Abstract
Evidence about the role of nutritional factors and microbiota in autoimmune diseases, and in rheumatoid arthritis (RA) in particular, has grown in recent years, however many controversies remain. The aim of this review is to summarize the role of nutrition and of the intestinal microbiota in the development of RA. We will focus on selected dietary patterns, individual foods and beverages that have been most consistently associated with RA or with the occurrence of systemic autoimmunity associated with RA. We will also review the evidence for a role of the intestinal microbiota in RA development. We propose that diet and digestive microbiota should be considered together in research, as they interact and may both be the target for future preventive interventions in RA.
1. Introduction
Rheumatoid arthritis (RA) is the most prevalent systemic autoimmune inflammatory disease affecting approximately 1% of the adult population worldwide [1]. However, the etiopathogenesis of RA is only partially understood. The current knowledge is that in genetically susceptible individuals, environmental factors induce a pathological activation of the immune system that eventually leads to clinical onset of RA [2]. The European League Against Rheumatism (EULAR) has proposed a terminology for specific preclinical phases of RA development, which are not necessarily consecutive or mutually exclusive (Figure 1) [2,3]. Interactions between genetic factors, environmental factors and the presence of autoantibodies lead to increased risk of developing RA.

Figure 1.
Proposed preclinical phases of RA development. Genetic, environmental factors and systemic autoimmunity interactions lead to RA development. The progression from one preclinical phase to another is not necessarily linear, and the phases may be overlapping.
Several studies suggest environmental factors play an important role in the etiology of the disease [4,5]. Smoking is the environmental factor more consistently associated with RA development [6]. However, patients are frequently also concerned about the effect of diet on the development of RA. Human diet has gone through extensive transformation globally, with increasing consumption of processed foods, salt and carbohydrate enriched products, contributing to the development of obesity and other chronic diseases, such as hypertension, type 2 diabetes mellitus and cardiovascular diseases. Some nutritional factors may contribute to the pathologic activation of immune system, eventually leading to RA, and some others may be protective. Studies have suggested that the initial steps of the pathological autoimmune response associated with RA take place at mucosal sites, such as intestinal or airway mucosa, rather than in the joints [7], and are associated with higher abundance of particular bacterial species [8]. Diet modifications affect the composition and function of the intestinal microbiota and it is possible that part of the observed effect of nutrition on RA is mediated by changes in the microbiota.
Although a number of studies have analyzed the impact of dietary factors and intestinal dysbiosis in RA development, many controversies remain. As many studies are performed cross-sectionally, it is often impossible to establish whether the described associations are causal or not. The aim of this manuscript is to review the role of nutritional factors and of intestinal microbiota in RA development. We will focus on selected dietary patterns, individual foods and beverages that have been most consistently associated with preclinical phases of RA, such as ‘systemic autoimmunity associated with RA’ and with the disease itself, whether protective or conferring an increased risk. We will then review the evidence of a role of the microbiota in RA development. We will discuss these associations in pre-clinical phases and in established RA separately. Associations reported in established disease are prone to reversed causation, which occurs when the disease status influences exposure, and could bias the association of dietary factors or microbiota observed in established RA [9]. The relevance of studying the role of diet and intestinal microbiota in RA development lies in their potential to be modified and to be used in preventive strategies.
2. Nutrition and Development of Systemic Autoimmunity Associated with RA
‘Systemic autoimmunity associated with RA’ is a pre-clinical phase of RA, often considered the immune onset of the disease and characterized by the presence of autoantibodies, such as the rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). Few studies have analyzed the impact of nutritional factors on the development of systemic autoimmunity associated with RA, in individuals at risk of RA.
Omega-3 and Omega-6 Fatty Acids
Omega-3 fatty acids have been suggested to be protective against the development of autoimmunity associated with RA. In a nested case-control study in the Studies of the Etiology of RA (SERA), healthy FDR-RA individuals who developed ACPAs had used less frequently omega-3 supplements (Odds ratio, OR 0.14, 95% Confidence Interval, CI 0.03–0.68) and had significantly lower concentrations of omega-3 fatty acids in red blood cell membranes than controls (30 cases vs 47 controls) [10]. The SERA research group further analyzed, in a larger number of FDR-RA individuals, whether omega-3 fatty acids were also associated with RF and whether these associations were modified by shared epitope (SE) positivity. Individuals with RF and SE positivity or with ACPA and SE positivity had lower concentrations of omega-3 fatty acids in red blood cell membranes (OR 0.27, 95% CI 0.10–0.79 and OR 0.42, 95% CI 0.20–0.98, respectively) [11]. These results suggest a potential protective effect of omega-3 fatty acids on RA-related autoimmunity, which may be more prominent in those with genetic susceptibility to RA.
3. Nutrition and Development of RA
Several studies have analyzed associations of dietary patterns, individual foods and beverages with established RA. We are going to review potentially protective and hazardous factors and discuss controversial factors.
3.1. Protective Factors
Alcohol
In animal models, adding small doses of ethanol to mice’s drinking water delays the onset of collagen-induced arthritis, suggesting preventive properties of low dose and persistent alcohol consumption [12]. In humans, moderate alcohol consumption (defined as 5.0–9.9 g/day) has been described as a protective factor against RA [13,14]. A meta-analysis of nine observational studies found a protective effect of alcohol on the development of RA (OR 0.78, 95% CI 0.63–0.96), and even more pronounced in ACPA-positive RA (OR 0.52, 95% CI 0.36–0.76) [15].
3.2. Hazardous Factors
3.2.1. Salt Consumption
High salt consumption has been suggested a risk factor for the development of RA, in particular in smokers [16,17]. In a nested case-control study from Sweden, 386 patients with RA were compared to 1886 matched controls [18]. High sodium intake doubled the risk of RA among smokers (OR 2.26, 95% CI 1.06–4.81) but not in nonsmokers. A study by same authors compared ACPA positive RA vs ACPA negative RA, and after stratification by salt consumption, ever-smokers with medium to high sodium consumption had an increased risk of ACPA-positive RA (OR 1.7, 95% CI 1.2–2.4) [19]. In a Spanish cohort study of 18,555 individuals, 392 persons developed RA [20]. Persons with high daily sodium intake (>4.55 g) had a higher risk of developing RA adjusting by confounders, such as physical activity, hypertension, cardiovascular diseases, diabetes, cancer and smoking (OR 1.5, 95% CI 1.1–2.1). However, in this study nonsmokers had a higher association than ever smokers.
3.2.2. Sugar-Sweetened Beverages
In the Nurses’ Health Study (NHS), regular consumption of sugar-sweetened sodas, meaning >1 daily serving, significantly increased the risk of developing RA [21]. The association was independent of obesity and other socio-economic factors and tended to be stronger for late-onset RA (HR 2.64, 95% CI 1.56–4.46). No causal relation was found with diet soda or between sugar-sweetened soda and seronegative RA [21]. An interaction between sugar sweetened soda consumption and smoking was described.
3.3. Controversial Factors
Despite the large number of studies examining the role of individual foods, dietary factors, dietary supplements and beverages in the development of RA, many controversies remain.
3.3.1. Controversial Dietary Factors
- Mediterranean diet is characterized by high consumption of vegetables, legumes, olive oil, alcohol, and fish. This dietary pattern has been associated with a number of chronic diseases, including RA. In the NHS, 913 incident cases of RA were documented during 3,511,050 cumulative person-years of follow-up. After adjustment for several lifestyle and dietary variables, adherence to Mediterranean dietary pattern was not associated with increased risk of RA in women [22]. A nested case-control study in the Swedish EIRA cohort, analyzed data of 1721 patients with incident RA and 3667 controls and found that a Mediterranean diet was inversely associated with the risk of RA, particularly among men (OR 0.49, 95% 0.33–0.73) and with RF and ACPA positivity (OR 0.69, 95% CI 0.54–0.88 and 0.72, 95% IC 0.57–0.92, respectively) [23]. Recently, a French cohort, the E3N study identified 480 incident cases among 62,629 women and found that a Mediterranean diet was associated with a decreased risk of RA among ever smokers (HR 0.86, 95% CI 0.84–0.99) [24].
