Relationship between Down-Regulation of Copper-Related Genes and Decreased Ferroportin Protein Level in the Duodenum of Iron-Deficient Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Biological Sample Collection
2.3. Measurement of Non-Heme and Heme Iron Content in the Liver
2.4. Measurement of Copper Content in the Tissues
2.5. Perls Staining
2.6. Quantitative Reverse Transcription PCR (RT-qPCR)
2.7. Protein Extracts Preparation and Western Blotting
2.8. Superoxide Dismutase 1 (Sod1) and Ceruloplasmin (Cp) Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Red Blood Cell (RBC) Parameters and Iron Status of Piglets During an Early Stage of Postnatal Development Indicate Iron Deficiency Anemia
3.2. Increased Copper Levels in Organs Controlling Iron Metabolism but Not in the Duodenum of Anemic Piglets
3.3. Iron Accumulation and Suppressed Hif-2α Transcriptional Activity in the Duodenum of Anemic Piglets
3.4. Decreased Expression of Copper Transporter Atp7b Contributes to Iron Retention in the Duodenum of Anemic Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Fox, P.L. The copper-iron chronicles: The story of an intimate relationship. Biometals 2003, 16, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Ece, A.; Uyanik, B.S.; Işcan, A.; Ertan, P.; Yiğitoğlu, M.R. Increased serum copper and decreased serum zinc levels in children with iron deficiency anemia. Biol. Trace Elem. Res. 1997, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ravia, J.J.; Stephen, R.M.; Ghishan, F.K.; Collins, J.F. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J. Biol. Chem. 2005, 280, 36221–36227. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; DeMars, L.C.S.; Johnson, W.T.; Lukaski, H.C. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J. Nutr. 2005, 135, 92–98. [Google Scholar] [CrossRef]
- Lee, G.R.; Nacht, S.; Lukens, J.N.; Cartwright, G.E. Iron metabolism in copper-deficient swine. J. Clin. Investig. 1968, 47, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Matak, P.; Zumerle, S.; Mastrogiannaki, M.; El Balkhi, S.; Delga, S.; Mathieu, J.R.R.; Canonne-Hergaux, F.; Poupon, J.; Sharp, P.A.; Vaulont, S.; et al. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2α and altered expression of iron absorption genes in mice. PLoS ONE 2013, 8, e59538. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Impact of copper deficiency in humans. Ann. N. Y. Acad. Sci. 2014, 1314, 1–5. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Drabek, J. Iron deficiency in suckling piglets: Ethiology, clinical aspects and diagnosis. Folia Vet. 2005, 49, 104–111. [Google Scholar]
