DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microalga Cultivation and Direct Ethylation of Biomass
2.3. Cell Culture and Treatments
2.4. Cell Viability
2.5. Lipid Extraction
2.6. Fatty Acid Analysis by Gas Chromatography with Flame Ionization Detector (GC-FID)
2.7. Interleukin 6(IL-6), Nitric Oxid, Prostaglandin E1, E2 and D2 Quantification
2.8. Determination of Intracellular Reactive Oxygen Species (ROS)
2.9. RNA Isolation, cDNA Preparation and Real-Time Polymerase Chain Reaction (PCR) Analysis of Gene Expression
2.10. Statistical Analysis
3. Results
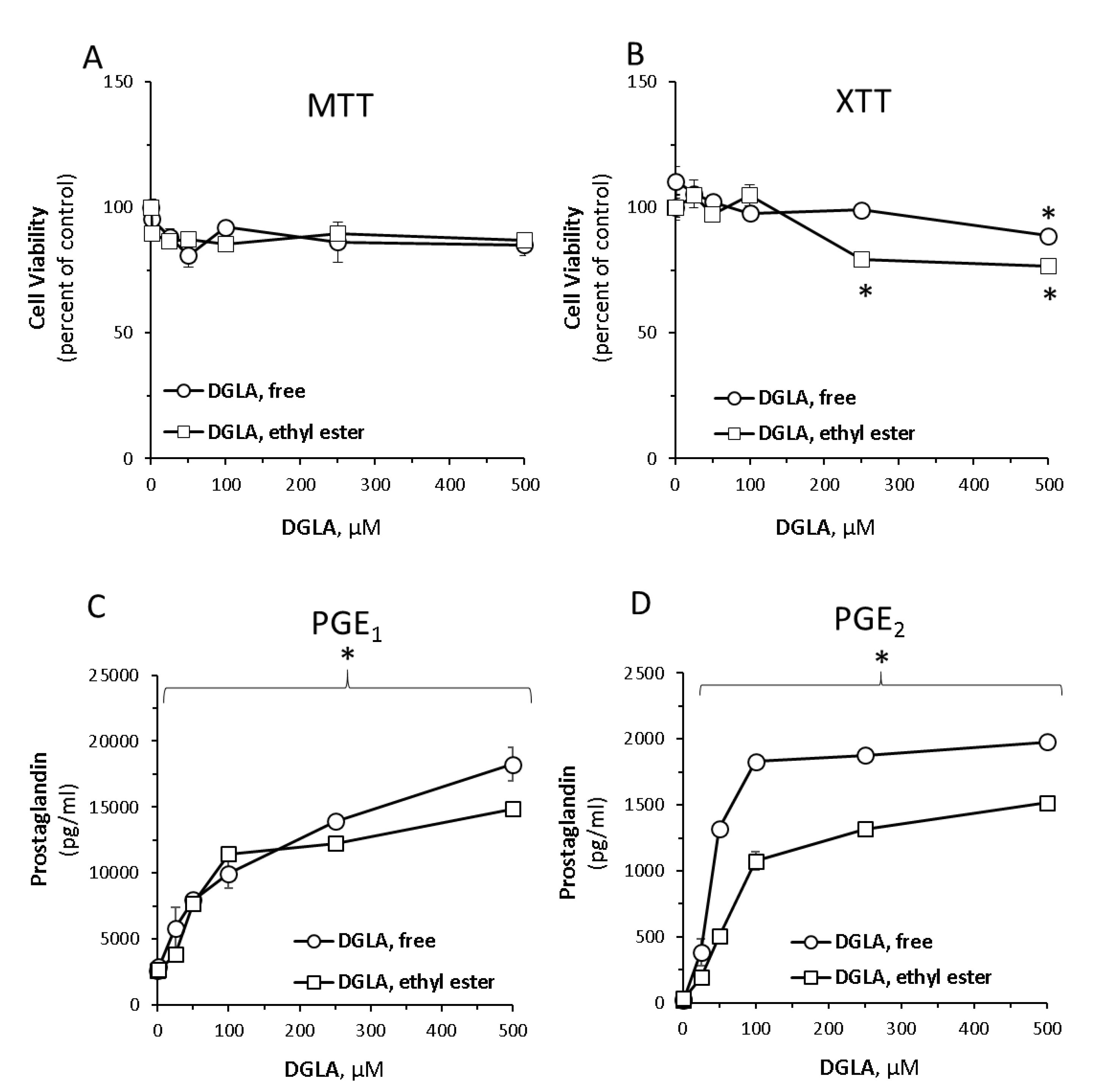
3.1. Dihomo-γ-Linolenic Acid (DGLA) Induces Prostaglandin Secretion in RAW264.7 Macrophage Cells
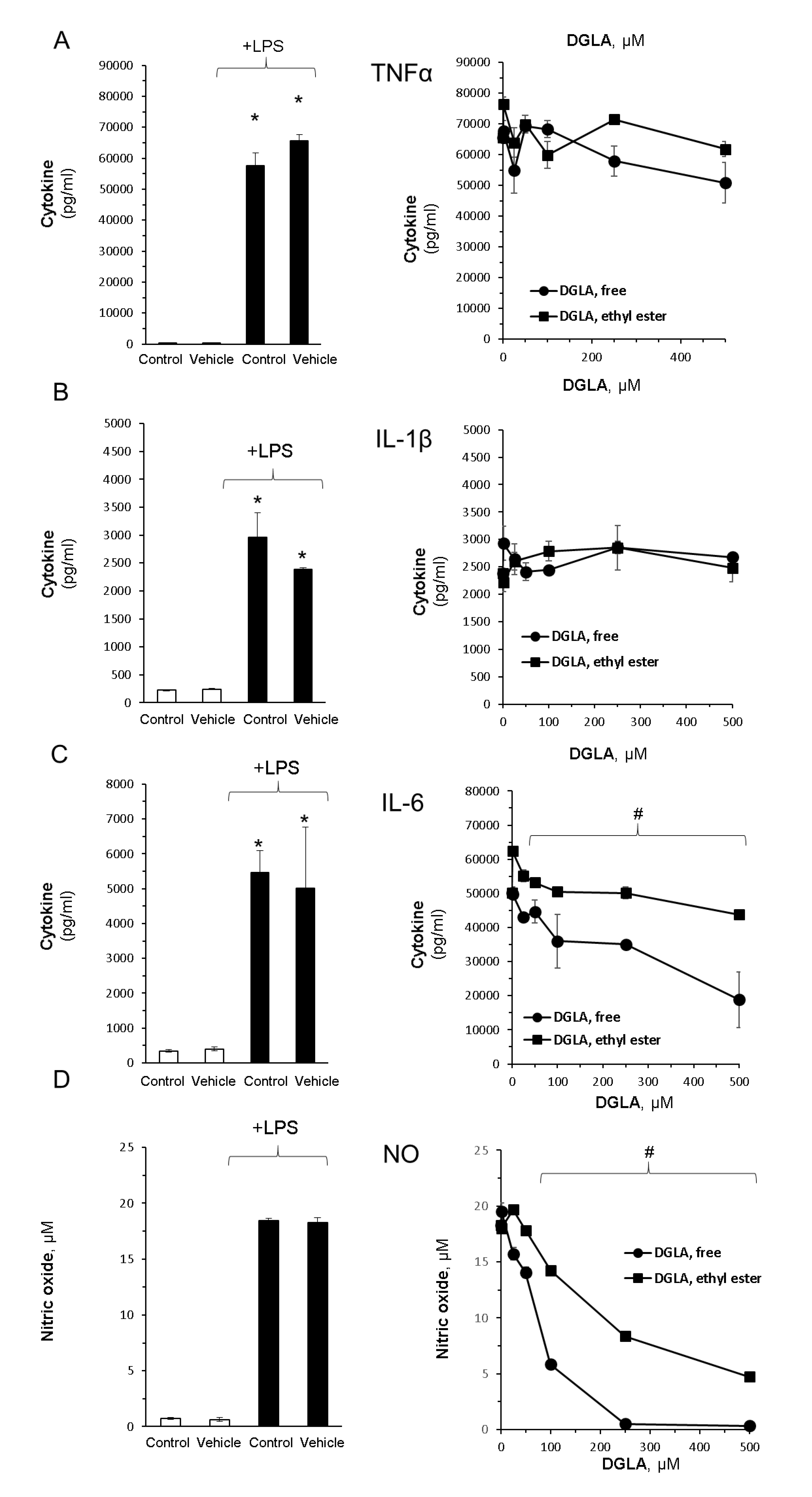
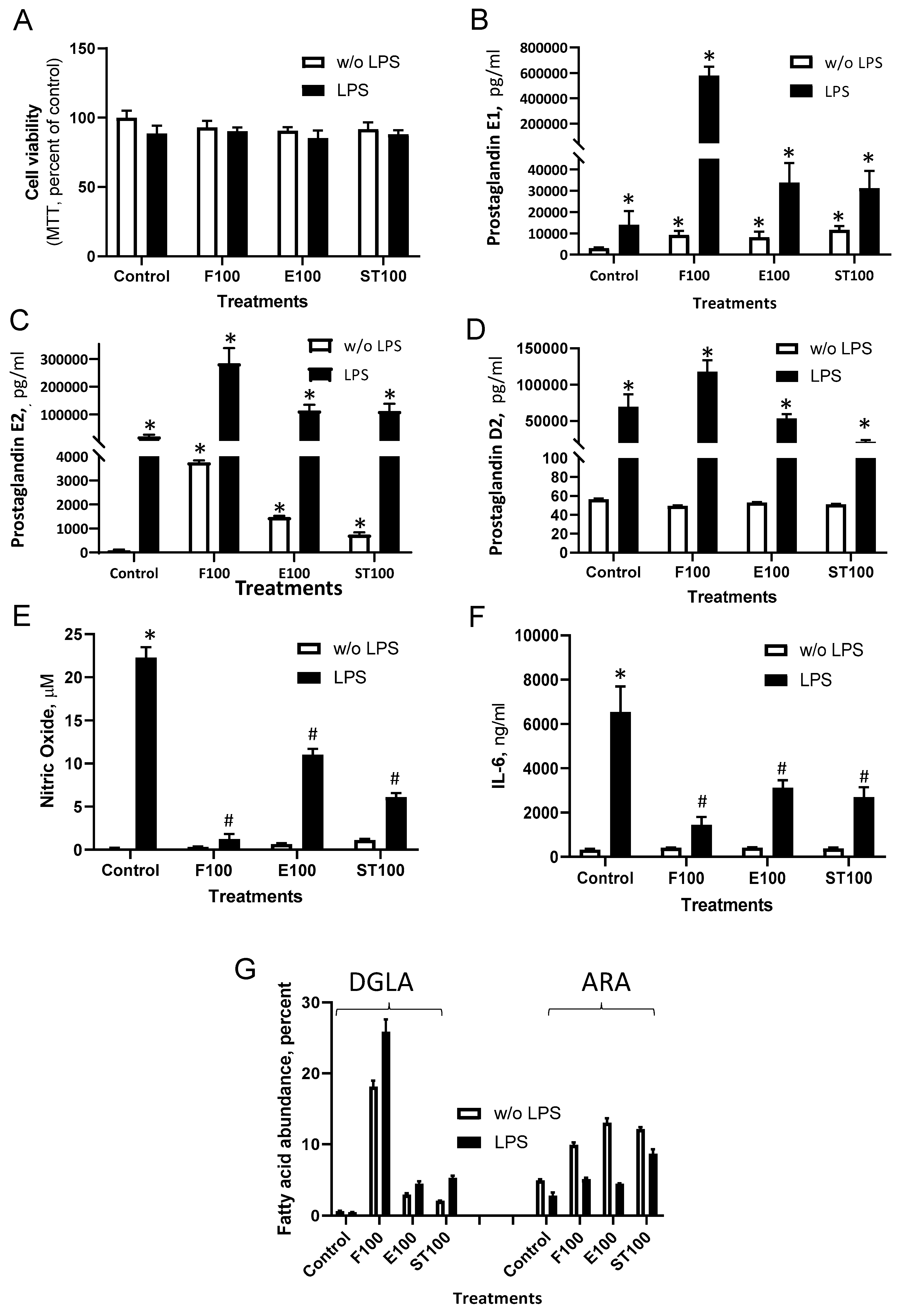
3.2. DGLA Supplementation Modulates Bacterial Lipopolysaccharide (LPS)-Induced Inflammation in RAW264.7 Macrophages
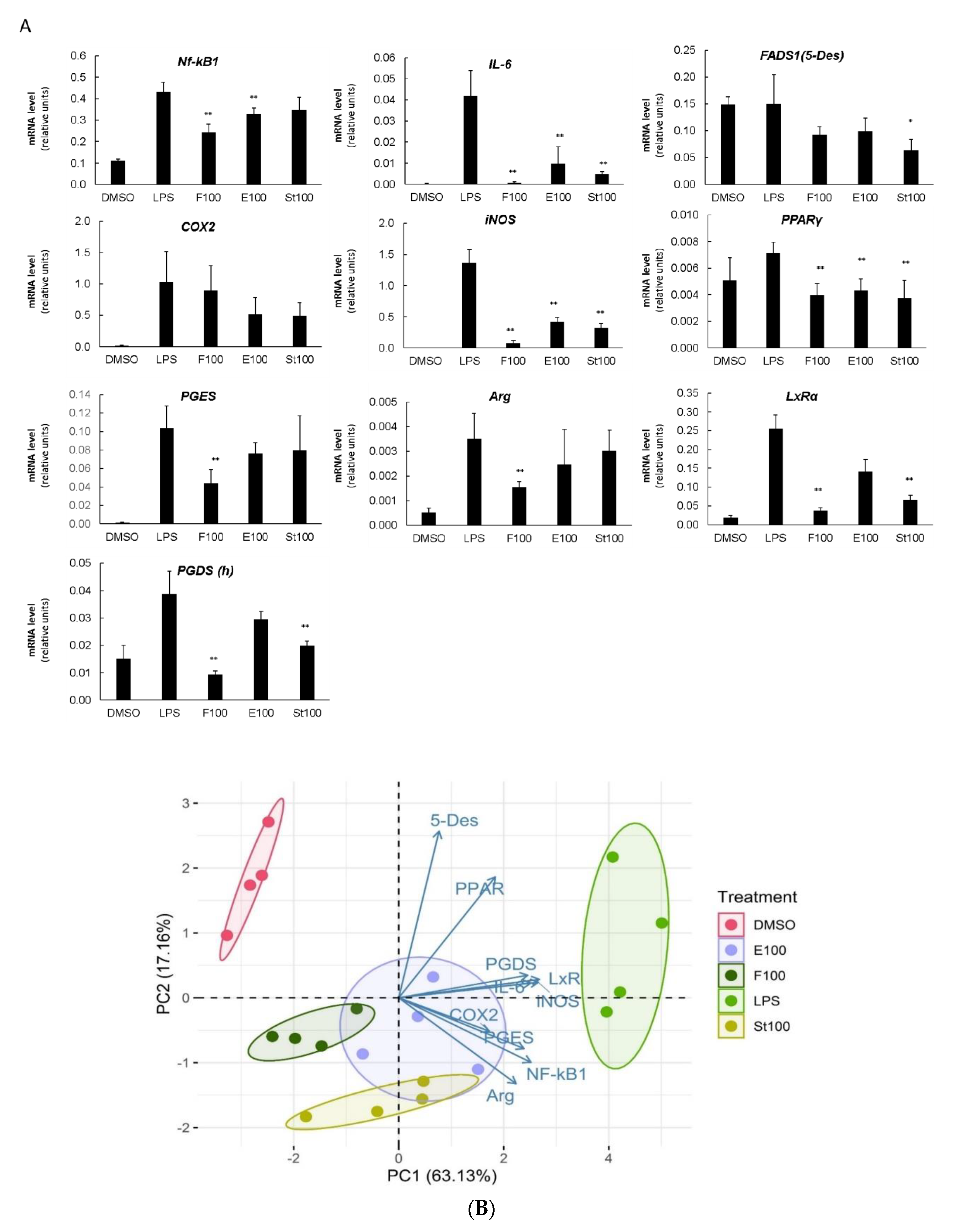
3.3. DGLA-Mediated Modulation of Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calder, P.C. Polyunsaturated fatty acids, inflammation, and immunity. Lipids 2001, 36, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.; Williams, J.O.; Ferekidis, N.; Ismail, A.; Chan, Y.-H.; Michael, D.R.; Guschina, I.A.; Tyrrell, V.J.; O’Donnell, V.B.; Harwood, J.L.; et al. Dihomo-γ-linolenic acid inhibits several key cellular processes associated with atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. Int. Rev. J. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary lipids induce ferroptosis in Caenorhabditis elegans and human cancer cells. Dev. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Iskandarov, U.; Khozin-Goldberg, I.; Cohen, Z. Selection of a DGLA-producing mutant of the microalga Parietochloris incisa: I. Identification of mutation site and expression of VLC-PUFA biosynthesis genes. Appl. Microbiol. Biotechnol. 