- Meat and dairy products consumption. During 12 years of follow-up in a Swedish cohort study (381,456 person-years), 368 individuals developed RA. No associations between the development of RA and the consumption of meat and meat products or total consumption of milk and dairy products were found (HR 1.08, 95% CI 0.77–1.53 and HR 1.09, 95% CI 0.76–1.55, respectively) [25]. Other analyses related to meat consumption are ongoing, such as a large prospective Danish cohort which aims to investigate the impact of fiber, red meat and processed meat on risk of late-onset chronic inflammatory diseases, including RA [26].
- Vegan diet. Vegan diet has been associated with reduced inflammation markers. In a randomized control trial, markers of inflammation relevant for RA were compared in individuals who were on vegan diet against individuals on meat-rich diet during four weeks. Vegan diet reduced neutrophils, monocytes and platelets related to branched-chain amino acids. These findings suggested a mode of action via the mTOR signaling pathway [27]. Another study reported improved signs and symptoms of RA with a gluten-free vegan diet and the effects on arthritis correlated with a reduction in antibodies to food antigens [28]. However, no protective effect of vegan diet on RA development has been demonstrated.
- Fasting has been reported as beneficial on RA disease activity [29,30,31]. A systematic review reported 31 studies examining the effects of fasting in patients with RA, but only four controlled studies analyzed follow-up data over at least three months after fasting, and showed statistically and clinically significant beneficial effects [32]. However, a protective effect of fasting on RA development has not yet been demonstrated.
- Elemental diet. A small study compared elemental diet with oral prednisolone for 2 weeks in RA patients. Elemental diet appeared as effective as a course of oral prednisolone 15 mg daily in improving subjective clinical parameters of RA [33]. In a smaller but longer study, patients with active RA were randomized either to a liquid elemental peptide-diet for four weeks or usual diet. Elemental diet produced transient but statistically significant improvement in pain and disability measured with Health Assessment Questionnaire (HAQ)-score [33]. Similarly, to the vegan diet and fasting, the role of elemental diet on RA development has not been explored.
3.3.2. Omega-3, Omega-6 Fatty Acids and Fish Consumption
A study compared polyunsaturated fatty acids (PUFA), including omega-3 and omega-6, between pre-RA individuals (measurement prior to disease onset) and matched controls from the European Prospective Investigation into Cancer and Nutrition (EPIC). Omega-6 PUFA levels of the erythrocyte were inversely associated with risk of RA, but no association were observed for omega-3 [34]. However, in a large cohort study from Sweden, in which 205 RA cases among 32,232 women were recorded, a similar analysis supported the hypothesis that omega-3 PUFA may play a role in RA development. In this study, long-lasting intake of omega-3 fatty acids higher than 0.21 g/day decreased the development of subsequent RA by 52% (95% CI 29–67%) [35], as well as a regular consumption of fish at least once per week (risk ratio (RR), 0.71, 95% CI 0.48–1.04). A meta-analysis examining the association between fish consumption and subsequent development of RA suggested a trend towards a protective effect with one to three portions of fish per week (RR 0.76, 95% CI 0.57–1.02) [36]. In a large prospective cohort study with 1080 incident RA cases in 3,863,909 person years of follow-up, no clear protective effect of omega-3 fatty acids intake on RA risk was found. However, authors reported a significant interaction between tobacco smoking and fish consumption. Frequent fish consumption among ever smokers women attenuated the strong association of smoking and RA, particularly in young-onset RA (diagnosed at 55 years of age or younger) [37].
3.3.3. Vitamin D
Vitamin D has immunomodulatory properties [38]. Low vitamin D levels may contribute to increased immune activation and may lead to RA development [39]. Several studies have reported vitamin D deficiency in RA patients, in up to 76% of patients and inverse association between vitamin D levels and disease activity [39,40,41]. However, the evidence is controversial as reverse causation may explain some of these findings and a beneficial effect of vitamin D supplementation on RA disease onset has not been demonstrated.
3.3.4. Coffee and Tea
In the large prospective NHS, the authors did not find a significant association between coffee, decaffeinated coffee, or tea consumption and the risk of RA in women [42]. Another prospective cohort study reported decaffeinated coffee consumption (≥4 cups by day) was associated with increased RA onset (RR 2.58, 95% CI 1.63–4.06), while tea consumption was inversely associated with RA (RR 0.39, 95% CI 0.16–0.97) [43].
3.3.5. Obesity
The role of obesity as a risk factor for RA is controversial, as it has been described as a risk factor in women, but as a protective factor in men [13,44,45]. Obese women (BMI ≥ 30.0 kg/m2) in the NHS tended to have an increased risk of RA, particularly those diagnosed at younger ages (HR 1.65, 95% CI 1.34–2.05) and in those obese during adolescence (HR 1.35, 95% CI 1.10–1.66) [13]. Similar results were found in Europe, with obesity increasing the risk for seronegative RA in women (HR 1.6, 95% CI 1.2–2.2) [44]. In men, the effect of obesity was less obvious and some studies have even described a reduced risk of RA in men [45]. From two large population-based health surveys (30,447 and 33,346 participants), excess weight or obesity in men was associated with a reduced risk of RA development (OR 0.33, 95%CI 0.14–0.76 and OR 0.60, 95%CI 0.39–0.91, respectively) [45].
4. Gut Microbiota in RA Development: Is Microbiota the Missing Link between Nutritional Factors and RA Onset?
4.1. Human Gut Microbiota in RA
Even though triggering of RA by micro-organisms has been speculated as early as 1896 [46], researchers only recently renewed interest in this hypothesis. Modern utilization of 16S and “shotgun” sequencing allowed comparison of healthy-control gut flora with microbiota from RA patients (Table 1). The subsequent findings have not been completely consistent across the literature, but a repeated feature has been the increase of Prevotella species in early RA [47,48,49,50], associated to a relative decrease in the Bacteroides genus [48,51]. The overrepresentation of Prevotella species is usually no longer found in established and treated RA patients [52,53,54]. In particular, Prevotella copri raised researcher’s interest, because of worsening arthritis when transferred in mice models [47,48]. To better distinguish causality from fortuitous association, Alpizar-Rodriguez et al. have studied gut microbiota in first-degree relatives of RA patients, who did not have the disease. They demonstrated an enrichment of Prevotella copri among individuals who displayed auto-immunity associated with RA or specific articular symptoms, compared to healthy seronegative subjects [8]. P. Wells et al. have further confirmed that healthy individuals genetically at risk for RA tended to host increased proportions of Prevotella species [55]. However, the specific expansion of P. copri has only been observed in pre-clinical stages or early untreated RA [55].

Table 1.
Up-to-date Human analysis of gut microbiota in rheumatoid arthritis (RA).