- Szudzik, M.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lenartowicz, M.; Lipiński, P. Iron Supplementation in Suckling Piglets: An Ostensibly Easy Therapy of Neonatal Iron Deficiency Anemia. Pharmaceuticals 2018, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, G.E.; Gubler, C.J.; Bush, J.A.; Wintrobe, M.W. Studies on copper metabolism. XVII. Further observations on the anemia of copper deficiency in swine. Blood 1956, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of iron and copper metabolism in the mammalian intestine and liver. Comp. Physiol. 2018, 14, 1433–1461. [Google Scholar]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Wyman, S.; Simpson, R.J.; McKie, A.T.; Sharp, P.A. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008, 582, 1901–1906. [Google Scholar] [CrossRef]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; DiRenzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef]
- Nose, Y.; Wood, L.K.; Kim, B.E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef]
- Wang, X.; Flores, S.R.; Ha, J.H.; Doguer, C.; Woloshun, R.R.; Xiang, P.; Groschet, A.; Vidyasagar, S.; Collins, J.F. Intestinal DMT1 is essential for optimal assimilation of dietary copper in male and female mice with iron-deficiency anemia. J. Nutr. 2018, 148, 1244–1252. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.H.; Barut, A.B.; Zapata, A.G.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Vulpe, C.D.; Kuo, Y.M.; Murphy, T.L.; Cowley, L.; Askwith, C.; Libina, N.; Gitschier, J.; Anderson, G.J. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999, 21, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Pierson, H.; Muchenditsi, A.; Kim, B.E.; Ralle, M.; Zachos, N.; Huster, D.; Lutsenko, S. The Function of ATPase Copper Transporter ATP7B in Intestine. Gastroenterology 2018, 154, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Investig. 2019, 129, 336–348. [Google Scholar] [CrossRef]
- Mastrogiannaki, M.; Matak, P.; Keith, B.; Simon, C.; Vaulont, S.; Peyssonnaux, C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Investig. 2009, 119, 1159–1166. [Google Scholar] [CrossRef]
- Taylor, M.; Qu, A.; Anderson, E.R.; Matsubara, T.; Martin, A.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 2011, 140, 2044–2055. [Google Scholar] [CrossRef]
- Shah, Y.M.; Matsubara, T.; Ito, S.; Yim, S.H.; Gonzalez, F.J. Intestinal Hypoxia-Inducible Transcription Factors Are Essential for Iron Absorption following Iron Deficiency. Cell Metab. 2009, 9, 152–164. [Google Scholar] [CrossRef]
- Xie, L.; Collins, J.F. Transcriptional regulation of the Menkes copper ATPase (Atp7a) gene by hypoxia-inducible factor (HIF-2α) in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C1298–C1305. [Google Scholar] [CrossRef]
- Pourvali, K.; Matak, P.; Latunde-Dada, G.O.; Solomou, S.; Mastrogiannaki, M.; Peyssonnaux, C.; Sharp, P.A. Basal expression of copper transporter 1 in intestinal epithelial cells is regulated by hypoxia-inducible factor 2α. FEBS Lett. 2012, 586, 2423–2427. [Google Scholar] [CrossRef]
