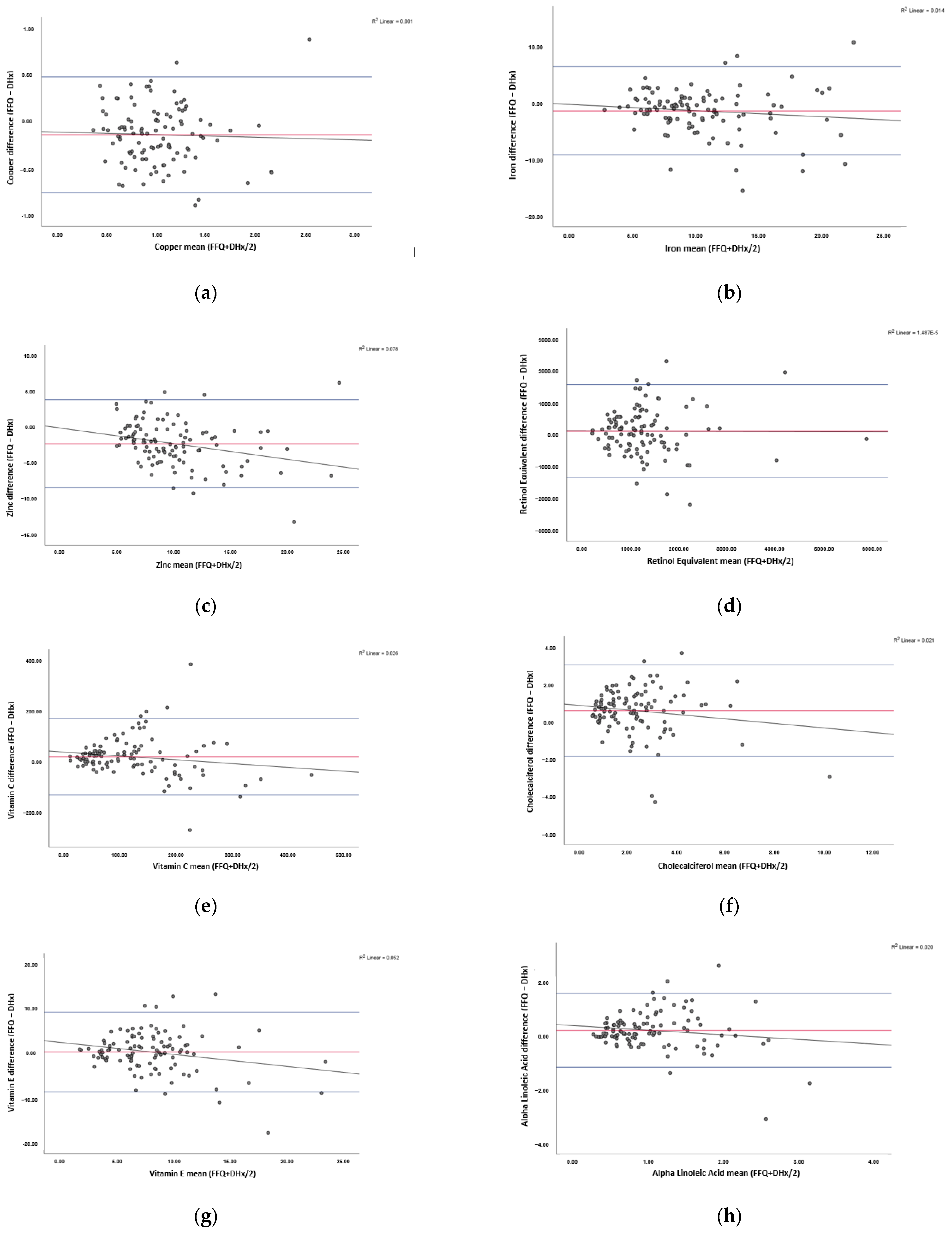
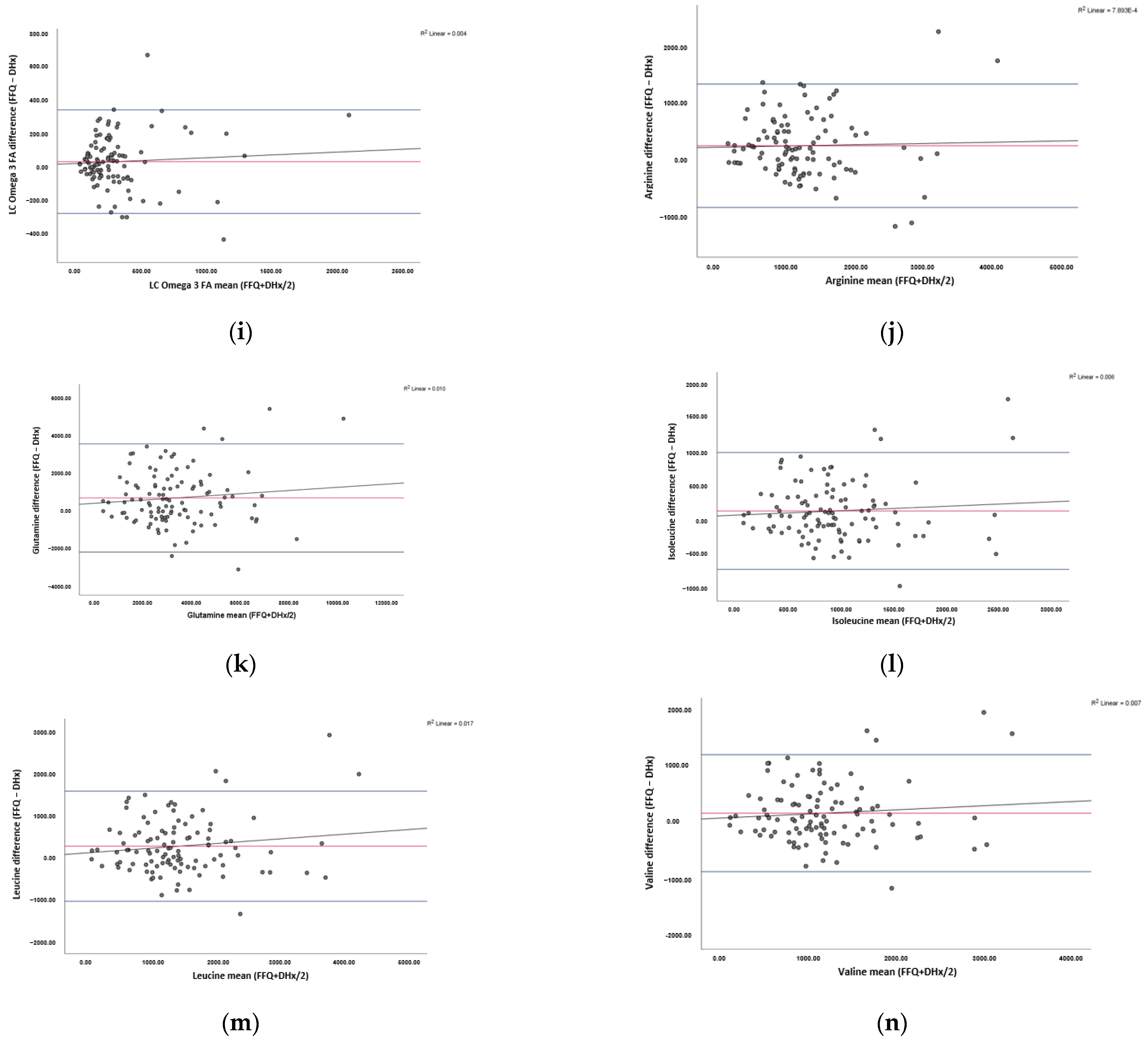
3.4. Agreement between the FFQ and Reference Method
3.5. Selectivity and Specificity
3.6. Supplementation
3.7. Limitations
3.8. Strengths
3.9. Implications for Practice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mohler, E.R. Peripheral Artery Disease, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef] [Green Version]
- Delaney, C.; Smale, M.; Miller, M.D. Nutritional Considerations for Peripheral Arterial Disease: A Narrative Review. Nutrients 2019, 11, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismaeel, A.; Brumberg, R.; Kirk, J.; Papoutsi, E.; Farmer, P.; Bohannon, W.; Smith, R.; Eidson, J.; Sawicki, I.; Koutakis, P. Oxidative Stress and Arterial Dysfunction in Peripheral Artery Disease. Antioxidants 2018, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimbrone, A.M.; García-Cardeña, A.G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olin, J.W.; White, C.J.; Armstrong, E.J.; Kadian-Dodov, D.; Hiatt, W.R. Peripheral Artery Disease: Evolving Role of Exercise, Medical Therapy, and Endovascular Options. J. Am. Coll. Cardiol. 2016, 67, 1338–1357. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360. [Google Scholar] [CrossRef] [Green Version]
- Hamer, M.; Steptoe, A. Influence of specific nutrients on progression of atherosclerosis, vascular function, haemostasis and inflammation in coronary heart disease patients: A systematic review. Br. J. Nutr. 2006, 95, 849–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, N.; Guevara-Cruz, M.; Velazquez-Villegas, L.; Tovar, A. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- National Heart Foundation of Australia. Dietary Position Statement—Heart Healthy Eating Patterns; National Heart Foundation of Australia: Sydney, Australia, 2019. [Google Scholar]