Some researchers have proposed a gut flora classification into “enterotypes” [56]. Even if this concept is still largely debated, an enterotype dominated by Prevotella species is often mentioned [57]. Given published association between RA genetic risk and Prevotella species, [55] we hypothesize that subjects genetically at risk for RA may simply be more susceptible to host gut flora enriched with Prevotella species. Subsequently, this “Prevotella-driven” microbiota would shift to a “pro-inflammatory” conformation, characterized by higher prevalence of P. copri, driving the progression to RA. Beyond mice models, the hypothesis of a pro-inflammatory role of P. copri is further supported by immune reactivity against P. copri peptides in RA subjects [58,59]. Nevertheless, additional immunological studies on human samples (blood, stool, or gut biopsies) are necessary to clarify the mechanism linking P. copri to the onset of RA. Finally, other bacterial species have been associated with human RA, such as Collinsella aerofaciens [53,54]. The latter also worsens arthritis in mice models [53,54], which underlines the fact that there may not be a single bacterial culprit, but probably a complex interplay between several microorganisms and host defenses.
4.2. Hypothesis Linking Gut Microbiota to RA
The intestinal epithelium layer and the underlying structures of the intestinal barrier protect the organism against invasion by micro-organisms and their toxins, while allowing the absorption of important quantities of nutrients and fluids. This selective permeability is modulated by various mechanisms, most of which remain to be understood [60].
Biological markers of the intestinal barrier function, such as zonulin, have been identified [61]. They allowed linking impaired intestinal barrier function to diseases, such as celiac disease [62] or depression [63]. Evidence for increased gut permeability in established RA is weak [30,64,65], and mostly biased because of non-steroidal anti-inflammatory drugs (NSAIDs) use. Indeed, NSAIDs are known to induce small bowel lesions, referred to as NSAID-induced enteropathy. These small bowel lesions include mucosal breaks [66], which are surprisingly prevalent in RA patients who chronically take NSAIDs [67]. Recent studies have demonstrated increased gut permeability both in early and in established RA patients, using zonulin as a serological marker [68]. This increase in gut-permeability has been demonstrated in about 1/3 pre-RA patients [68]. This finding was further supported by ileus mucosal biopsies of RA patients, manifesting lower expression of tight-junction proteins and increasing levels of immune cells in the lamina propria compared to healthy controls [68]. To evaluate potential bacterial translocation, Ayyappan et al. [69] compared serum from RA patients to healthy age-sex matched controls and assessed various serological antimicrobial response factors. Among others, these authors found significant elevation of sCD14, as previously reported [70,71], higher levels of LBP and Lysozyme [72], in line with the hypothesis of an increased intestinal permeability in these patients.
Beyond P. copri or C. aerofaciens isolates, the “whole” pre-RA or RA-derived microbiota also worsen arthritis when transferred to mice models [47,51,53]. For instance, Prevotella species have been found invading the mucus layer in irritable bowel disease models [73] or in human colonic cancer related studies [74,75]. As suggested by Palm et al. [76], the ability of particular strains to invade a normally sterile environment could be the common denominator of “auto-immunogenic” or “pro-inflammatory” microbes. Conversely, some species of Prevotella exert protective effects, attenuating arthritis in mice [77]. This illustrates that besides P. copri, other bacteria are capable of modulating intestinal epithelium integrity and have beneficial immunomodulatory effects.
More recently, Balakrishnan et al. [78] demonstrated on a humanized mouse model that gavage with some RA-associated bacteria (Eggerthella lenta or Collinsella aerofaciens) also increased the gut permeability, compared to gavage with non-associated bacterial species (Prevotella histicola or Bifidobacterium sp.). Their results confirmed that RA-associated bacteria are not just “inflammation-associated” taxa, but rather active contributors to the chronic inflammatory state in mice. Tajik et al. studied a mice model of collagen-induced-arthritis [68] and demonstrated that intestinal inflammation and increased gut permeability precede the onset of arthritis. Interestingly, mice-to-mice fecal microbiota transplantation also transferred the leaky barrier and mucosal inflammation [68]. Finally, they were able to attenuate the development of arthritis by preventively targeting intestinal barrier dysfunction, using butyrate or zonulin-inhibitors [68]. If future research confirms gut permeability as a relevant therapeutic target in RA, various interventions could be considered to modulate the epithelial gut barrier function (Table 2).

Table 2.
Main factors influencing intestinal barrier function.
The role of hormonal factors in the female over-representation of RA is not fully understood [79,80]. Beyond estrogen bioavailability, the increased female risk of RA could also be linked to gut microbiota. While to date, little is known about the contribution of sex-dependent differences in human microbiota in RA, sex-specific variations in gut microbiota are well established [81], and some intestinal bacteria can interact with sex-hormones [82]. Mice models suggested a contribution of the microbiota to the female sex-bias of auto-immune diseases, since female mice with arthritis had significantly less microbial diversity than male individuals and a different microbiota composition [83]. Moreover, non-obese diabetic female mice usually have 1.3 to 4.4 times higher incidence of type 1 diabetes (T1D), but germ-free mice loose this gender bias (female to male ratio 1.1–1.2) [82]. Transfer of gut microbiota, in mice models, from adult males to immature females, produced an elevation of testosterone and metabolomic changes resulting in a robust T1D protection [84].
4.3. The Gut-Joint Axis and Autoimmunity Onset
Hypotheses linking “pro-inflammatory” microbes to RA generally suppose an activation of autoreactive T-cells in the intestine, which later migrate to the joints and exacerbate inflammation. Such systemic spread of local immune cells have been shown by Teng et al. in the K/BxN mice model [103]. Indeed, arthritis triggered by segmented-filamentous-bacteria-containing feces was actually driven by the migration of T-follicular-helper cells from the Peyer-Patches to the peripheral lymphoid tissues; where auto-antibodies are produced [103].
Strong evidence for immune cell migration from the gut to the joints is still lacking in human RA. However, patient-derived gut immunoblasts have been known for a long time to strongly bind high-endothelial-venules of synovium [104,105]. This could partly explain the tendency for gut-joint axis in rheumatic disease. Interestingly, May et al. have shown in one enterogenic-SpA patient that a few expanded T-cell clones in the inflamed synovium were also overrepresented in the gut and in the peripheral blood [106]. The latter suggests that the systemic spread of mucosal expanded T-cell clones occurs in humans; but similar findings have yet to be established in RA.
Once the gut permeability has been impaired, several mechanisms are suspected to activate self-reactive T-cells, linking auto-immunity with microbial homeostasis:
- Molecular mimicry: A loss of tolerance against autoantigens because of structural similitude with a bacterial antigen. A classic example is rheumatic fever, which follows a group A Streptococcal infection. Several structural similarities with human proteins characterize Group A Streptococcus M5 protein; for instance, cardiac myosin, which will then also be targeted by antibodies generated against the Streptococcus [107,108]. A similar cross-reactional mechanism is also suspected to occur with intestinal bacteria in less acute contexts, such as lupus [109] or anti-phospholipid antibody syndrome [110]. After the auto-immune response is initiated, it can amplify itself by subsequently targeting other adjacent epitopes (“epitope spreading”).
- Neo-autoantigens generation: This represents a variant of the cross-reaction described above. The "cross-reactive" epitope, instead of being a bacterial structure, would be a human epitope modified by a microbe or by an inflammatory reaction of the host against the microbe (for instance: A citrullinated protein) [111].
- Activation of dual TCR cells: Some T-lymphocytes are known to have two T-receptors, with two different affinities. One of these receptors could have an affinity for a ’self’ structure’, but the T-cell would be activated via its other T-receptor, recognizing a bacterial epitope. The subsequent immune reaction against the microbial peptide could then collaterally target host structures [112,113].
- A-specific activation: Some bacterial antigens can a-specifically activate lymphocytes in a via innate immunity receptors (e.g., the "Toll Like Receptors"). If these lymphocytes have T receptors with affinity for host structures, their "accidental" activation by this non-specific mechanism could trigger autoimmune disorders [111,113].