- Lipinski, P.; Starzyński, R.R.; Canonne-Hergaux, F.; Tudek, B.; Oliński, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef]
- Svoboda, M.; Vaňhara, J.; Berlinská, J. Parenteral iron administration in suckling piglets—A review. Acta Vet. Brno 2017, 86, 249–261. [Google Scholar] [CrossRef]
- Torrance, J.D.; Bothwell, T.H. Iron stores. Methods Hematol. 1980, 1, 90–115. [Google Scholar]
- Canonne-Hergaux, F.; Fleming, M.D.; Levy, J.E.; Gauthier, S.; Ralph, T.; Picard, V.; Andrews, N.C.; Gros, P. The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood 2000, 96, 3964–3970. [Google Scholar] [PubMed]
- Staroń, R.; Lipiński, P.; Lenartowicz, M.; Bednarz, A.; Gajowiak, A.; Smuda, E.; Krzeptowski, W.; Pieszka, M.; Korolonek, T.; Hamza, I.; et al. Dietary hemoglobin rescues young piglets from severe iron deficiency anemia: Duodenal expression profile of genes involved in heme iron absorption. PLoS ONE 2017, 12, e0181117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Wilcock, P.; Bedford, M.R. Iron status of piglets and impact of phytase superdosing on iron physiology: A review. Anim. Feed. Sci. Technol. 2018, 235, 8–14. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal. Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Ma, X.; Das, N.K.; Castillo, C.; Gourani, A.; Perekatt, A.O.; Verzi, M.P.; Shah, Y.M. SMAD Family Member 3 (SMAD3) and SMAD4 repress HIF2α-dependent iron-regulatory genes. J. Biol. Chem. 2019, 294, 3974–3986. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.K.; Steimle, B.L.; Kosman, D.J.; Dlouhy, A.C.; Parker, H.V. Fluorescence resonance energy transfer links membrane ferroportin, hephaestin but not ferroportin, amyloid precursor protein complex with iron efflux. J. Biol. Chem. 2019, 294, 4202–4214. [Google Scholar]
- Egeli, A.K.; Framstad, T.; Morberg, H. Clinical biochemistry, haematology and body weight in piglets. Acta Vet. Scand. 1998, 39, 381–393. [Google Scholar] [PubMed]
- Kapitsinou, P.P.; Liu, Q.; Unger, T.L.; Rha, J.; Davidoff, O.; Keith, B.; Epstein, J.A.; Moores, S.L.; Erickson-Miller, C.L.; Haase, V.H. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010, 116, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, C.; Mayeux, P. The molecular biology of erythropoietin. Nephrol. Dial. Transpl. 1999, 14, 22–28. [Google Scholar] [CrossRef]
- Egeli, A.K.; Framstad, T. An evaluation of iron-dextran supplementation in piglets administered by injection on the first, third or fourth day after birth. Res. Vet. Sci. 1999, 66, 179–184. [Google Scholar] [CrossRef]
- Starzyński, R.R.; Laarakkers, C.M.; Tjalsma, H.; Swinkels, D.W.; Pieszka, M.; Styś, A.; Mickiewicz, M.; Lipiński, P. Iron supplementation in suckling piglets: How to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS ONE 2013, 8, e64022. [Google Scholar] [CrossRef]