- Collins, C.; Burrows, T.; Rollo, M. Dietary Patterns and Cardiovascular Disease Outcomes: An Evidence Check Rapid Review; National Heart Foundation of Australia: Sydney, Australia, 2017. [Google Scholar]
- Casas, R.; Estruch, R.; Sacanella, E. Influence of Bioactive Nutrients on the Atherosclerotic Process: A Review. Nutrients 2018, 10, 1630. [Google Scholar] [CrossRef] [Green Version]
- Adiamah, A.; Skořepa, P.; Weimann, A.; Lobo, D.N. The Impact of Preoperative Immune Modulating Nutrition on Outcomes in Patients Undergoing Surgery for Gastrointestinal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 247–256. [Google Scholar] [CrossRef]
- Wong, C.S.; Aly, E.H. The effects of enteral immunonutrition in upper gastrointestinal surgery: A systematic review and meta-analysis. Int. J. Surg. 2016, 29, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-H.; Chen, J.-W.; Tsai, C.; Chiang, M.-C.; Young, M.S.; Lin, S.-J. l-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clin. Nutr. 2005, 24, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Bright, B.C.; Ort, K.A.; Montgomery, P.S. Dietary Intake of Participants with Peripheral Artery Disease and Claudication. Angiology 2011, 62, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.; Delaney, C.; Suen, J.; Miller, M. Nutritional status of patients admitted to a metropolitan tertiary care vascular surgery unit. Asia Pac. J. Clin. Nutr. 2019, 28, 64. [Google Scholar] [CrossRef] [PubMed]
- DAPA Measurement Tool Kit. Dietary Assessment. Available online: https://dapa-toolkit.mrc.ac.uk/diet/subjective-methods/introduction (accessed on 20 November 2020).
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlois, L.M.; Duprez, L.D.; Delanghe, L.J.; De Buyzere, L.M.; Clement, L.D. Serum Vitamin C Concentration Is Low in Peripheral Arterial Disease and Is Associated with Inflammation and Severity of Atherosclerosis. Circ. J. Am. Heart Assoc. 2001, 103, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Samman, S.; Herbert, J.; Petocz, P.; Lyons-Wall, P.M. Development and Validation of a Short Questionnaire for Estimating the Intake of Zinc. Biol. Trace Elem. Res. 2010, 134, 226–234. [Google Scholar] [CrossRef]