- Antigen dissemination: A damaged intestinal mucosa allows bacterial components or even whole microorganisms to be translocated into the circulation («leaky gut» [114]). It is not uncommon to find these immunogenic structures in serum or synovial fluid [115]. This may contribute to local inflammation (i.e., if antigen has a tropism for synovium) or loss of tolerance [116].
4.4. Interaction between Diet and Microbiota in RA
Diet is a key factor able to shape gut microbiota. However, most of the interactions between specific diets and resulting gut-microbiota alterations remain poorly understood and published findings can be conflicting. The existing experiments aimed at shaping the gut microbiota using diet have focused mainly on the metabolic syndrome. Some findings might, however, be interesting for the field of RA. For instance, healthy subjects exhibit improved glucose metabolism after a diet supplemented with barley kernel-based bread (very rich in fibers) and display an expanded prevalence of Prevotella species, specially P copri [117]. This association of P. copri with a beneficial effect contradicts the observed ability of P copri to drive insulin resistance in type II diabetic patients and in mice models [118]. We also report above that Mediterranean diet seems to be protective for RA, while this diet is also known to increase the abundance of fiber degrading bacteria, such as specific Prevotella species [119]. A strain-level analysis could help make sense of this contradictory findings. Indeed, diets rich in fibers may select P. copri strains with higher potential for complex carbohydrate degradation, while P. copri strains associated with an omnivore diet showed a higher prevalence of genes related to branched-chain-amino-acid (BCAA) biosynthesis, and subjects harboring these genes in their microbiome had a higher BCAA urinary level [120] (circulating BCAA are associated with metabolic syndrome [121]). The latter underlines the necessity of a strain-level-identification approach to study association between P. copri and RA. Indeed, using shotgun sequencing, Scher et al. revealed that new onset RA patients do not exactly host the same strains as control patients [48].
The beneficial effect of dietary fibers is probably the best studied interaction linking diet with microbiota composition [122]. Beyond selective growth of beneficial strains, the production of short-chain-fatty-acids (SCFA) by the gut flora from the ingested plant fibers is also a key element. SCFA are the main energy source of colonocytes [123] and are currently studied for various other beneficial effects, such as regulating IgA secretion [124] or improving intestinal barrier function [68,100]. Based on these findings, Häger et al. conducted the first feasibility study of fiber supplementation in RA patients [102]. The preliminary results showed reduced serum zonulin and calprotectin levels at the end of the follow-up, as well as modest improvement in physical function and quality of life (n = 36 patients) [102]. The beneficial effect of fiber supplementation remains to be confirmed in larger studies and the long-term benefit of diets on the microbiota better understood. For instance, a whole-grain diet in obese individuals led to reduced body weight and inflammatory markers, but authors did not observe major changes in microbiota composition after eight weeks [125]. Similarly, Fragiadakis noticed significant changes in gut microbiota after low-carb or low-fat diets, but these changes tended to regress to baseline after one year of follow-up, despite maintenance of diet by participants [126]. This underlines that the gut microbiota might be more resilient to dietary changes; or that significant changes occur on the functional level, which are not identified by 16S-based methodology.
Beyond diets, specific nutrients may also impact the composition of the gut microbiota. Mice model with serum-transfer induced arthritis have increased intestinal permeability, plasma endotoxins, and a reduced intestinal concentration of omega3-derived cytokine called “resolvin” [99]. Subsequent inoculation with Porphyromonas gingivalis exacerbated these changes, while administration of the resolvin restored the barrier function and was associated with reduced joint inflammation and swelling [99]. This could elegantly explain the observed beneficial effect of omega-3 supplementation [127]. Salt consumption has also been linked with microbiota alterations, in both murine models and human subjects [128,129]. Notably, Wilck et al. have demonstrated that a high-salt diet in mice markedly shifts gut microbiota, in particular reducing the abundance of Lactobacillus species [129], and associates with an increase in splenic Th17-cells. A 14-day high-salt diet challenge in healthy male volunteers reproduced this Lactobacillus depletion and elevated circulating Th17 cells [125]. Salt consumption and relation with microbiota in the context of RA have never been studied, but it might be a relevant question, since Th17 cell involvement is a recurrent finding in mice models of arthritis [47,130,131,132]. How moderate alcohol consumption could be a protective factor for RA is not fully understood. However, Caslin et al. have recently studied the effect of moderate alcohol intake in a mice model of autoimmune encephalomyelitis [133]. Surprisingly, moderate alcohol intake induced greater disease remission in male mice; the latter seems to associate with sex-specific gut microbiota alteration [133]. Alcohol consumption has also been identified as a protective factor in human RA [134]. The benefit of ethanol in arthritis mice models is well-established, and was recently shown to result from a direct effect on T follicular helper cells [135]. A possible link to the gut microbiota has not been studied. Finally, probiotics such as Lactobacillus species are known to exert beneficial effects in arthritis mice models [136,137,138,139]. However, effectiveness in treating established RA is still unclear. Mohammed et al. have conducted a meta-analysis including 361 patients from 6 randomized trials (probiotics tested—Lactobacillus species, Bifidobacterium bifidum and Bacillus coagulans) [140]. They concluded that probiotic supplementation reduced reported levels of IL-6, but did not significantly change disease activity [140]. To our knowledge, no human trial has tested probiotics in a preventive or pre-RA context, nor assessed the effect of other potentially beneficial species such as Prevotella histicola [77]. Figure 2 shows putative dietary factors involved in RA development and their potential effects on the microbiota.
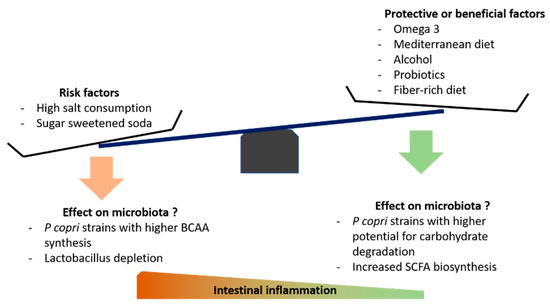
Figure 2.
Some putative dietary factors involved in the risk of RA development and their potential effects on the microbiota. “Beneficial factors” refers to interventions that have shown modest but positive effects in established RA. BCAA—Branched Chain Amino Acids. SCFA—Short Chain Fatty Acids. P copri—Prevotella copri.
5. Discussion and Future Perspectives
A number of clinical studies have examined the role of diet in RA development. However, due to study design and the struggle to differentiate diet from associated confounding factors (i.e., healthy lifestyle or socio-economic status), it is often problematic to establish whether the associations between diet and RA are causal or not. The rare interventional studies focusing on diet in RA are hampered by low sample size, poor methodology, short-term follow-up, and a focus on established RA populations. Other explanations for the contrasting results of the literature relating to diet may be the diverse dietary interventions, and the difficulty to measure the real consumption of individual foods. In clinical studies, a single type of food or nutrient may confer only a modest effect, difficult to demonstrate reliably, unless several dietary factors are grouped together and demonstrate a stronger combined effect [141,142]. To allow appropriate causal inferences, future research needs to overcome imperfect diet-compliance and limited follow-up of nutrition clinical trials. One possibility could be to improve existing “artificial guts” to study the impact of dietary changes. Edward G. et al. were able to study microbiota changes following a fat-only diet in such a setting [143]; experiments that could help understand how dietary patterns, or even fasting, could selectively promote or reduce the abundance of a given bacterial taxa. Finally, intestinal inflammation associated with specific ‘dysbiosis’ suggests new possible targets. A recent study suggested that supplementation with intestinal alkaline phosphatase, which in animal models can reduce lipo-polysaccharide production, may decrease intestinal inflammation [144]. There is only limited evidence about the effectiveness of such intervention in humans, but the administration of exogenous alkaline phosphatase to patients with ulcerative colitis was well tolerated [145].
