Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Anthropometric Determinations
2.4. PROP Taster Status Classification
2.5. Taste Sensitivity Measurements
2.6. Oleic Acid DetectionThreshold Assessments
2.7. Olfactory Function Assessments
2.8. Molecular Analysis
2.9. Statistical Analyses
3. Results
3.1. Participants’ Demographic, Clinical and Genetic Features
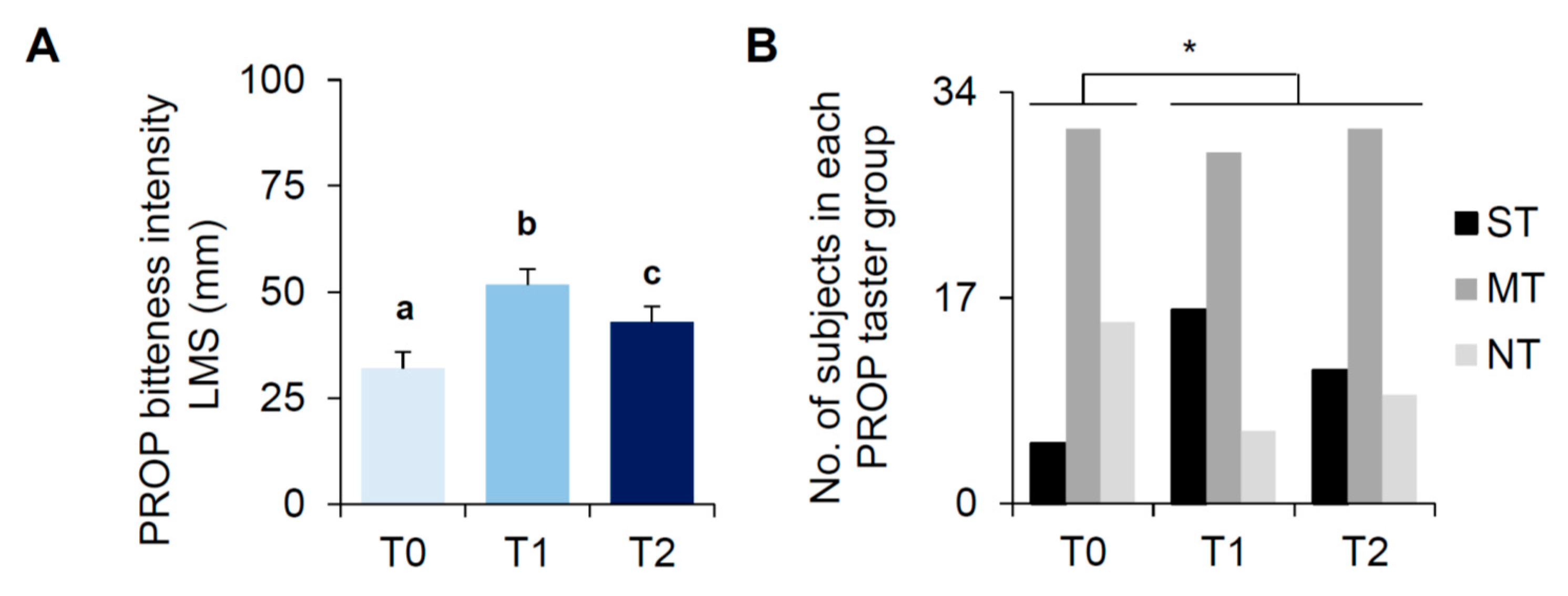
3.2. Bariatric Surgery-Induced Effects on PROP Tasting
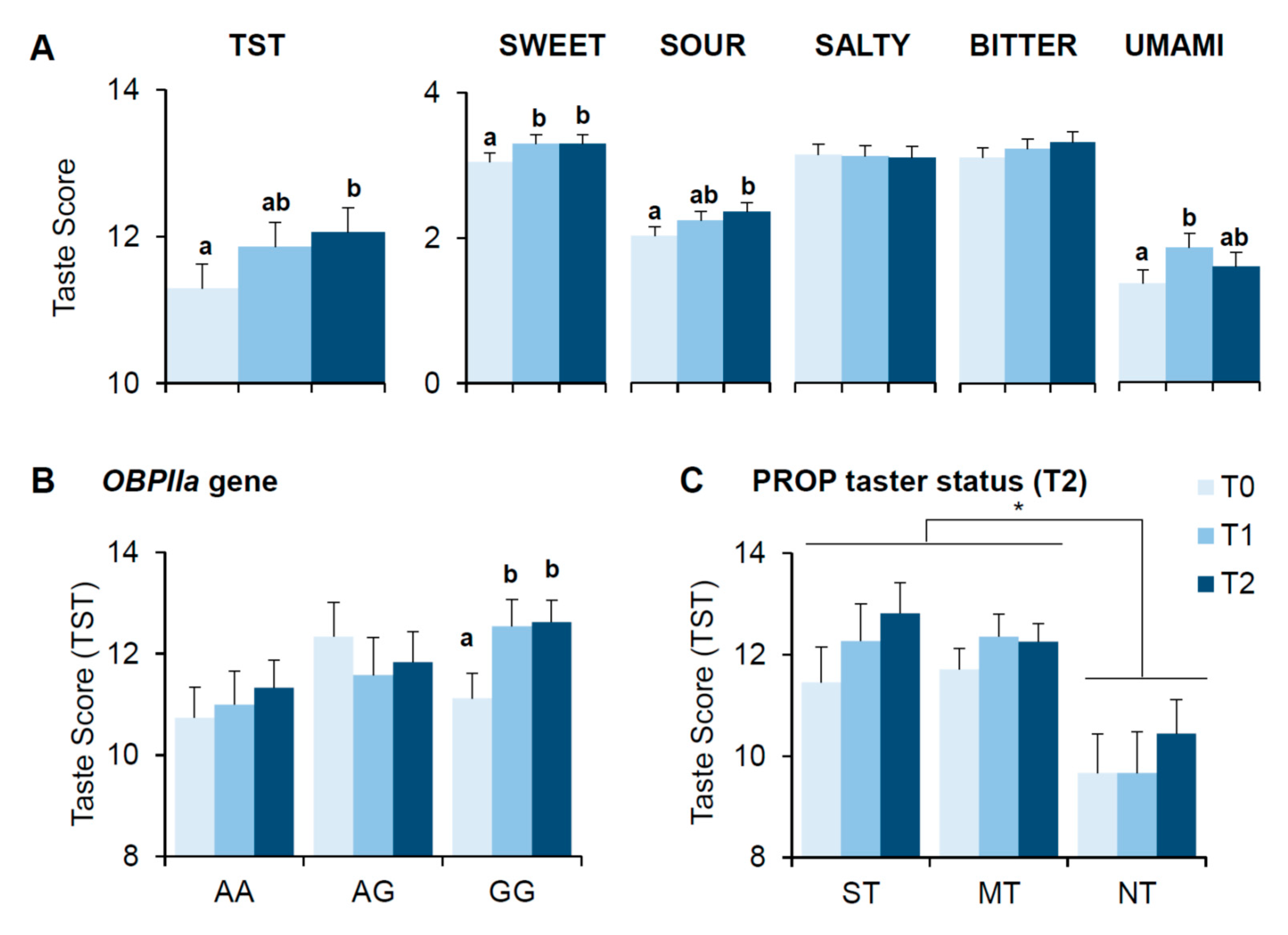
3.3. Bariatric Surgery-Induced Effects on Scores of Taste Perception
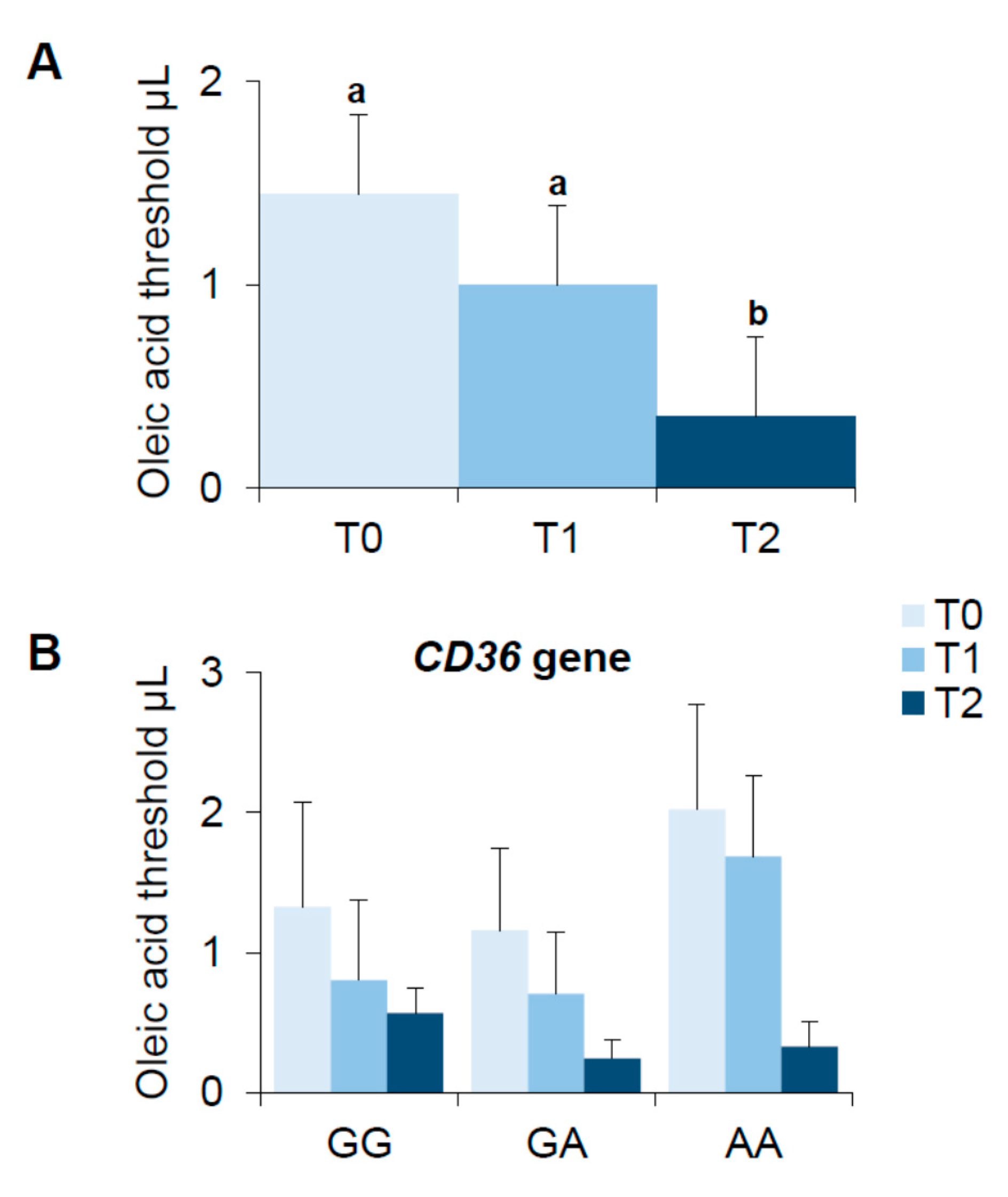
3.4. Bariatric Surgery-Induced Effect on Oleic Acid Detection Thresholds
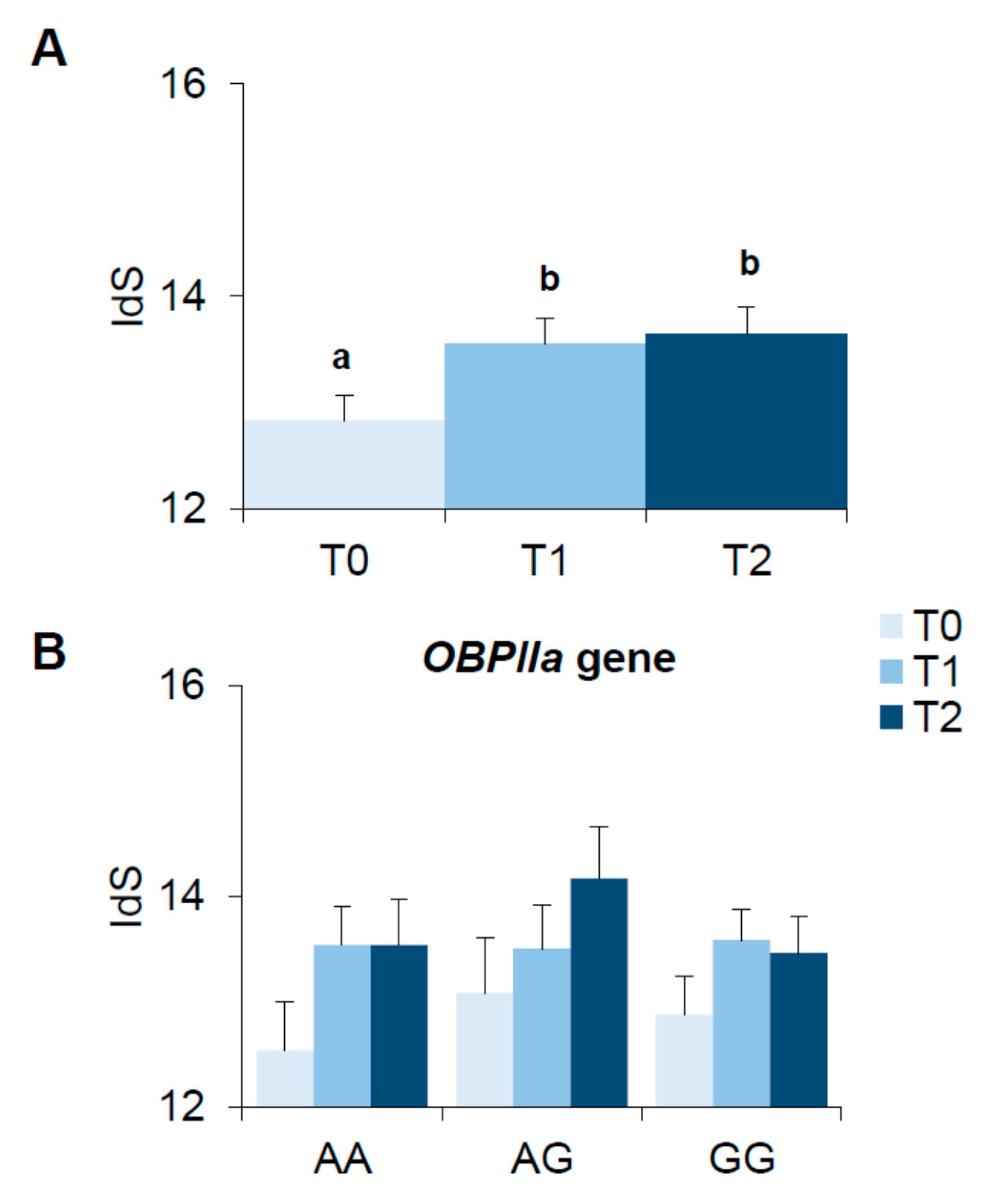
3.5. Bariatric Surgery-Induced Effect on Olfactory Function
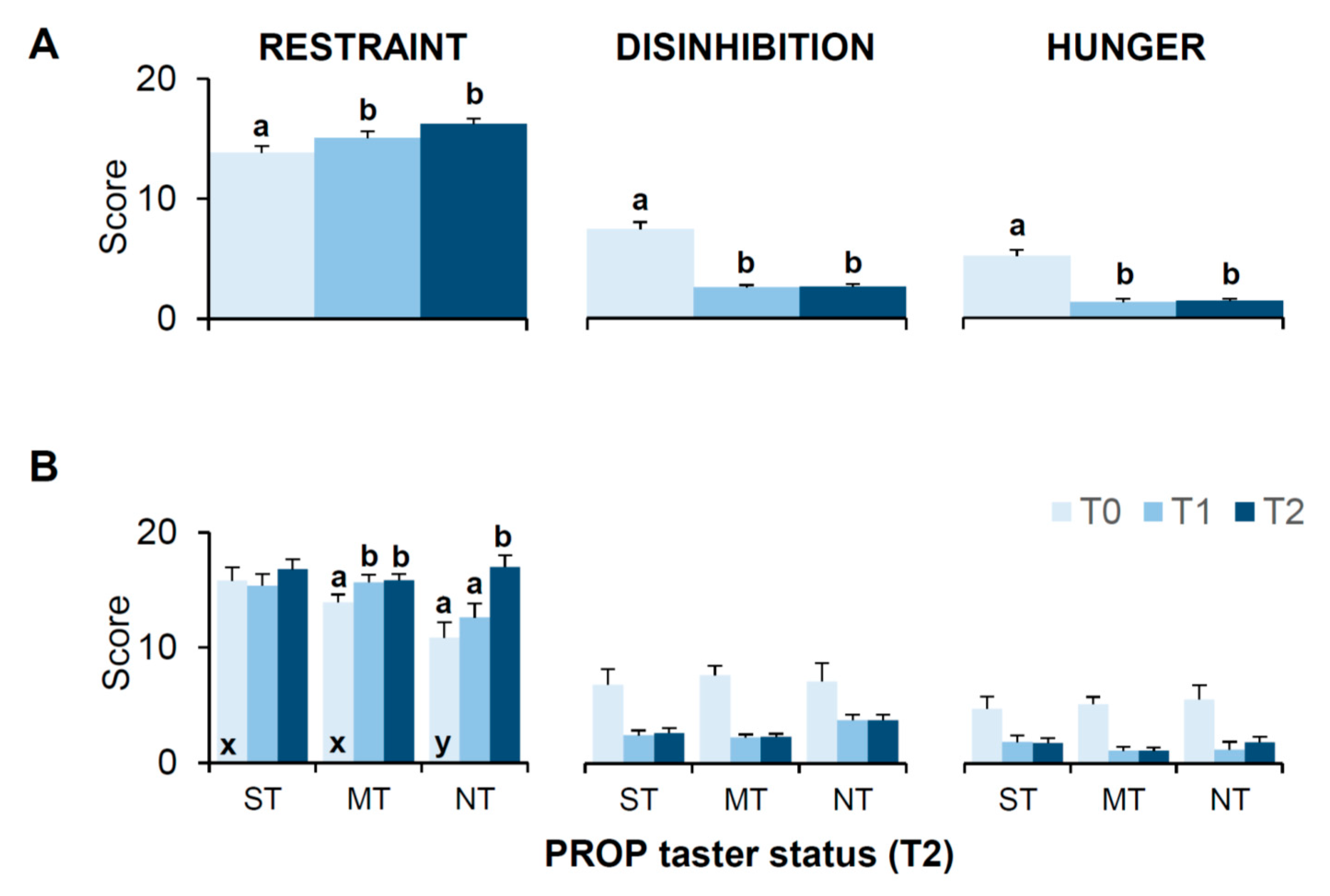
3.6. Bariatric Surgery-Induced Effects on Scores of the 3-Factor Eating Questionnaire (TFEQ)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bjorntorp, P. Definition and classification of obesity. In Eating Disorders and Obesity: A Comprehensive Handbook; Fairburn, C.G., Brownell, K.D., Eds.; The Guilford Press: New York, NY, USA, 2002; Volume 2, pp. 377–381. [Google Scholar]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Kurth, C.; Holden-Wiltse, J.; Saari, J. Food preferences in human obesity: Carbohydrates versus fats. Appetite 1992, 18, 207–221. [Google Scholar] [CrossRef]
- Macdiarmid, J.I.; Vail, A.; Cade, J.E.; Blundell, J.E. The sugar-fat relationship revisited: Differences in consumption between men and women of varying BMI. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 1053–1061. [Google Scholar] [CrossRef]
- Rissanen, A.; Hakala, P.; Lissner, L.; Mattlar, C.E.; Koskenvuo, M.; Ronnemaa, T. Acquired preference especially for dietary fat and obesity: A study of weight-discordant monozygotic twin pairs. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 973–977. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef]
- Drewnowski, A.; Grinker, J.A.; Hirsch, J. Obesity and flavor perception: Multidimensional scaling of soft drinks. Appetite 1982, 3, 361–368. [Google Scholar] [CrossRef]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.J. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef]
- Miras, A.D.; le Roux, C.W. Bariatric surgery and taste: Novel mechanisms of weight loss. Curr. Opin. Gastroenterol. 2010, 26, 140–145. [Google Scholar] [CrossRef]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child. 2012, 97, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher Body Mass Index Is Associated with Subjective Olfactory Dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered olfactory acuity in the morbidly obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924–935. [Google Scholar] [CrossRef]
- Shin, A.C.; Berthoud, H.R. Food reward functions as affected by obesity and bariatric surgery. Int. J. Obes. 2011, 35 (Suppl. S3), S40–S44. [Google Scholar] [CrossRef]
- Vignini, A.; Borroni, F.; Sabbatinelli, J.; Pugnaloni, S.; Alia, S.; Taus, M.; Ferrante, L.; Mazzanti, L.; Fabri, M. General Decrease of Taste Sensitivity Is Related to Increase of BMI: A Simple Method to Monitor Eating Behavior. Dis. Markers 2019, 2019, 2978026. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef]
- Stewart, J.E.; Newman, L.P.; Keast, R.S. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Banos, R.; Botella, C.; de la Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G.; et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS ONE 2017, 12, e0171204. [Google Scholar] [CrossRef]