- Sublette, M.E.; Segal-Isaacson, C.J.; Cooper, T.B.; Fekri, S.; Vanegas, N.; Galfalvy, H.C.; Oquendo, M.A.; Mann, J.J. Validation of a Food Frequency Questionnaire to Assess Intake of n-3 Polyunsaturated Fatty Acids in Subjects with and without Major Depressive Disorder. J. Am. Diet. Assoc. 2011, 111, 117–123.e112. [Google Scholar] [CrossRef] [Green Version]
- Cancer Council Victoria. Dietary Questionnaires. Available online: https://www.cancervic.org.au/research/epidemiology/nutritional_assessment_services (accessed on 13 July 2020).
- Satia, J.A.; Watters, J.L.; Galanko, J.A. Validation of an antioxidant nutrient questionnaire in whites and African Americans. J. Am. Diet. Assoc. 2009, 109, 502–508.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Conesa, M.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Baranowski, T.; Subar, A.F.; Tooze, J.A.; Frongillo, E.A. Best Practices for Conducting and Interpreting Studies to Validate Self-Report Dietary Assessment Methods. J. Acad. Nutr. Diet. 2019, 119, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia & New Zealand. Austalian Food Composition Database. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/pages/default.aspx (accessed on 13 July 2020).
- Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results—Foods and Nutrients. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/2011-12#history-of-changes (accessed on 8 December 2020).
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; Australian Government Department of Health and Ageing, New Zealand Ministry of Health, Eds.; National Health and Medical Research Council Canberra: Canberra, Australia, 2006.