It is simplistic and reductive to systematically refer to the “microbiota” to explain the unknown. However, given its role at the crossroads of many metabolic systems, the gut microbiota in relation to intestinal epithelial homeostasis is a promising key element to articulate various risk-factors interactions. As rightfully underlined by Harald Brüssow [146], the whole “microbiome” research field has partly become entangled in terminological and logical issues. In particular, the term ”dysbiosis” is pointed out as spurious or misleading, since it lacks proper consensual definition. This vague concept of “an imbalanced” gut flora leads to probably spurious conclusions, since case-control studies implicitly label any microbiota differences observed in diseased individuals, compared to healthy controls, as “dysbiosis”. We should rather postulate that a variety of bacterial species could have the ability to promote auto-immunity, as they interact with gut mucosa and local lymphoid organs. P. copri and Collinsella aerofaciens gained credibility as potential “pathobionts”, since worsening arthritis when transferred to mice models. Adequate mice studies should, for each identified bacterial taxa, confirm the “arthritogenic” potential. Ideally, these pro-inflammatory pathobionts should also be studied on human gut mucosa samples. Selective fluorescent marking could help confirm the extent to which they invade the mucus layer or submucosa.
We have listed several individual foods, dietary patterns, dietary supplements, and beverages that have been associated with RA development in clinical studies. An appealing hypothesis would be that these dietary factors are effective by modifying the gut microbiota and modulating the intestinal barrier integrity, thus changing the antigenic load and subsequent immune dysregulation. While the evidence suggesting a role of nutritional factors in RA disease progression and outcomes is increasing, randomized trials with “anti-inflammatory” diets in established RA have only shown modest effects [147,148,149]. Bustamante et al. are attempting an approach that would optimize the nutritional strategy based on evidence [150]. Dietary counseling in conjunction with disease modifying anti-rheumatic drugs could become part of the management of established RA. Other targeted preventive strategies could also be proposed to manipulate microbiota, such as male to female FMT, or supplementation with a combination of nutrients and prebiotics, to obtain a synergistic effect. Before a “preventive” diet for RA can gain acceptance, clinical trials during the pre-clinical stages of RA are needed to establish if lifestyle-related interventions are ultimately able to prevent or delay the onset of RA in high risk individuals.
6. Conclusions
Although published literature is still limited, interest in the role of diet and microbiota in the development of RA is growing. Several studies have recently suggested that the use of omega-3 and moderate alcohol consumption may have a protective effect on RA development, particularly among smokers or individuals at high risk. We postulate that the microbiota and intestinal barrier homeostasis may be a missing link between the various nutritional factors and the development of RA. Modification of microbiota using dietary interventions and focusing on the improvement of the intestinal barrier function may become an important part of the future “preventive” nutritional strategies. Longitudinal cohort studies during the preclinical phases of RA, studying dietary patterns and microbiota changes concurrently, are needed to better understand the causality of these associations. Clinical trials in individuals at risk of RA need to be conducted in order to determine the feasibility and the efficacy of such interventions.
Author Contributions
All authors have contributed with research of articles, writing, review and editing. D.A.-R., B.G. and A.F. planned and conceptualized the manuscript. D.A.-R. and B.G. produced the initial draft, which was reviewed and edited by A.F. Figures were produced by D.A.-R. and B.G. and edited by A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Swiss National Science Foundation (Grant 320030_192471/1).
Acknowledgments
We thank Jorge Alberto Barragan-Garfias, for his help with research of articles.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Mankia, K.; Emery, P. Preclinical Rheumatoid Arthritis: Progress Toward Prevention. Arthritis Rheumatol. 2016, 68, 779–788. [Google Scholar] [CrossRef]
- Svendsen, A.J.; Holm, N.V.; Kyvik, K.; Petersen, P.H.; Junker, P. Relative importance of genetic effects in rheumatoid arthritis: Historical cohort study of Danish nationwide twin population. BMJ 2002, 324, 264–266. [Google Scholar] [CrossRef]
- Hensvold, A.H.; Magnusson, P.K.E.; Joshua, V.; Hansson, M.; Israelsson, L.; Ferreira, R.; Jakobsson, P.J.; Holmdahl, R.; Hammarström, L.; Malmström, V.; et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: An epidemiological investigation in twins. Ann. Rheum. Dis. 2015, 74, 375–380. [Google Scholar] [CrossRef]
- Sugiyama, D.; Nishimura, K.; Tamaki, K.; Tsuji, G.; Nakazawa, T.; Morinobu, A.; Kumagai, S. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2010, 69, 70–81. [Google Scholar] [CrossRef]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78. [Google Scholar] [CrossRef]
- Jiang, X.; Alfredsson, L. Modifiable environmental exposure and risk of rheumatoid arthritis—current evidence from genetic studies. Arthritis Res. Ther. 2020, 22, 154. [Google Scholar] [CrossRef]
- Gan, R.W.; Young, K.A.; Zerbe, G.O.; Demoruelle, M.K.; Weisman, M.H.; Buckner, J.H.; Gregersen, P.K.; Mikuls, T.R.; O’Dell, J.R.; Keating, R.M.; et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: A nested case-control study. Rheumatology (Oxford) 2016, 55, 367–376. [Google Scholar] [CrossRef]
- Gan, R.W.; Demoruelle, M.K.; Deane, K.D.; Weisman, M.H.; Buckner, J.H.; Gregersen, P.K.; Mikuls, T.R.; O’Dell, J.R.; Keating, R.M.; Fingerlin, T.E.; et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 147–152. [Google Scholar] [CrossRef]
- Jonsson, I.-M.; Verdrengh, M.; Brisslert, M.; Lindblad, S.; Bokarewa, M.; Islander, U.; Carlsten, H.; Ohlsson, C.; Nandakumar, K.S.; Holmdahl, R.; et al. Ethanol prevents development of destructive arthritis. Proc. Natl. Acad. Sci. USA 2007, 104, 258–263. [Google Scholar] [CrossRef]
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef]
- Källberg, H.; Jacobsen, S.; Bengtsson, C.; Pedersen, M.; Padyukov, L.; Garred, P.; Frisch, M.; Karlson, E.W.; Klareskog, L.; Alfredsson, L. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: Results from two Scandinavian case-control studies. Ann. Rheum. Dis. 2009, 68, 222–227. [Google Scholar] [CrossRef]
- Scott, I.C.; Tan, R.; Stahl, D.; Steer, S.; Lewis, C.M.; Cope, A.P. The protective effect of alcohol on developing rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology (Oxford) 2013, 52, 856–867. [Google Scholar] [CrossRef]
- Sigaux, J.; Semerano, L.; Favre, G.; Bessis, N.; Boissier, M.-C. Salt, inflammatory joint disease, and autoimmunity. Joint Bone Spine 2018, 85, 411–416. [Google Scholar] [CrossRef]
- Sharif, K.; Amital, H.; Shoenfeld, Y. The role of dietary sodium in autoimmune diseases: The salty truth. Autoimmun. Rev. 2018, 17, 1069–1073. [Google Scholar] [CrossRef]