- Guild, A.A. Olfactory acuity in normal and obese human subjects: Diurnal variations and the effect of d-amphetamine sulphate. J. Laryngol. Otol. 1956, 70, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R.; Tepper, B.J.; Rietzschel, J.; Prescott, J. Human hedonic responses to sweetness: Role of taste genetics and anatomy. Physiol. Behav. 2007, 91, 264–273. [Google Scholar] [CrossRef]
- Prescott, J.; Soo, J.; Campbell, H.; Roberts, C. Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol. Behav. 2004, 82, 459–469. [Google Scholar] [CrossRef]
- Melis, M.; Yousaf, N.Y.; Mattes, M.Z.; Cabras, T.; Messana, I.; Crnjar, R.; Tomassini Barbarossa, I.; Tepper, B.J. Sensory perception of and salivary protein response to astringency as a function of the 6-n-propylthioural (PROP) bitter-taste phenotype. Physiol. Behav. 2017, 173, 163–173. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I. Taste Perception of Sweet, Sour, Salty, Bitter, and Umami and Changes Due to l-Arginine Supplementation, as a Function of Genetic Ability to Taste 6-n-Propylthiouracil. Nutrients 2017, 9, 541–558. [Google Scholar] [CrossRef]
- Bartoshuk, L.M. The biological basis of food perception and acceptance. Food Qual. Prefer. 1993, 4, 21–32. [Google Scholar] [CrossRef]
- Melis, M.; Sollai, G.; Muroni, P.; Crnjar, R.; Barbarossa, I.T. Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients 2015, 7, 2068–2084. [Google Scholar] [CrossRef]
- Hayes, J.E.; Duffy, V.B. Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses 2007, 32, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Bartoshuk, L.M. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 2000, 100, 647–655. [Google Scholar] [CrossRef]
- Keller, K.L.; Steinmann, L.; Nurse, R.J.; Tepper, B.J. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite 2002, 38, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Neilland, M.; Ullrich, N.V.; Koelliker, Y.; Belzer, L.M. Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite 2011, 56, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Nurse, R.J. PROP taster status is related to fat perception and preference. Ann. N. Y. Acad. Sci. 1998, 855, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, I.T.; Carta, G.; Murru, E.; Melis, M.; Zonza, A.; Vacca, C.; Muroni, P.; Di Marzo, V.; Banni, S. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition 2013, 29, 531–536. [Google Scholar] [CrossRef]
- Carta, G.; Melis, M.; Pintus, S.; Pintus, P.; Piras, C.A.; Muredda, L.; Demurtas, D.; Di Marzo, V.; Banni, S.; Barbarossa, I.T. Participants with Normal Weight or with Obesity Show Different Relationships of 6-n-Propylthiouracil (PROP) Taster Status with BMI and Plasma Endocannabinoids. Sci. Rep. 2017, 7, 1361. [Google Scholar] [CrossRef]
- Lucock, M.; Ng, X.; Boyd, L.; Skinner, V.; Wai, R.; Tang, S.; Naylor, C.; Yates, Z.; Choi, J.H.; Roach, P.; et al. TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps. Food Funct. 2011, 2, 457–465. [Google Scholar] [CrossRef]
- Adappa, N.D.; Truesdale, C.M.; Workman, A.D.; Doghramji, L.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Cowart, B.J.; Cohen, N.A. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2016, 6, 783–791. [Google Scholar] [CrossRef]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2014, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Cohen, N.A. Bitter taste receptors in innate immunity: T2R38 and chronic rhinosinusitis. J. Rhinol. Otol. 2017, 5, 12–18. [Google Scholar] [CrossRef]