- Geisser, P.; Baer, M.; Schaub, E. Structure/histotoxicity relationship of parenteral iron preparations. Arzneimittelforschung 1992, 42, 1439–1452. [Google Scholar]
- Sherman, A.R.; Tissue, N.T. Tissue iron, copper and zinc levels in offspring of iron-sufficient and iron-deficient rats. J. Nutr. 1981, 111, 266–275. [Google Scholar] [CrossRef]
- Huster, D.; Finegold, M.J.; Morgan, C.T.; Burkhead, J.L.; Nixon, R.; Vanderwerf, S.M.; Gilliam, C.T.; Lutsenko, S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006, 168, 423–434. [Google Scholar] [CrossRef]
- Jończy, A.; Lipiński, P.; Ogórek, M.; Starzyński, R.R.; Krzysztofik, D.; Bednarz, A.; Krzeptowski, W.; Szudzik, M.; Haberkiewicz, O.; Miłoń, A.; et al. Functional iron deficiency in toxic milk mutant mice (tx-J) despite high hepatic ferroportin: A critical role of decreased GPI-ceruloplasmin expression in liver macrophages. Metallomics 2019, 11, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, J.D. Aceruloplasminemia. Pediatric Res. 1998, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.N.; Lu, Y.; Jiang, L.; Kim, C.; Collins, J.F. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 2011, 118, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Fetherolf, M.M.; Boyd, S.D.; Taylor, A.B.; Kim, H.J.; Wohlschlegel, J.A.; Blackburn, N.J.; Hart, P.J.; Winge, D.R.; Winkler, D.D. Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site. J. Biol. Chem. 2017, 292, 12025–12040. [Google Scholar] [CrossRef]
- Lassi, K.C.; Prohaska, J.R. Erythrocyte copper chaperone for superoxide dismutase is increased following marginal copper deficiency in adult and postweanling mice. J. Nutr. 2012, 142, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R.; Geissler, J.; Brokate, B.; Broderius, M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp. Biol. Med. 2003, 228, 959–966. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Converso-Baran, K.; Michele, D.E.; Shah, Y.M. A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling. J. Biol. Chem. 2019, 294, 14991–15002. [Google Scholar] [CrossRef]
- Shah, Y.M.; Xie, L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 2014, 146, 630–642. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- D’Anna, M.C.; Veuthey, T.V.; Roque, M.E. Immunolocalization of ferroportin in healthy and anemic mice. J. Histochem. Cytochem. 2009, 57, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rausa, M.; Pagani, A.; Nai, A.; Campanella, A.; Gilberti, M.E.; Apostoli, P.; Camaschella, C.; Silvestri, L. Bmp6 expression in murine liver non parenchymal cells: A mechanism to control their high iron exporter activity and protect hepatocytes from iron overload? PLoS ONE 2015, 10, e0122696. [Google Scholar] [CrossRef] [PubMed]