- Sundström, B.; Johansson, I.; Rantapää-Dahlqvist, S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: Results from a nested case-control study. Rheumatology (Oxford) 2015, 54, 487–493. [Google Scholar] [CrossRef]
- Jiang, X.; Sundström, B.; Alfredsson, L.; Klareskog, L.; Rantapää-Dahlqvist, S.; Bengtsson, C. High sodium chloride consumption enhances the effects of smoking but does not interact with SGK1 polymorphisms in the development of ACPA-positive status in patients with RA. Ann. Rheum. Dis. 2016, 75, 943–946. [Google Scholar] [CrossRef]
- Salgado, E.; Bes-Rastrollo, M.; de Irala, J.; Carmona, L.; Gómez-Reino, J.J. High Sodium Intake Is Associated With Self-Reported Rheumatoid Arthritis: A Cross Sectional and Case Control Analysis Within the SUN Cohort. Medicine 2015, 94, e924. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Hu, F.B.; Karlson, E.W.; Lu, B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res. 2015, 67, 597–606. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D.; EIRA study group. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar] [CrossRef]
- Nguyen, Y.; Salliot, C.; Gelot, A.; Gambaretti, J.; Mariette, X.; Boutron-Ruault, M.-C.; Seror, R. Mediterranean diet and risk of rheumatoid arthritis: Findings from the French E3N-EPIC cohort study. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Sundström, B.; Ljung, L.; Di Giuseppe, D. Consumption of Meat and Dairy Products Is Not Associated with the Risk for Rheumatoid Arthritis among Women: A Population-Based Cohort Study. Nutrients 2019, 11, 2825. [Google Scholar] [CrossRef]
- Rasmussen, N.F.; Rubin, K.H.; Stougaard, M.; Tjønneland, A.; Stenager, E.; Lund Hetland, M.; Glintborg, B.; Bygum, A.; Andersen, V. Impact of red meat, processed meat and fibre intake on risk of late-onset chronic inflammatory diseases: Prospective cohort study on lifestyle factors using the Danish ’Diet, Cancer and Health’ cohort (PROCID-DCH): Protocol. BMJ Open 2019, 9, e024555. [Google Scholar] [CrossRef]
- Lederer, A.-K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids—A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef]
- Hafström, I.; Ringertz, B.; Spångberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford) 2001, 40, 1175–1179. [Google Scholar]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Sundqvist, T.; Lindström, F.; Magnusson, K.-E.; Sköldstam, L.; Stjernström, I.; Tagesson, C. Influence of Fasting on Intestinal Permeability and Disease Activity in Patients with Rheumatoid Arthritis. Scand. J. Rheumatol. 1982, 11, 33–38. [Google Scholar] [CrossRef]
- Sköldstam, L.; Larsson, L.; Lindström, F.D. Effects of Fasting and Lactovegetarian Diet on Rheumatoid Arthritis. Scand. J. Rheumatol. 1979, 8, 249–255. [Google Scholar] [CrossRef]
- Müller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Podas, T.; Nightingale, J.M.D.; Oldham, R.; Roy, S.; Sheehan, N.J.; Mayberry, J.F. Is rheumatoid arthritis a disease that starts in the intestine? A pilot study comparing an elemental diet with oral prednisolone. Postgrad. Med. J. 2007, 83, 128–131. [Google Scholar] [CrossRef]
- De Pablo, P.; Romaguera, D.; Fisk, H.L.; Calder, P.C.; Quirke, A.-M.; Cartwright, A.J.; Panico, S.; Mattiello, A.; Gavrila, D.; Navarro, C.; et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case-control study. Ann. Rheum. Dis. 2018, 77, 981–987. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Wallin, A.; Bottai, M.; Askling, J.; Wolk, A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: A prospective cohort study of women. Ann. Rheum. Dis. 2014, 73, 1949–1953. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Crippa, A.; Orsini, N.; Wolk, A. Fish consumption and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res. Ther. 2014, 16, 446. [Google Scholar] [CrossRef]
- Sparks, J.A.; O’Reilly, É.J.; Barbhaiya, M.; Tedeschi, S.K.; Malspeis, S.; Lu, B.; Willett, W.C.; Costenbader, K.H.; Karlson, E.W. Association of fish intake and smoking with risk of rheumatoid arthritis and age of onset: A prospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 2. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Watad, A.; Neumann, S.G.; Simon, M.; Brown, S.B.; Abu Much, A.; Harari, A.; Tiosano, S.; Amital, H.; Shoenfeld, Y. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr. Opin. Rheumatol. 2017, 29, 378–388. [Google Scholar] [CrossRef]
- Raczkiewicz, A.; Kisiel, B.; Kulig, M.; Tłustochowicz, W. Vitamin D status and its association with quality of life, physical activity, and disease activity in rheumatoid arthritis patients. J. Clin. Rheumatol. 2015, 21, 126–130. [Google Scholar] [CrossRef]
- Buondonno, I.; Rovera, G.; Sassi, F.; Rigoni, M.M.; Lomater, C.; Parisi, S.; Pellerito, R.; Isaia, G.C.; D’Amelio, P. Vitamin D and immunomodulation in early rheumatoid arthritis: A randomized double-blind placebo-controlled study. PLoS ONE 2017, 12, e0178463. [Google Scholar] [CrossRef] [PubMed]
- Karlson, E.W.; Mandl, L.A.; Aweh, G.N.; Grodstein, F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Merlino, L.; Mudano, A.S.; Burma, M.; Folsom, A.R.; Saag, K.G. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2002, 46, 83–91. [Google Scholar] [CrossRef]
- Wesley, A.; Bengtsson, C.; Elkan, A.C.; Klareskog, L.; Alfredsson, L.; Wedrén, S.; Epidemiological Investigation of Rheumatoid Arthritis Study Group. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: Results from a population-based case-control study. Arthritis Care Res. 2013, 65, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Turesson, C.; Bergström, U.; Pikwer, M.; Nilsson, J.-Å.; Jacobsson, L.T.H. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology (Oxford) 2016, 55, 307–314. [Google Scholar] [CrossRef]
- Bannatyne, G.A.; Wohlmann, A.S. Rheumatoid Arthritis: Its Clinical History, Etiology, and Pathology. Lancet 1896, 147, 1120–1125. [Google Scholar] [CrossRef][Green Version]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. ELife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.-W.; You, H.J.; Park, S.-J.; Lee, J.; Ju, J.H.; Park, M.S.; Jin, H.; Cho, M.L.; Kwon, B.; et al. Gut Microbial Composition and Function Are Altered in Patients with Early Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 693. [Google Scholar] [CrossRef]
- Kishikawa, T.; Maeda, Y.; Nii, T.; Motooka, D.; Matsumoto, Y.; Matsushita, M.; Matsuoka, H.; Yoshimura, M.; Kawada, S.; Teshigawara, S.; et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis. 2020, 79, 103–111. [Google Scholar] [CrossRef]