2011, 90, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Chapkin, R.S. Importance of Dietary g -Linolenic Acid in Human Health and Nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Layé, S. N-3 polyunsaturated fatty acids and their derivates reduce neuroinflammation during aging. Nutrients 2020, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Duffin, K.L.; Obukowicz, M.G.; Hummert, S.L.; Fujiwara, H.; Needleman, P.; Raz, A. Differential metabolism of dihomo-γ-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: Implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem. J. 2002, 365, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Y.; Brooks, A.; Guo, B.; Miskimins, K.W.; Qian, S.Y. Knockdown delta-5-desaturase promotes the formation of a novel free radical byproduct from COX-catalyzed ω-6 peroxidation to induce apoptosis and sensitize pancreatic cancer cells to chemotherapy drugs. Free Radic. Biol. Med. 2016, 97, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, H.; Sakuradani, E.; Nakatani, M.; Ando, A.; Okuda, T.; Sakamoto, T.; Ochiai, M.; Shimizu, S.; Ogawa, J. Gene targeting in the oil-producing fungus Mortierella alpina 1S-4 and construction of a strain producing a valuable polyunsaturated fatty acid. Curr. Genet. 2015, 61, 579–589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jareonkitmongkol, S.; Sakuradani, E.; Shimizu, S. A Novel A5-Desaturase-Defective Mutant of Mortierella alpina 1S-4 and Its Dihomo-y-Linolenic Acid Productivity. Appl. Environ. Microbiol. 1993, 59, 4300–4304. [Google Scholar] [CrossRef] [PubMed]
- Holladay, C.S.; Wright, R.M.; Spangelo, B.L. Arachidonic acid stimulates interleukin-6 release from rat peritoneal macrophages in vitro: Evidence for a prostacyclin-dependent mechanism. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 915–922. [Google Scholar] [CrossRef]