- Myles, P.S.; Cui, J.I. Using the Bland–Altman method to measure agreement with repeated measures. BJA Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Standards Australia New Zealand. Nutrition Information User Guide to Standard 1.2.8—Nutrition Information Requirements, Part B—Nutrition Claims. Available online: https://www.foodstandards.gov.au/code/userguide/Documents/Userguide_Prescribed%20Nutrition%20Information%20Nov%2013%20Dec%202013.pdf (accessed on 13 July 2020).
- Henríquez-Sánchez, P.; Sánchez-Villegas, A.; Doreste-Alonso, J.; Ortiz-Andrellucchi, A.; Pfrimer, K.; Serra-Majem, L. Dietary assessment methods for micronutrient intake: A systematic review on vitamins. Br. J. Nutr. 2009, 102, S10–S37. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.S.; Gibson, P.R. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J. Am. Diet. Assoc. 2010, 110, 1469–1476. [Google Scholar] [CrossRef]
- Harmouche-Karaki, M.; Mahfouz, M.; Obeyd, J.; Salameh, P.; Mahfouz, Y.; Helou, K. Development and validation of a quantitative food frequency questionnaire to assess dietary intake among Lebanese adults. Nutr. J. 2020, 19, 65. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Davis, C.G.; Lee, S.G.; Fernandez, M.L.; Koo, S.I.; Cho, E.; Chun, O.K. Validation of an FFQ to assess antioxidant intake in overweight postmenopausal women. Public Health Nutr. 2014, 17, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Food Standards Australia New Zealand. AUSNUT 2011–13 Dietary Supplement Nutrient Database. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/ausnutdatafiles/Pages/dietarysupplementnutrient.aspx (accessed on 17 December 2020).
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
| Immune-Modulating Nutrient | Immune Pathway/Mechanism |
|---|---|
| Glutamine | Modulates immune system and suppresses acute inflammatory responses when administered to gastrointestinal surgical patients, reducing infectious complications and length of stay [14,15], highlighting potential in PAD population to impede the development and progression of atherosclerosis through anti-inflammatory and antioxidant properties, mitigating the pro-inflammatory and oxidative state of atherosclerosis [2]. |
| Arginine | |
| Nucleotides | |
| Omega-3 fatty acids (LC n-3 FA) | |
| Vitamin E | Reduces suppression of adhesion molecules resulting in reduced recruitment of monocytes [9] |
| Vitamin C | Increases endothelial cell production of NO, thus blocking pro-inflammatory and adhesion molecules [9,16]. |
| B-vitamins | |
| L-arginine | |
| Vitamin D | Decreases levels and uptake of LDL which contribute to inflammation and disease progression [8,9,13]. |
| LC n-3 FA | |
| Zinc | Decreases inflammation through modulating the release of pro-inflammatory cytokines [8,9,13]. |
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Age (years) | 72 ± 11.49 |
| Gender | |
| Male | 88 (83) |
| Female | 18 (17) |
| Living situation | |
| Home | 99 (93.4) |
| Residential Aged Care | 6 (5.7) |
| Other | 1 (0.9) |
| Recruitment location | |
| Inpatient | 11 (10.4) |
| Outpatient | 95 (89.6) |
| Time for FFQ (minutes) | 8.25 ± 2.68 |
| Nutrient | Bias | Upper LOA | Lower LOA | Clinically Acceptable Bias (±) a | Clinically Acceptable LOA (±) b | T-Statistic | p-Value c |
|---|---|---|---|---|---|---|---|
| Copper (mg) | −0.14 | 0.47 | −0.75 | 0.28 | 1.10 | −0.35 | 0.73 |
| Iron (mg) | −1.53 | 6.24 | −9.29 | 2.04 | 8.17 | −1.22 | 0.23 |