- Melis, M.; Errigo, A.; Crnjar, R.; Pes, G.M.; Tomassini Barbarossa, I. TAS2R38 bitter taste receptor and attainment of exceptional longevity. Sci. Rep. 2019, 9, 18047. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Grzeschuchna, L.; Sollai, G.; Hummel, T.; Tomassini Barbarossa, I. Taste disorders are partly genetically determined: Role of the TAS2R38 gene, a pilot study. Laryngoscope 2019. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Melis, M.; Sarchioto, M.; Melis, M.; Melis, M.; Morelli, M.; Tomassini Barbarossa, I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov. Disord. 2018, 33, 1331–1339. [Google Scholar] [CrossRef]
- Vascellari, S.; Melis, M.; Cossu, G.; Melis, M.; Serra, A.; Palmas, V.; Perra, D.; Oppo, V.; Fiorini, M.; Cusano, R.; et al. Genetic variants of TAS2R38 bitter taste receptor associate with distinct gut microbiota traits in Parkinson’s disease: A pilot study. Int. J. Biol. Macromol. 2020, 165, 665–674. [Google Scholar] [CrossRef]
- Oppo, V.; Melis, M.; Melis, M.; Tomassini Barbarossa, I.; Cossu, G. “Smelling and Tasting” Parkinson’s disease: Using Senses to Improve the Knowledge of the Disease. Front. Aging Neurosci. 2020, 12, 43. [Google Scholar] [CrossRef]
- Brewer, W.J.; Lin, A.; Moberg, P.J.; Smutzer, G.; Nelson, B.; Yung, A.R.; Pantelis, C.; McGorry, P.D.; Turetsky, B.I.; Wood, S.J. Phenylthiocarbamide (PTC) perception in ultra-high risk for psychosis participants who develop schizophrenia: Testing the evidence for an endophenotypic marker. Psychiatry Res. 2012, 199, 8–11. [Google Scholar] [CrossRef]
- Moberg, P.J.; McGue, C.; Kanes, S.J.; Roalf, D.R.; Balderston, C.C.; Gur, R.E.; Kohler, C.G.; Turetsky, B.I. Phenylthiocarbamide (PTC) perception in patients with schizophrenia and first-degree family members: Relationship to clinical symptomatology and psychophysical olfactory performance. Schizophr. Res. 2007, 90, 221–228. [Google Scholar] [CrossRef][Green Version]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.; Kim, U.K.; Bamshad, M.J.; Larsen, J.; Jorde, L.B.; Drayna, D. Natural Selection and Molecular Evolution in PTC, a Bitter-Taste Receptor Gene. Am. J. Hum. Genet. 2004, 74, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Boxer, E.E.; Garneau, N.L. Rare haplotypes of the gene TAS2R38 confer bitter taste sensitivity in humans. SpringerPlus 2015, 4, 505. [Google Scholar] [CrossRef]
- Keller, K.L.; Liang, L.C.; Sakimura, J.; May, D.; van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity 2012, 20, 1066–1073. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef]
- Karmous, I.; Plesnik, J.; Khan, A.S.; Sery, O.; Abid, A.; Mankai, A.; Aouidet, A.; Khan, N.A. Orosensory detection of bitter in fat-taster healthy and obese participants: Genetic polymorphism of CD36 and TAS2R38. Clin. Nutr. 2017, 37, 313–320. [Google Scholar] [CrossRef]
- Daoudi, H.; Plesnik, J.; Sayed, A.; Sery, O.; Rouabah, A.; Rouabah, L.; Khan, N.A. Oral Fat Sensing and CD36 Gene Polymorphism in Algerian Lean and Obese Teenagers. Nutrients 2015, 7, 9096–9104. [Google Scholar] [CrossRef]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005. [Google Scholar] [CrossRef]
- Reed, D.R.; Xia, M.B. Recent advances in fatty acid perception and genetics. Adv. Nutr. 2015, 6, 353s–360s. [Google Scholar] [CrossRef]
- Mrizak, I.; Sery, O.; Plesnik, J.; Arfa, A.; Fekih, M.; Bouslema, A.; Zaouali, M.; Tabka, Z.; Khan, N.A. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br. J. Nutr. 2015, 113, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.; Melis, M.; Scoular, K.; Driver, M.; Schaich, K.M.; Keller, K.L.; Tomassini Barbarossa, I.; Tepper, B.J. Effects of CD36 Genotype on Oral Perception of Oleic Acid Supplemented Safflower Oil Emulsions in Two Ethnic Groups: A Preliminary Study. J. Food Sci. 2018, 83, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Melis, M.; Mastinu, M.; Pani, D.; Cosseddu, P.; Bonfiglio, A.; Crnjar, R.; Tepper, B.J.; Tomassini Barbarossa, I. Human Tongue Electrophysiological Response to Oleic Acid and Its Associations with PROP Taster Status and the CD36 Polymorphism (rs1761667). Nutrients 2019, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Love-Gregory, L.; Sherva, R.; Schappe, T.; Qi, J.S.; McCrea, J.; Klein, S.; Connelly, M.A.; Abumrad, N.A. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 2011, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Murugesan, G.; Chen, K.; Zhang, L.; Wang, Q.; Febbraio, M.; Anselmo, R.M.; Marchant, K.; Barnard, J.; Silverstein, R.L. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood 2011, 117, 6355–6366. [Google Scholar] [CrossRef]
- Melis, M.; Carta, G.; Pintus, S.; Pintus, P.; Piras, C.A.; Murru, E.; Manca, C.; Di Marzo, V.; Banni, S.; Tomassini Barbarossa, I. Polymorphism rs1761667 in the CD36 Gene Is Associated to Changes in Fatty Acid Metabolism and Circulating Endocannabinoid Levels Distinctively in Normal Weight and Obese Subjects. Front. Physiol. 2017, 8, 1006. [Google Scholar] [CrossRef]
- Tomassini Barbarossa, I.; Ozdener, M.H.; Melis, M.; Love-Gregory, L.; Mitreva, M.; Abumrad, N.A.; Pepino, M.Y. Variant in a common odorant-binding protein gene is associated with bitter sensitivity in people. Behav. Brain Res. 2017, 329, 200–204. [Google Scholar] [CrossRef]
- Melis, M.; Mastinu, M.; Arca, M.; Crnjar, R.; Tomassini Barbarossa, I. Effect of chemical interaction between oleic acid and L-Arginine on oral perception, as a function of polymorphisms of CD36 and OBPIIa and genetic ability to taste 6-n-propylthiouracil. PLoS ONE 2018, 13, e0194953. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Magri, S.; Usai, P.; Hummel, T.; Tomassini Barbarossa, I.; Crnjar, R. Association between the rs2590498 polymorphism of Odorant Binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behav. Brain Res. 2019, 372. [Google Scholar] [CrossRef]
- Melis, M.; Sollai, G.; Masala, C.; Pisanu, C.; Cossu, G.; Melis, M.; Sarchioto, M.; Oppo, V.; Morelli, M.; Crnjar, R.; et al. Odor identification performance in idiopathic Parkinson’s disease is associated with gender and the genetic variability of the olfactory binding protein. Chem. Senses 2019, 44, 311–318. [Google Scholar] [CrossRef]
- Brolin, R.E. Bariatric surgery and long-term control of morbid obesity. JAMA 2002, 288, 2793–2796. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, G.S.; Smastuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofso, D.; Lindberg, M.; Hertel, J.K.; Hjelmesaeth, J. Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018, 319, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e245. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Teh, J. A New Emerging procedure—Sleeve Gastrectomy. In Essentials and Controversies in Bariatric Surgery; Huang, C.-K., Ed.; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Boughter, J.D., Jr.; Madan, A.K. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg. Obes. Relat. Dis. 2006, 2, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Murty, G.; Bowrey, D.J. Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes. Surg. 2014, 24, 1463–1468. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef]
- Ochner, C.N.; Kwok, Y.; Conceicao, E.; Pantazatos, S.P.; Puma, L.M.; Carnell, S.; Teixeira, J.; Hirsch, J.; Geliebter, A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011, 253, 502–507. [Google Scholar] [CrossRef]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef]
- Thirlby, R.C.; Bahiraei, F.; Randall, J.; Drewnoski, A. Effect of Roux-en-Y gastric bypass on satiety and food likes: The role of genetics. J. Gastrointest. 2006, 10, 270–277. [Google Scholar] [CrossRef]
- le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Lowenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef] [PubMed]