- Honda, K.L.; Lamon, S.; Matthan, N.R.; Wu, D.; Lichtenstein, A.H. EPA and DHA Exposure Alters the Inflammatory Response but not the Surface Expression of Toll-like Receptor 4 in Macrophages. Lipids 2015, 50, 121–129. [Google Scholar] [CrossRef]
- Astarita, G.; Kendall, A.C.; Dennis, E.A.; Nicolaou, A. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 456–468. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 879–886. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Cohen, Z. Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). Phytochemistry 2002, 60, 135–143. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary supplementation with ω6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to streptococcal infection. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Pal-Nath, D.; Didi-Cohen, S.; Shtaida, N.; Nath, P.R.; Samani, T.; Boussiba, S.; Khozin-Goldberg, I. Improved productivity and oxidative stress tolerance under nitrogen starvation is associated with the ablated Δ5 desaturation in the green microalga Lobosphaera incisa. Algal Res. 2017, 26, 25–38. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Hwang, D.; Jang, B.C.; Yu, G.; Boudreau, M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: Mediation through both mitogen-activated protein kinase and NF-κB signaling pathways in macrophages. Biochem. Pharmacol. 1997, 54, 87–96. [Google Scholar] [CrossRef]
- von Schacky, C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc. Health Risk Manag. 2006, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Reichart, D.; Dumlao, D.S.; Glass, C.K.; Dennis, E.A. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J. Leukoc. Biol. 2011, 90, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.S.; Levine, L.A. Pharmacological erection program using prostaglandin E1. J. Urol. 1991, 146, 786–789. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Significance of lipoxygenase-derived monohydroxy fatty acids in cutaneous biology. Prostaglandins Other Lipid Mediat. 2000, 63, 3–13. [Google Scholar] [CrossRef]