- Tong, Y.; Tang, H.; Li, Y.; Su, L.C.; Wu, Y.; Bozec, A.; Zaiss, M.; Qing, P.; Zhao, H.; Tan, C.; et al. Gut Microbiota Dysbiosis in the High-Risk Individual for Ra Triggers the Mucosal Immunity Perturbation and Promotes Rheumatoid Arthritis Development. Research Square 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Mena-Vázquez, N.; Ruiz-Limón, P.; Moreno-Indias, I.; Manrique-Arija, S.; Tinahones, F.J.; Fernández-Nebro, A. Expansion of Rare and Harmful Lineages is Associated with Established Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.M.; Adebayo, A.S.; Bowyer, R.C.E.; Freidin, M.B.; Finckh, A.; Strowig, T.; Lesker, T.R.; Alpizar-Rodriguez, D.; Gilbert, B.; Kirkham, B.; et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: A cross-sectional study. Lancet Rheumatol. 2020, 2, e418–e427. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.L.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Investig. 2017, 127, 2946–2956. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Heyman, M.; Abed, J.; Lebreton, C.; Cerf-Bensussan, N. Intestinal permeability in coeliac disease: Insight into mechanisms and relevance to pathogenesis. Gut 2012, 61, 1355–1364. [Google Scholar] [CrossRef]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Gibson, R.A.; Brooks, P.M. Abnormal bowel permeability in ankylosing spondylitis and rheumatoid arthritis. J. Rheumatol. 1985, 12, 299–305. [Google Scholar] [PubMed]
- Fagiolo, U.; Paganelli, R.; Ossi, E.; Quinti, I.; Cancian, M.; D’Offizi, G.P.; Fiocco, U. Intestinal Permeability and Antigen Absorption in Rheumatoid Arthritis. Int Arch. Allergy Appl. Immunol. 1989, 89, 98–102. [Google Scholar] [CrossRef]
- Maiden, L.; Thjodleifsson, B.; Theodors, A.; Gonzalez, J.; Bjarnason, I. A Quantitative Analysis of NSAID-Induced Small Bowel Pathology by Capsule Enteroscopy. Gastroenterology 2005, 128, 1172–1178. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanigawa, T.; Nadatani, Y.; Nagami, Y.; Sugimori, S.; Okazaki, H.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; et al. Risk factors for severe nonsteroidal anti-inflammatory drug-induced small intestinal damage. Dig. Liver Dis. 2013, 45, 390–395. [Google Scholar] [CrossRef]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Ayyappan, P.; Harms, R.Z.; Seifert, J.A.; Bemis, E.A.; Feser, M.L.; Deane, K.D.; Demoruelle, M.K.; Mikuls, T.R.; Holers, V.M.; Sarvetnick, N.E. Heightened Levels of Antimicrobial Response Factors in Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 427. [Google Scholar] [CrossRef]
- Yu, S.; Nakashima, N.; Xu, B.H.; Matsuda, T.; Izumihara, A.; Sunahara, N.; Nakamura, T.; Tsukano, M.; Matsuyama, T. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: In the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumatol. Int. 1998, 17, 237–243. [Google Scholar] [CrossRef]
- Mikuls, T.R.; LeVan, T.D.; Sayles, H.; Yu, F.; Caplan, L.; Cannon, G.W.; Kerr, G.S.; Reimold, A.M.; Johnson, D.S.; Thiele, G.M. Soluble CD14 and CD14 Polymorphisms in Rheumatoid Arthritis. J. Rheumatol. 2011, 38, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Torsteinsdóttir, I.; Håkansson, L.; Hällgren, R.; Gudbjörnsson, B.; Arvidson, N.-G.; Venge, P. Serum lysozyme: A potential marker of monocyte/macrophage activity in rheumatoid arthritis. Rheumatology (Oxford) 1999, 38, 1249–1254. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Tran van Nhieu, J.; Furet, J.P. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A Coating Identifies Colitogenic Bacteria in Inflammatory Bowel Disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A.; Luckey, D.H.; Jeraldo, P.R.; Lamba, A.; Patel, R.; Luthra, H.S.; Mangalam, A.; Taneja, V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016, 68, 2878–2888. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Luckey, D.; Taneja, V. Autoimmunity-Associated Gut Commensals Modulate Gut Permeability and Immunity in Humanized Mice. Mil. Med. 2019, 184, 529–536. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Mueller, R.B.; Möller, B.; Dudler, J.; Ciurea, A.; Zufferey, P.; Kyburz, D.; Walker, U.A.; von Mühlenen, I.; Roux-Lombard, P.; et al. Female hormonal factors and the development of anti-citrullinated protein antibodies in women at risk of rheumatoid arthritis. Rheumatology (Oxford) 2017, 56, 1579–1585. [Google Scholar] [CrossRef]
- Alpízar-Rodríguez, D.; Pluchino, N.; Canny, G.; Gabay, C.; Finckh, A. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatology (Oxford) 2017, 56, 1254–1263. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Wood, R.; Ritchlin, C.T.; Schwarz, E.; Rahimi, H. Sexually Dimorphic Dysbiosis of Gut Microbiota in Tumor Necrosis Factor Transgenic Mice with Inflammatory-Erosive Arthritis. ACR Meeting Abstracts. Available online: https://acrabstracts.org/abstract/sexually-dimorphic-dysbiosis-of-gut-microbiota-in-tumor-necrosis-factor-transgenic-mice-with-inflammatory-erosive-arthritis/ (accessed on 19 July 2020).
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McKoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Tanigawa, T.; Watanabe, T.; Nakata, A.; Sugimura, N.; Itani, S.; Higashimori, A.; Nadatani, Y.; Otani, K.; Taira, K.; et al. Involvement of gliadin, a component of wheat gluten, in increased intestinal permeability leading to non-steroidal anti-inflammatory drug-induced small-intestinal damage. PLoS ONE 2019, 14, e0211436. [Google Scholar] [CrossRef]
- Demaude, J.; Salvador-Cartier, C.; Fioramonti, J.; Ferrier, L.; Bueno, L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: Implications for delayed epithelial barrier dysfunction. Gut 2006, 55, 655–661. [Google Scholar] [CrossRef]
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. (Lond) 2010, 7, 15. [Google Scholar] [CrossRef]
- Kavanagh, K.; Wylie, A.T.; Tucker, K.L.; Hamp, T.J.; Gharaibeh, R.Z.; Fodor, A.A.; Cullen, J.M. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am. J. Clin. Nutr. 2013, 98, 349–357. [Google Scholar] [CrossRef]
- Bergheim, I.; Weber, S.; Vos, M.; Krämer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Hamada, K.; Shitara, Y.; Sekine, S.; Horie, T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother. Pharmacol. 2010, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, C.; Mahoney, D. Low-Dose Methotrexate-Induced Changes in Intestinal Permeability Determined by Polyethylene Glycol Polymers. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lagishetty, V.; Misharin, A.V.; Liu, N.Q.; Lisse, T.S.; Chun, R.F.; Ouyang, Y.; McLachlan, S.M.; Adams, J.S.; Hewison, M. Vitamin D Deficiency in Mice Impairs Colonic Antibacterial Activity and Predisposes to Colitis. Endocrinology 2010, 151, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Salim Rasoel, S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef]
- Pagnini, C.; Saeed, R.; Bamias, G.; Arseneau, K.O.; Pizarro, T.T.; Cominelli, F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 454–459. [Google Scholar] [CrossRef]
- Noth, R.; Stüber, E.; Häsler, R.; Nikolaus, S.; Kühbacher, T.; Hampe, J.; Bewig, B.; Schreiber, S.; Arlt, A. Anti-TNF-α antibodies improve intestinal barrier function in Crohn’s disease. J. Crohns Colitis 2012, 6, 464–469. [Google Scholar] [CrossRef]
- Graham, W.V.; He, W.; Marchiando, A.M.; Zha, J.; Singh, G.; Li, H.-S.; Biswas, A.; Ong, M.L.D.M.; Jiang, Z.H.; Choi, W.; et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nature Med. 2019, 25, 690–700. [Google Scholar] [CrossRef]
- Flak, M.B.; Colas, R.A.; Muñoz-Atienza, E.; Curtis, M.A.; Dalli, J.; Pitzalis, C. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight 2019, 4, e125191. [Google Scholar] [CrossRef]
- Venkatraman, A.; Ramakrishna, B.S.; Pulimood, A.B.; Patra, S. Increased Permeability in Dextran Sulphate Colitis in Rats: Time Course of Development and Effect of Butyrate. Scand. J. Gastroenterol. 2000, 35, 1053–1059. [Google Scholar] [CrossRef]