- Ammon, B.S.; Bellanger, D.E.; Geiselman, P.J.; Primeaux, S.D.; Yu, Y.; Greenway, F.L. Short-term pilot study of the effect of sleeve gastrectomy on food preference. Obes. Surg. 2015, 25, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Burge, J.C.; Schaumburg, J.Z.; Choban, P.S.; DiSilvestro, R.A.; Flancbaum, L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J. Am. Diet. Assoc. 1995, 95, 666–670. [Google Scholar] [CrossRef]
- Zerrweck, C.; Zurita, L.; Alvarez, G.; Maydon, H.G.; Sepulveda, E.M.; Campos, F.; Caviedes, A.; Guilbert, L. Taste and Olfactory Changes Following Laparoscopic Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2016, 26, 1296–1302. [Google Scholar] [CrossRef]
- Scruggs, D.M.; Buffington, C.; Cowan, G.S., Jr. Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes. Surg. 1994, 4, 24–28. [Google Scholar] [CrossRef]
- Bueter, M.; Miras, A.D.; Chichger, H.; Fenske, W.; Ghatei, M.A.; Bloom, S.R.; Unwin, R.J.; Lutz, T.A.; Spector, A.C.; le Roux, C.W. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and Gustatory Function after Bariatric Surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef]
- Altun, H.; Hanci, D.; Altun, H.; Batman, B.; Serin, R.K.; Karip, A.B.; Akyuz, U. Improved Gustatory Sensitivity in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Ann. Otol. Rhinol. Laryngol. 2016, 125, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Hanci, D.; Altun, H.; Altun, H.; Batman, B.; Karip, A.B.; Serin, K.R. Laparoscopic Sleeve Gastrectomy Improves Olfaction Sensitivity in Morbidly Obese Patients. Obes. Surg. 2016, 26, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Jurowich, C.F.; Seyfried, F.; Miras, A.D.; Bueter, M.; Deckelmann, J.; Fassnacht, M.; Germer, C.T.; Thalheimer, A. Does bariatric surgery change olfactory perception? Results of the early postoperative course. Int. J. Colorectal Dis. 2014, 29, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Rieber, N.; Sauer, H.; Klosterhalfen, S.; Mack, I.; Zipfel, S.; Teufel, M. Almost nothing—not even bariatric surgery for obesity—Changes olfactory sensitivity. J. Res. Obes. 2014, 2014, 491890. [Google Scholar] [CrossRef][Green Version]
- Zerrweck, C.; Gallardo, V.C.; Calleja, C.; Sepúlveda, E.; Guilber, L. Gross Olfaction Before and After Laparoscopic Gastric Bypass. Obes. Surg. 2017, 27, 2988–2992. [Google Scholar] [CrossRef]
- Richardson, B.E.; Vanderwoude, E.A.; Sudan, R.; Leopold, D.A.; Thompson, J.S. Gastric Bypass Does Not Influence Olfactory Function in Obese Patients. Obes. Surg. 2012, 22, 283–286. [Google Scholar] [CrossRef]
- Padiglia, A.; Zonza, A.; Atzori, E.; Chillotti, C.; Calò, C.; Tepper, B.J.; Barbarossa, I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010, 92, 539–545. [Google Scholar] [CrossRef]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; d’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef]
- Tepper, B.J.; Ullrich, N.V. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol. Behav. 2002, 75, 305–312. [Google Scholar] [CrossRef]
- Laessle, R.G.; Tuschl, R.J.; Kotthaus, B.C.; Pirke, K.M. Behavioral and biological correlates of dietary restraint in normal life. Appetite 1989, 12, 83–94. [Google Scholar] [CrossRef]
- Alexander, J.M.; Tepper, B.J. Use of reduced-calorie/reduced-fat foods by young adults: Influence of gender and restraint. Appetite 1995, 25, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Trail, A.C.; Shaffer, S.E. Diet and physical activity in restrained eaters. Appetite 1996, 27, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Westenhoefer, J. Dietary restraint and disinhibition: Is restraint a homogeneous construct? Appetite 1991, 16, 45–55. [Google Scholar] [CrossRef]