- Petrak, J.; Vyoral, D. Hephaestin—A ferroxidase of cellular iron export. Int. J. Biochem. Cell Biol. 2005, 37, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Lu, Y.; Darshan, D.; Frazer, D.M.; Wilkins, S.J.; Wolkow, N.; Bell, A.G.; Hsu, J.; Yu, C.C.; Chen, H.; et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS ONE 2014, 9, e987922014. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, T.; Attieh, Z.K.; Fox, T.C.; McKie, A.T.; Anderson, G.J.; Vulpe, C.D. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood 2003, 102, 1893–1899. [Google Scholar] [CrossRef]
- Nittis, T.; Gitlin, J.D. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J. Biol. Chem. 2004, 279, 25696–25702. [Google Scholar] [CrossRef]
- Barnes, N.; Tsivkovskii, R.; Tsivkovskaia, N.; Lutsenko, S. The copper-transporting ATPases, Menkes and Wilson disease proteins, have distinct roles in adult and developing cerebellum. J. Biol. Chem. 2005, 280, 9640–9645. [Google Scholar] [CrossRef]


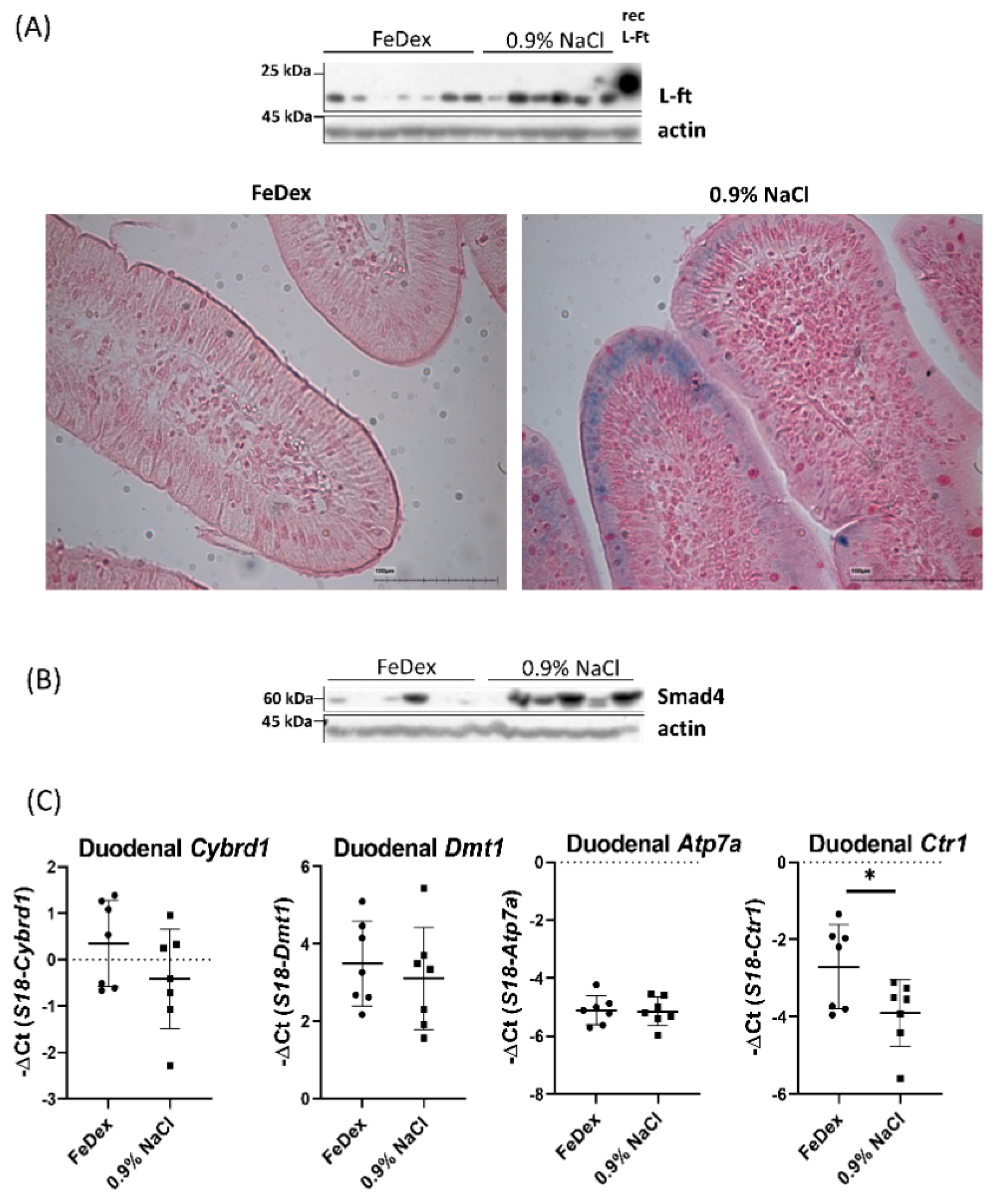
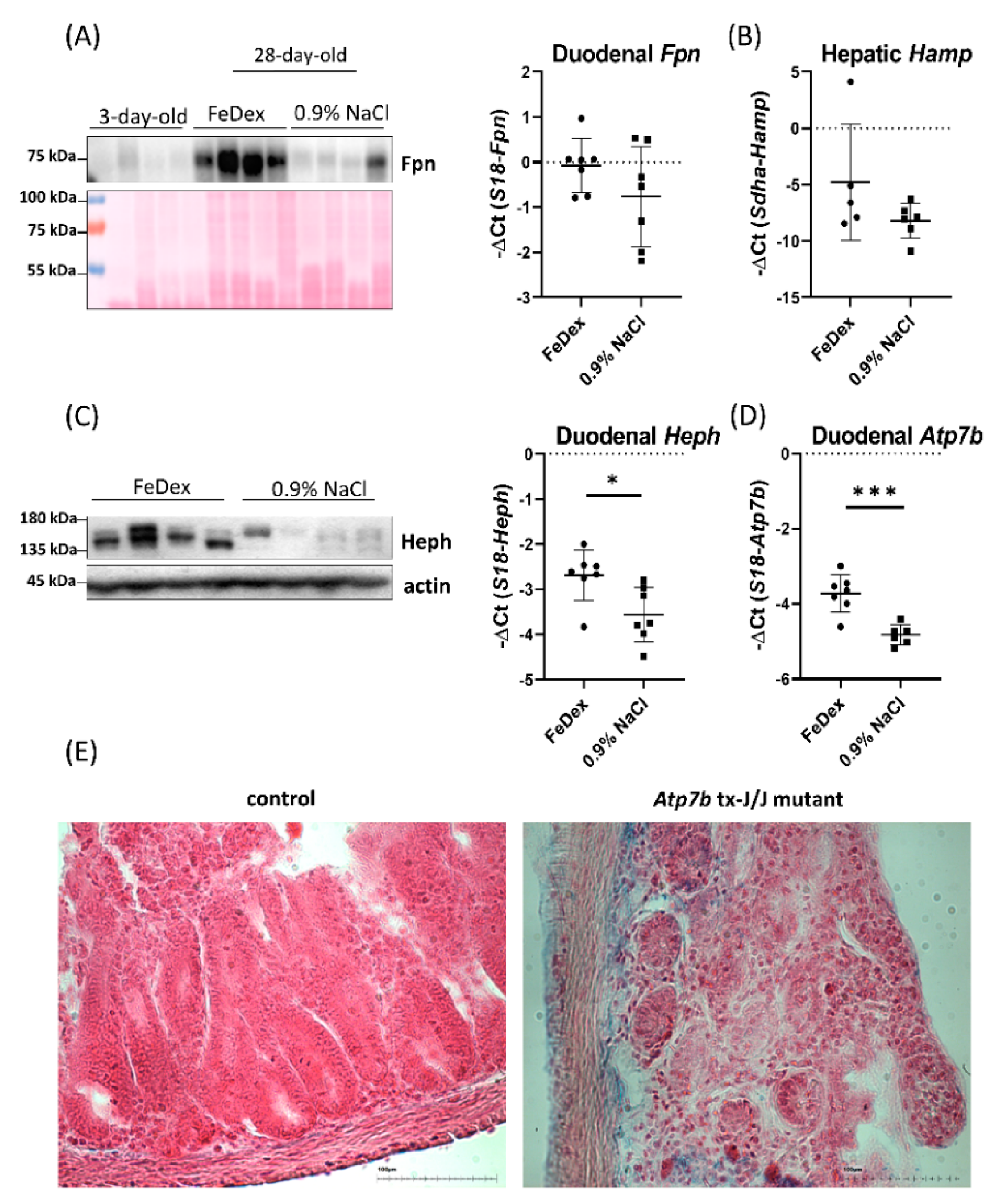
| RBC Indices/Group | RBC (106/mm3) | HB (g/dL) | HCT (%) | MCV (µm3) | |
|---|---|---|---|---|---|
| three-day-old | 3.44 ± 0.97 | 6.83 ± 1.66 | 19.79 ± 5.34 | 57.67 ± 4.44 | |
| 28-day-old | 0.9% NaCl | 3.00 ± 0.92 | 4.11 ± 0.97 ‡*** | 10.67 ± 3.05 ‡*** | 35.89 ± 1.69 §*** |
| FeDex | 6.13 ± 0.66 †*** | 10.73 ± 1.31 †*** | 33.41 ± 4.08 †*** | 54.56 ± 5.90 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jończy, A.; Mazgaj, R.; Starzyński, R.R.; Poznański, P.; Szudzik, M.; Smuda, E.; Kamyczek, M.; Lipiński, P. Relationship between Down-Regulation of Copper-Related Genes and Decreased Ferroportin Protein Level in the Duodenum of Iron-Deficient Piglets. Nutrients 2021, 13, 104. https://doi.org/10.3390/nu13010104
Jończy A, Mazgaj R, Starzyński RR, Poznański P, Szudzik M, Smuda E, Kamyczek M, Lipiński P. Relationship between Down-Regulation of Copper-Related Genes and Decreased Ferroportin Protein Level in the Duodenum of Iron-Deficient Piglets. Nutrients. 2021; 13(1):104. https://doi.org/10.3390/nu13010104
Chicago/Turabian StyleJończy, Aneta, Rafał Mazgaj, Rafał Radosław Starzyński, Piotr Poznański, Mateusz Szudzik, Ewa Smuda, Marian Kamyczek, and Paweł Lipiński. 2021. "Relationship between Down-Regulation of Copper-Related Genes and Decreased Ferroportin Protein Level in the Duodenum of Iron-Deficient Piglets" Nutrients 13, no. 1: 104. https://doi.org/10.3390/nu13010104
APA StyleJończy, A., Mazgaj, R., Starzyński, R. R., Poznański, P., Szudzik, M., Smuda, E., Kamyczek, M., & Lipiński, P. (2021). Relationship between Down-Regulation of Copper-Related Genes and Decreased Ferroportin Protein Level in the Duodenum of Iron-Deficient Piglets. Nutrients, 13(1), 104. https://doi.org/10.3390/nu13010104