- Rutkowska, M.; Fereniec-Gołębiewska, L. ACEA (arachidonyl-2-chloroethylamide), the selective cannabinoid CB1 receptor agonist, protects against aspirin-induced gastric ulceration. Pharmazie 2006, 61, 341–342. [Google Scholar]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Klinger, C.N.; Felix, K.M.; Bradley, C.P.; Wu, E.; Tran, N.L.; Umesaki, Y.; Wu, H.J. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 2016, 44, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Andrew, D.E.; Butcher, E.C.; Jalkanen, S. Dual Binding Capacity of Mucosal Immunoblasts to Mucosal and Synovial Endothellum in Humans: Dissection of the Molecular Mechanisms. J. Exp. Med. 1995, 181, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Rajala, P.; Jalkanen, S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J. Clin. Investig. 1997, 99, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- May, E.; Märker-Hermann, E.; Wittig, B.M.; Zeitz, M.; Meyer zum Büschenfelde, K.; Duchmann, R. Identical T-cell expansions in the colon mucosa and the synovium of a patient with enterogenic spondyloarthropathy. Gastroenterology 2000, 119, 1745–1755. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Faé, K.C.; da Silva, D.D.; Oshiro, S.E.; Tanaka, A.C.; Pomerantzeff, P.M.A.; Douay, C.; Charron, D.; Toubert, A.; Cunningham, M.W.; Kalil, J.; et al. Mimicry in Recognition of Cardiac Myosin Peptides by Heart-Intralesional T Cell Clones from Rheumatic Heart Disease. J. Immunol. 2006, 176, 5662–5670. [Google Scholar] [CrossRef]
- Greiling, T.M.; Dehner, C.; Chen, X.; Hughes, K.; Iñiguez, A.J.; Boccitto, M.; Ruiz, D.Z.; Renfroe, S.C.; Vieira, S.M.; Ruff, W.E.; et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018, 10, eaan2306. [Google Scholar] [CrossRef]
- Ruff, W.E.; Dehner, C.; Kim, W.J.; Pagovich, O.; Aguiar, C.L.; Yu, A.T.; Roth, A.S.; Vieira, S.M.; Kriegel, C.; Adeniyi, O.; et al. Pathogenic Autoreactive T and B Cells Cross-React with Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe 2019, 26, 100–113. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Yue, Y.; Zhang, Z.; Su, K. Microbial Infection and Rheumatoid Arthritis. J. Clin. Cell Immunol. 2013, 4, 174. [Google Scholar]
- Bradley, C.P.; Teng, F.; Felix, K.M.; Sano, T.; Naskar, D.; Block, K.E.; Huang, H.; Knox, K.S.; Littman, D.R.; Wu, H.J. Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell Host Microbe 2017, 22, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Ost, K.S.; Round, J.L. Communication Between the Microbiota and Mammalian Immunity. Annu. Rev. Microbiol. 2018, 72, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef]
- Reichert, S.; Haffner, M.; Keyßer, G.; Schäfer, C.; Stein, J.M.; Schaller, H.-G.; Wienke, A.; Strauss, H.; Heide, S.; Schulz, S. Detection of oral bacterial DNA in synovial fluid. J. Clin. Periodontol. 2013, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; Storia, A.L.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Tett, A.; Tarallo, S.; Naccarati, A.; De Angelis, M.; Neviani, E.; Cocolin, L.; Gobbetti, M.; Segata, N.; et al. Distinct Genetic and Functional Traits of Human Intestinal Prevotella copri Strains Are Associated with Different Habitual Diets. Cell Host Microbe 2019, 25, 444–453. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Fragiadakis, G.K.; Wastyk, H.C.; Robinson, J.L.; Sonnenburg, E.D.; Sonnenburg, J.L.; Gardner, C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020, 111, 1127–1136. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- De Aquino, S.G.; Abdollahi-Roodsaz, S.; Koenders, M.I.; van de Loo, F.A.J.; Pruijn, G.J.M.; Marijnissen, R.J.; Walgreen, B.; Helsen, M.M.; van den Bersselaar, L.A.; de Molon, R.S.; et al. Periodontal Pathogens Directly Promote Autoimmune Experimental Arthritis by Inducing a TLR2- and IL-1-Driven Th17 Response. J. Immunol. 2014, 192, 4103–4111. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.B.; Koenders, M.I.; Devesa, I.; Roelofs, M.F.; Radstake, T.R.D.J.; Heuvelmans-Jacobs, M.; Akira, S.; Nicklin, M.J.; Ribeiro-Dias, F.; et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Investig. 2008, 118, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Caslin, B.; Maguire, C.; Karmakar, A.; Mohler, K.; Wylie, D.; Melamed, E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc. Natl. Acad. Sci. USA 2019, 116, 25808–25815. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.J.; Gabay, C.; Scherer, A.; Finckh, A.; Swiss Clinical Quality Management Project in Rheumatoid Arthritis. The effect of alcohol on radiographic progression in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Azizov, V.; Dietel, K.; Steffen, F.; Dürholz, K.; Meidenbauer, J.; Lucas, S.; Frech, M.; Omata, Y.; Tajik, N.; Knipfer, L.; et al. Ethanol consumption inhibits TFH cell responses and the development of autoimmune arthritis. Nat. Commun. 2020, 11, 1998. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Endo-Tanaka, K.; Yokokura, T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998, 63, 635–644. [Google Scholar] [CrossRef]
- So, J.-S.; Kwon, H.-K.; Lee, C.-G.; Yi, H.-J.; Park, J.-A.; Lim, S.-Y.; Hwang, K.C.; Jeon, Y.H.; Im, S.H. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol. Immunol. 2008, 45, 2690–2699. [Google Scholar] [CrossRef]
- Baharav, E.; Mor, F.; Halpern, M.; Weinberger, A. Lactobacillus GG Bacteria Ameliorate Arthritis in Lewis Rats. J. Nutr. 2004, 134, 1964–1999. [Google Scholar] [CrossRef]
- Rodríguez-Cabezas, M.E.; Fisac, F.; Bailon, E.; Comalada, M.; Camuesco, D.; Xaus, J.; Concha, A.; Talavera, P.; Nieto, A.; Zarzuelo, A.; et al. Lactobacillus fermentum exerts a beneficial effect in an experimental model of rheumatoid arthritis in mice. Proc. Nutr. Soc. 2008, 67. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Khalil A., M.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, S.F.; Tjønneland, A.M.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J. Rheumatol. 2005, 32, 1249–1252. [Google Scholar]
- Pattison, D.J.; Harrison, R.A.; Symmons, D.P.M. The role of diet in susceptibility to rheumatoid arthritis: A systematic review. J. Rheumatol. 2004, 31, 1310–1319. [Google Scholar] [PubMed]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525–e01528. [Google Scholar] [CrossRef] [PubMed]
- Hamarneh, S.R.; Kim, B.-M.; Kaliannan, K.; Morrison, S.A.; Tantillo, T.J.; Tao, Q.; Mohamed, M.M.R.; Ramirez, J.M.; Karas, A.; Liu, W.; et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig. Dis. Sci. 2017, 62, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Drastich, P.; Konecny, M.; Gionchetti, P.; Urban, O.; Cantoni, F.; Bortlik, M.; Duricova, D.; Bulitta, M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel. Dis. 2010, 16, 1180–1186. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Cutolo, M.; Nikiphorou, E. Don’t neglect nutrition in rheumatoid arthritis! RMD Open 2018, 4, e000591. [Google Scholar] [CrossRef]
- Dey, M.; Cutolo, M.; Nikiphorou, E. Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients 2020, 12, 3155. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)—a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Agustín-Perez, M.; Cedola, F.; Coras, R.; Narasimhan, R.; Golshan, S.; Guma, M. Design of an anti-inflammatory diet (ITIS diet) for patients with rheumatoid arthritis. Contemp. Clin. Trials Commun. 2020, 17, 100524. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).