- Bryant, E.J.; King, N.A.; Blundell, J.E. Disinhibition: Its effects on appetite and weight regulation. Obes. Rev. 2008, 9, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lawson, O.J.; Williamson, D.A.; Champagne, C.M.; DeLany, J.P.; Brooks, E.R.; Howat, P.M.; Wozniak, P.J.; Bray, G.A.; Ryan, D.H. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes. Res. 1995, 3, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Figura, A.; Rose, M.; Ordemann, J.; Klapp, B.F.; Ahnis, A. Changes in self-reported eating patterns after laparoscopic sleeve gastrectomy: A pre-post analysis and comparison with conservatively treated patients with obesity. Surg. Obes. Relat. Dis. 2017, 13, 129–137. [Google Scholar] [CrossRef]
- Rieber, N.; Giel, K.E.; Meile, T.; Enck, P.; Zipfel, S.; Teufel, M. Psychological dimensions after laparoscopic sleeve gastrectomy: Reduced mental burden, improved eating behavior, and ongoing need for cognitive eating control. Surg. Obes. Relat. 2013, 9, 569–573. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Wilson, G.T.; Brolin, R.E.; Bradley, L. Effects of bariatric surgery on binge eating and related psychopathology. Eat. Weight Disord. 1999, 4, 1–5. [Google Scholar] [CrossRef]
- Burgmer, R.; Grigutsch, K.; Zipfel, S.; Wolf, A.M.; de Zwaan, M.; Husemann, B.; Albus, C.; Senf, W.; Herpertz, S. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes. Surg. 2005, 15, 684–691. [Google Scholar] [CrossRef]
- Mack, I.; Ölschläger, S.; Sauer, H.; von Feilitzsch, M.; Weimer, K.; Junne, F.; Peeraully, R.; Enck, P.; Zipfel, S.; Teufel, M. Does Laparoscopic Sleeve Gastrectomy Improve Depression, Stress and Eating Behaviour? A 4-Year Follow-up Study. Obes. Surg. 2016, 26, 2967–2973. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Zhao, L.; Kirkmeyer, S.V.; Tepper, B.J. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol. Behav. 2003, 78, 625–633. [Google Scholar] [CrossRef]
- Barbarossa, I.T.; Melis, M.; Mattes, M.Z.; Calò, C.; Muroni, P.; Crnjar, R.; Tepper, B.J. The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol. Behav. 2015, 138, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Melis, M.; Pani, D.; Cosseddu, P.; Usai, I.; Crnjar, R.; Bonfiglio, A.; Tomassini Barbarossa, I. First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Sci. Rep. 2017, 7, 40353. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; Shaffer, G.S.; Gilmore, M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses 1993, 18, 683–702. [Google Scholar] [CrossRef]
- Tepper, B.J.; Christensen, C.M.; Cao, J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001, 73, 571–577. [Google Scholar] [CrossRef]
- Landis, B.N.; Welge-Luessen, A.; Bramerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef]
- Mueller, C.; Kallert, S.; Renner, B.; Stiassny, K.; Temmel, A.F.; Hummel, T.; Kobal, G. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 2003, 41, 2–6. [Google Scholar]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Eibenstein, A.; Fioretti, A.B.; Lena, C.; Rosati, N.; Amabile, G.; Fusetti, M. Modern psychophysical tests to assess olfactory function. Neurol. Sci. 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.L. The relationship between chemical constitution and taste. Proc. Natl. Acad. Sci. USA 1932, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.; Kalmus, H. Chemical specificity in genetical differences of taste sensitivity. Ann. Eugen. 1949, 15, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Reed, D.R. The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 2001, 28, 111–142. [Google Scholar]