- Amagai, Y.; Oida, K.; Matsuda, A.; Jung, K.; Kakutani, S.; Tanaka, T.; Matsuda, K.; Jang, H.; Ahn, G.; Xia, Y.; et al. Dihomo-γ-linolenic acid prevents the development of atopic dermatitis through prostaglandin D1 production in NC/Tnd mice. J. Dermatol. Sci. 2015, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Kawashima, H.; Tanaka, T.; Shiraishi-Tateishi, A.; Kiso, Y. Uptake of dihomo-γ-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 23–29. [Google Scholar] [CrossRef]
- Gomolka, B.; Siegert, E.; Blossey, K.; Schunck, W.-H.; Rothe, M.; Weylandt, K.H.; Chen, C.; Zhuo, S.; Ye, Y.; He, Q.; et al. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostaglandins Other Lipid Mediat. 2011, 94, 81–87. [Google Scholar] [CrossRef]
- Natarajan, K.; Abraham, P.; Kota, R.; Isaac, B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem. Toxicol. 2018, 118, 766–783. [Google Scholar] [CrossRef]
- Mendez, M.; LaPointe, M.C. PPARγ Inhibition of Cyclooxygenase-2, PGE2 Synthase, and Inducible Nitric Oxide Synthase in Cardiac Myocytes. Hypertension 2003, 42, 844–850. [Google Scholar] [CrossRef]
- A-González, N.; Castrillo, A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 982–994. [Google Scholar] [CrossRef]
- Bordoni, A.; Di Nunzio, M.; Danesi, F.; Biagi, P.L. Polyunsaturated fatty acids: From diet to binding to PPARs and other nuclear receptors. Genes Nutr. 2006, 1, 95–106. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Arnett, E.; Schlesinger, L.S. Macrophage nuclear receptors: Emerging key players in infectious diseases. PLoS Pathog. 2019, 15, e1007585. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A paradigm for gene regulation: Inflammation, NF-κB and PPAR. In Advances in Experimental Medicine and Biology; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; Volume 544, pp. 181–196. [Google Scholar]