| Zinc (mg) | −2.33 | 3.76 | −8.42 | 2.10 | 8.39 | −2.96 | 0.00 |
| Retinol Equivalents (µg) | 115.94 | 1566.58 | −1334.69 | 1197.99 | 4791.95 | −0.04 | 0.97 |
| Vitamin C (mg) | 16.06 | 166.50 | −134.39 | 24.38 | 97.54 | −1.66 | 0.10 |
| Cholecalciferol (D3) (µg) | 0.58 | 3.01 | −1.86 | 2.70 | 10.79 | −1.49 | 0.14 |
| Vitamin E (mg) | 0.34 | 9.19 | −8.51 | 2.75 | 10.98 | −2.39 | 0.02 |
| Alpha Linolenic Acid (g) | 0.17 | 1.52 | −1.19 | 0.27 | 1.07 | −1.47 | 0.15 |
| Total LC n-3 FA (mg) | 29.32 | 337.09 | −278.46 | 542.96 | 2171.83 | 0.65 | 0.52 |
| Arginine (mg) | 241.96 | 1329.48 | −845.55 | 316.90 | 1267.62 | 0.29 | 0.78 |
| Glutamic Acid (mg) | 564.67 | 3417.36 | −2288.03 | 687.80 | 2751.19 | 1.02 | 0.31 |
| Isoleucine (mg) | 145.55 | 995.88 | −704.79 | 203.51 | 814.04 | 0.78 | 0.44 |
| Leucine (mg) | 261.33 | 1563.47 | −1040.80 | 312.40 | 1249.60 | 1.34 | 0.48 |
| Valine (mg) | 182.90 | 1217.14 | −851.33 | 243.73 | 974.92 | 0.89 | 0.38 |
| Average Nutrient Intake | Proportion Inadequate Intake (%) ‡ | Sensitivity and Specificity | |||||
|---|---|---|---|---|---|---|---|
| Nutrient | NRV a | FFQ | Diet History | FFQ | Diet History | Sensitivity (%) b | Specificity (%) c |
| Copper (mg) | 1.7 | 0.94 | 1.08 | 94.34 | 89.62 | 98.95 | 45.45 |
| Iron (mg) | 8 | 9.69 | 11.22 | 43.40 | 26.42 | 89.29 | 73.08 |
| Zinc (mg) | 14 | 8.94 | 11.27 | 86.79 | 71.70 | 96.05 | 36.67 |
| Retinol Equivalents (µg) | 900 | 1339.56 | 1223.61 | 33.02 | 41.51 | 50.00 | 79.03 |
| Vitamin C (mg) | 45 | 128.49 | 112.43 | 17.92 | 27.36 | 48.28 | 94.81 |
| Cholecalciferol (D3) (µg) | 15 | 2.53 | 1.95 | 100.00 | 100.00 | 100.00 | - |
| Vitamin E (mg) | 10 | 8.30 | 7.96 | 68.00 | 69.81 | 75.68 | 50.00 |
| Alpha Linolenic Acid (g) | 1.3 | 1.12 | 0.95 | 66.04 | 75.47 | 67.65 | 34.72 |
| Total LC n-3 FA (mg) | 160 | 325.63 | 296.31 | 24.53 | 32.08 | 58.82 | 91.67 |
| Arginine (mg) | NA | 1365.69 | 1123.73 | ||||
| Glutamic Acid (mg) | NA | 3578.00 | 3013.33 | ||||
| Isoleucine (mg) | NA | 1029.00 | 883.45 | ||||
| Leucine (mg) | NA | 1601.91 | 1340.58 | ||||
| Valine (mg) | NA | 1276.19 | 1093.29 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, B.J.; Delaney, C.L.; Boffo, J.E.; Miller, M.D. Development and Validation of a Short Food Frequency Questionnaire to Measure Dietary Intake of a Selection of Immune-Modulating Nutrients in Patients with Established Peripheral Arterial Disease. Nutrients 2021, 13, 3316. https://doi.org/10.3390/nu13103316
Collins BJ, Delaney CL, Boffo JE, Miller MD. Development and Validation of a Short Food Frequency Questionnaire to Measure Dietary Intake of a Selection of Immune-Modulating Nutrients in Patients with Established Peripheral Arterial Disease. Nutrients. 2021; 13(10):3316. https://doi.org/10.3390/nu13103316
Chicago/Turabian StyleCollins, Bianca J., Christopher L. Delaney, Jade E. Boffo, and Michelle D. Miller. 2021. "Development and Validation of a Short Food Frequency Questionnaire to Measure Dietary Intake of a Selection of Immune-Modulating Nutrients in Patients with Established Peripheral Arterial Disease" Nutrients 13, no. 10: 3316. https://doi.org/10.3390/nu13103316
APA StyleCollins, B. J., Delaney, C. L., Boffo, J. E., & Miller, M. D. (2021). Development and Validation of a Short Food Frequency Questionnaire to Measure Dietary Intake of a Selection of Immune-Modulating Nutrients in Patients with Established Peripheral Arterial Disease. Nutrients, 13(10), 3316. https://doi.org/10.3390/nu13103316