- Des Gachons, C.P.; Beauchamp, G.K.; Breslin, P.A. The genetics of bitterness and pungency detection and its impact on phytonutrient evaluation. Ann. N. Y. Acad. Sci. 2009, 1170, 140–144. [Google Scholar] [CrossRef]
- Jeon, T.-I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Investig. 2008, 118, 3693–3700. [Google Scholar] [CrossRef]
- Hubert, P.A.; Papasavas, P.; Stone, A.; Swede, H.; Huedo-Medina, T.B.; Tishler, D.; Duffy, V.B. Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women. Nutrients 2019, 11, 804. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Drayna, D.; Coon, H.; Kim, U.K.; Elsner, T.; Cromer, K.; Otterud, B.; Baird, L.; Peiffer, A.P.; Leppert, M. Genetic analysis of a complex trait in the Utah Genetic Reference Project: A major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum. Genet. 2003, 112, 567–572. [Google Scholar] [CrossRef]
- Prodi, D.A.; Drayna, D.; Forabosco, P.; Palmas, M.A.; Maestrale, G.B.; Piras, D.; Pirastu, M.; Angius, A. Bitter taste study in a Sardinian genetic isolate supports the association of phenylthiocarbamide sensitivity to the TAS2R38 bitter receptor gene. Chem. Senses 2004, 29, 697–702. [Google Scholar] [CrossRef]
- Reed, D.R.; Zhu, G.; Breslin, P.A.; Duke, F.F.; Henders, A.K.; Campbell, M.J.; Montgomery, G.W.; Medland, S.E.; Martin, N.G.; Wright, M.J. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum. Mol. Genet. 2010, 19, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Atzori, E.; Cabras, S.; Zonza, A.; Calò, C.; Muroni, P.; Nieddu, M.; Padiglia, A.; Sogos, V.; Tepper, B.J.; et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS ONE 2013, 8, e74151. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Reed, D.; Williams, A. Supertasting, earaches and head injury: Genetics and pathology alter our taste worlds. Neurosci. Biobehav. Rev. 1996, 20, 79–87. [Google Scholar] [CrossRef]
- Mennella, J.; Pepino, M.Y.; Duke, F.; Reed, D. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010, 11, 60. [Google Scholar] [CrossRef]
- Bell, K.I.; Tepper, B.J. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotype. Am. J. Clin. Nutr. 2006, 84, 245–251. [Google Scholar] [CrossRef]
- Tepper, B.J. Does genetic taste sensitivity to PROP influence food preferences and body weight? Appetite 1999, 32, 422. [Google Scholar] [CrossRef]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef]
- Forrai, G.; Bánkövi, G. Taste perception for phenylthiocarbamide and food choice—A Hungarian twin study. Acta Physiol. Hung. 1984, 64, 33–40. [Google Scholar]
- Beauchamp, G.K. Sensory and receptor responses to umami: An overview of pioneering work. Am. J. Clin. Nutr. 2009, 90, 723s–727s. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Maedge, J.; Lam, L.; Blasche, G.; Shakeri-Leidenmühler, S.; Kundi, M.; Ludvik, B.; Langer, F.B.; Prager, G.; Schindler, K.; et al. Salt taste after bariatric surgery and weight loss in obese persons. PeerJ 2016, 4, e2086. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Reed, D.R.; Snyder, D.J.; Bartoshuk, L.M. Bitter Receptor Gene (TAS2R38), 6-n-Propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcohol. Clin. Exp. Res. 2004, 28, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Lucchina, L.A.; Prutkin, J.; Fast, K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann. N. Y. Acad. Sci. 1998, 855, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Keast, R.S.J. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhou, L.H.; Ban, X.; Liu, D.X.; Jiang, W.; Liu, X.M. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 2011, 113, 663–667. [Google Scholar] [CrossRef]
- Tucker, K.; Cavallin, M.A.; Jean-Baptiste, P.; Biju, K.C.; Overton, J.M.; Pedarzani, P.; Fadool, D.A. The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel. Results Probl. Cell Differ. 2010, 52, 147–157. [Google Scholar] [CrossRef]