- Von Knethen, A.; Brüne, B. Cyclooxygenase-2: An essential regulator of NO-mediated apoptosis. FASEB J. 1997, 11, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Vio, C.P.; Quiroz-Munoz, M.; Cuevas, C.A.; Cespedes, C.; Ferreri, N.R. Prostaglandin E 2 EP3 receptor regulates cyclooxygenase-2 expression in the kidney. Am. J. Physiol. Physiol. 2012, 303, F449–F457. [Google Scholar] [CrossRef]
- Horrobin, D.F. The regulation of prostaglandin biosynthesis: Negative feedback mechanisms and the selective control of formation of 1 and 2 series prostaglandins: Relevance to inflammation and immunity. Med. Hypotheses 1980, 6, 687–709. [Google Scholar] [CrossRef]
- Tang, T.; Scambler, T.E.; Smallie, T.; Cunliffe, H.E.; Ross, E.A.; Rosner, D.R.; O’Neil, J.D.; Clark, A.R. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E2, dual specificity phosphatase 1 and tristetraprolin. Sci. Rep. 2017, 7, 4350. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Hüll, M.; Lieb, K.; Schumann, G.; Berger, M.; Bauer, J. Potential link between interleukin-6 and arachidonic acid metabolism in Alzheimer’s disease. J. Neural Transm. Suppl. 1998, 269–278. [Google Scholar]
- Dooper, M.M.B.W.; Van Riel, B.; Graus, Y.M.F.; M’Rabet, L. Dihomo-γ-linolenic acid inhibits tumour necrosis factor-α production by human leucocytes independently of cyclooxygenase activity. Immunology 2003, 110, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Farrokhnia, F.; Makarem, J.; Khan, Z.H.; Mohagheghi, M.; Maghsoudlou, M.; Abdollahi, A. The effects of prostaglandin E 1 on interleukin-6, pulmonary function and postoperative recovery in oesophagectomised patients. Anaesth. Intensive Care 2009, 37, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xie, Y.; Lai, S.C.; Liu, B.F.; He, Y.R.; Hu, H.; Cao, Y. Benefits of anti-inflammatory therapy in the treatment of ischemia/reperfusion injury in the renal microvascular endothelium of rats with return of spontaneous circulation. Mol. Med. Rep. 2017, 15, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, C.; Billiar, T.R. Nitric Oxide in Trauma and Sepsis; Zuckschwerdt: Munich, Germany, 2001. [Google Scholar]
- Massaro, M.; Habib, A.; Lubrano, L.; Del Turco, S.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKCε inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Soon, A.L.; Hye, J.K.; Ki, C.C.; Jong, C.B.; Ji, K.P.; Jeong, K.S.; Won, J.C.; Jong, H.L.; Won, Y.P. DHA and EPA down-regulate COX-2 expression through suppression of NF-κB activity in LPS-treated human umbilical vein endothelial cells. Korean J. Physiol. Pharmacol. 2009, 13, 301–307. [Google Scholar]
- Narayanan, B.A.; Narayanan, N.K.; Simi, B.; Reddy, B.S. Modulation of Inducible Nitric Oxide Synthase and Related Proinflammatory Genes by the Omega-3 Fatty Acid Docosahexaenoic Acid in Human Colon Cancer Cells. Cancer Res. 2003, 63, 972–979. [Google Scholar]
- Siendones, E.; Fouad, D.; Díaz-Guerra, M.J.M.; De La Mata, M.D.L.; Boscá, L.; Muntané, J. PGE1-induced NO reduces apoptosis by D-galactosamine through attenuation of NF-κB and NOS-2 expression in rat hepatocytes. Hepatology 2004, 40, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Moulin, D.; Sebillaud, S.; Koufany, M.; Galteau, M.M.; Netter, P.; Terlain, B.; Jouzeau, J.Y. Contrasting effects of peroxisome-proliferator-activated receptor (PPAR)gamma agonists on membrane-associated prostaglandin E2 synthase-1 in IL-1beta-stimulated rat chondrocytes: Evidence for PPARgamma-independent inhibition by 15-deoxy-Delta12,14prostagl. Arthritis Res. Ther. 2005, 7, R1325. [Google Scholar] [CrossRef]