- Neumann, M.; Holzapfel, C.; Müller, A.; Hilbert, A.; Crosby, R.D.; de Zwaan, M. Features and Trajectories of Eating Behavior in Weight-Loss Maintenance: Results from the German Weight Control Registry. Obesity 2018, 26, 1501–1508. [Google Scholar] [CrossRef]
- Hays, N.P.; Roberts, S.B. Aspects of Eating Behaviors “Disinhibition” and “Restraint” Are Related to Weight Gain and BMI in Women. Obesity 2008, 16, 52–58. [Google Scholar] [CrossRef]
- Brockmeyer, T.; Hamze Sinno, M.; Skunde, M.; Wu, M.; Woehning, A.; Rudofsky, G.; Friederich, H.C. Inhibitory Control and Hedonic Response towards Food Interactively Predict Success in a Weight Loss Programme for Adults with Obesity. Obes. Facts 2016, 9, 299–309. [Google Scholar] [CrossRef]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-Loss Maintenance for 10 Years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef]
- Zoon, H.F.A.; de Bruijn, S.E.M.; Smeets, P.A.M.; de Graaf, C.; Janssen, I.M.C.; Schijns, W.; Aarts, E.O.; Jager, G.; Boesveldt, S. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav. Brain Res. 2018, 353, 194–202. [Google Scholar] [CrossRef] [PubMed]
| Clinical Features | |||
|---|---|---|---|
| Surgery (n) | SG (21) | RYGB (30) | |
| Age (y) | 42.28 ± 2.33 | 46.83 ± 2.08 | |
| Female (n) | 14 | 22 | |
| Male (n) | 7 | 8 | |
| Genetic Features | |||
| TAS2R38 n (%) | PAV/PAV 10 (21.28) | PAV/AVI 19 (40.42) | AVI/AV I18 (38.30) |
| CD36 n (%) | GG 14 (27.45) | GA 23 (45.10) | AA 14 (27.45) |
| OBPIIa n (%) | AA 15 (29.41) | AG 12 (23.53) | GG 24 (47.06) |
| PROP Status | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Super-Taster | Medium Taster | Non-Taster | |||||
| TAS2R38 | n | % | n | % | n | % | |
| T0 | |||||||
| Genotype | |||||||
| PAV/PAV | 1 | 25.0 | 9 | 32.1 | 0 | 0.0 | 0.00098 |
| PAV/AVI | 2 | 50.0 | 13 | 46.4 | 4 | 26.7 | |
| AVI/AVI | 1 | 25.0 | 6 | 21.4 | 11 | 73.3 | |
| Haplotype | |||||||
| PAV | 4 | 50.0 | 31 | 55.4 | 4 | 13.3 | 0.0003 |
| AVI | 4 | 50.0 | 25 | 44.6 | 26 | 86.7 | |
| T1 | |||||||
| Genotype | |||||||
| PAV/PAV | 4 | 26.7 | 6 | 23.1 | 0 | 0.0 | 0.0019 |
| PAV/AVI | 8 | 53.3 | 11 | 42.3 | 0 | 0.0 | |
| AVI/AVI | 3 | 20.0 | 9 | 34.6 | 6 | 100.0 | |
| Haplotype | |||||||
| PAV | 16 | 53.3 | 23 | 44.2 | 0 | 0.0 | 0.0026 |
| AVI | 14 | 46.7 | 29 | 55.8 | 12 | 100.0 | |
| T2 | |||||||
| Genotype | |||||||
| PAV/PAV | 4 | 40.0 | 6 | 21.4 | 0 | 0.0 | 0.0004 |
| PAV/AVI | 6 | 60.0 | 12 | 42.9 | 1 | 11.1 | |
| AVI/AVI | 0 | 0.0 | 10 | 35.7 | 8 | 88.9 | |
| Haplotype | |||||||
| PAV | 14 | 70.0 | 24 | 42.9 | 1 | 5.6 | 0.000004 |
| AVI | 6 | 30.0 | 32 | 57.1 | 17 | 94.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, M.; Pintus, S.; Mastinu, M.; Fantola, G.; Moroni, R.; Pepino, M.Y.; Tomassini Barbarossa, I. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients 2021, 13, 250. https://doi.org/10.3390/nu13010250
Melis M, Pintus S, Mastinu M, Fantola G, Moroni R, Pepino MY, Tomassini Barbarossa I. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients. 2021; 13(1):250. https://doi.org/10.3390/nu13010250
Chicago/Turabian StyleMelis, Melania, Stefano Pintus, Mariano Mastinu, Giovanni Fantola, Roberto Moroni, Marta Yanina Pepino, and Iole Tomassini Barbarossa. 2021. "Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes" Nutrients 13, no. 1: 250. https://doi.org/10.3390/nu13010250
APA StyleMelis, M., Pintus, S., Mastinu, M., Fantola, G., Moroni, R., Pepino, M. Y., & Tomassini Barbarossa, I. (2021). Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients, 13(1), 250. https://doi.org/10.3390/nu13010250