- Maione, F.; Casillo, G.M.; Raucci, F.; Iqbal, A.J.; Mascolo, N. The functional link between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the onset of inflammation. Pharmacol. Res. 2020, 157, 104807. [Google Scholar] [CrossRef]
- Oishi, Y.; Spann, N.J.; Link, V.M.; Shimano, H.; Saghatelian, A.; Glass, C.K. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2017, 25, 412–427. [Google Scholar] [CrossRef]
- Uehara, Y.; Miura, S.I.; von Eckardstein, A.; Abe, S.; Fujii, A.; Matsuo, Y.; Rust, S.; Lorkowski, S.; Assmann, G.; Yamada, T.; et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis 2007, 191, 11–21. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Nayak, S.; Al Ashhab, A.; Zilberg, D.; Khozin-Goldberg, I. Dietary Supplementation with Omega-6 LC-PUFA-Rich Microalgae Regulates Mucosal Immune Response and Promotes Microbial Diversity in the Zebrafish Gut. Biology 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novichkova, E.; Chumin, K.; Eretz-Kdosha, N.; Boussiba, S.; Gopas, J.; Cohen, G.; Khozin-Goldberg, I. DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients 2020, 12, 2892. https://doi.org/10.3390/nu12092892
Novichkova E, Chumin K, Eretz-Kdosha N, Boussiba S, Gopas J, Cohen G, Khozin-Goldberg I. DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients. 2020; 12(9):2892. https://doi.org/10.3390/nu12092892
Chicago/Turabian StyleNovichkova, Ekaterina, Katya Chumin, Noy Eretz-Kdosha, Sammy Boussiba, Jacob Gopas, Guy Cohen, and Inna Khozin-Goldberg. 2020. "DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages" Nutrients 12, no. 9: 2892. https://doi.org/10.3390/nu12092892
APA StyleNovichkova, E., Chumin, K., Eretz-Kdosha, N., Boussiba, S., Gopas, J., Cohen, G., & Khozin-Goldberg, I. (2020). DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients, 12(9), 2892. https://doi.org/10.3390/nu12092892





