Vitamin D Correction Down-Regulates Serum Amyloid P Component Levels in Vitamin D Deficient Arab Adults: A Single-Arm Trial
Abstract
1. Introduction
2. Methods
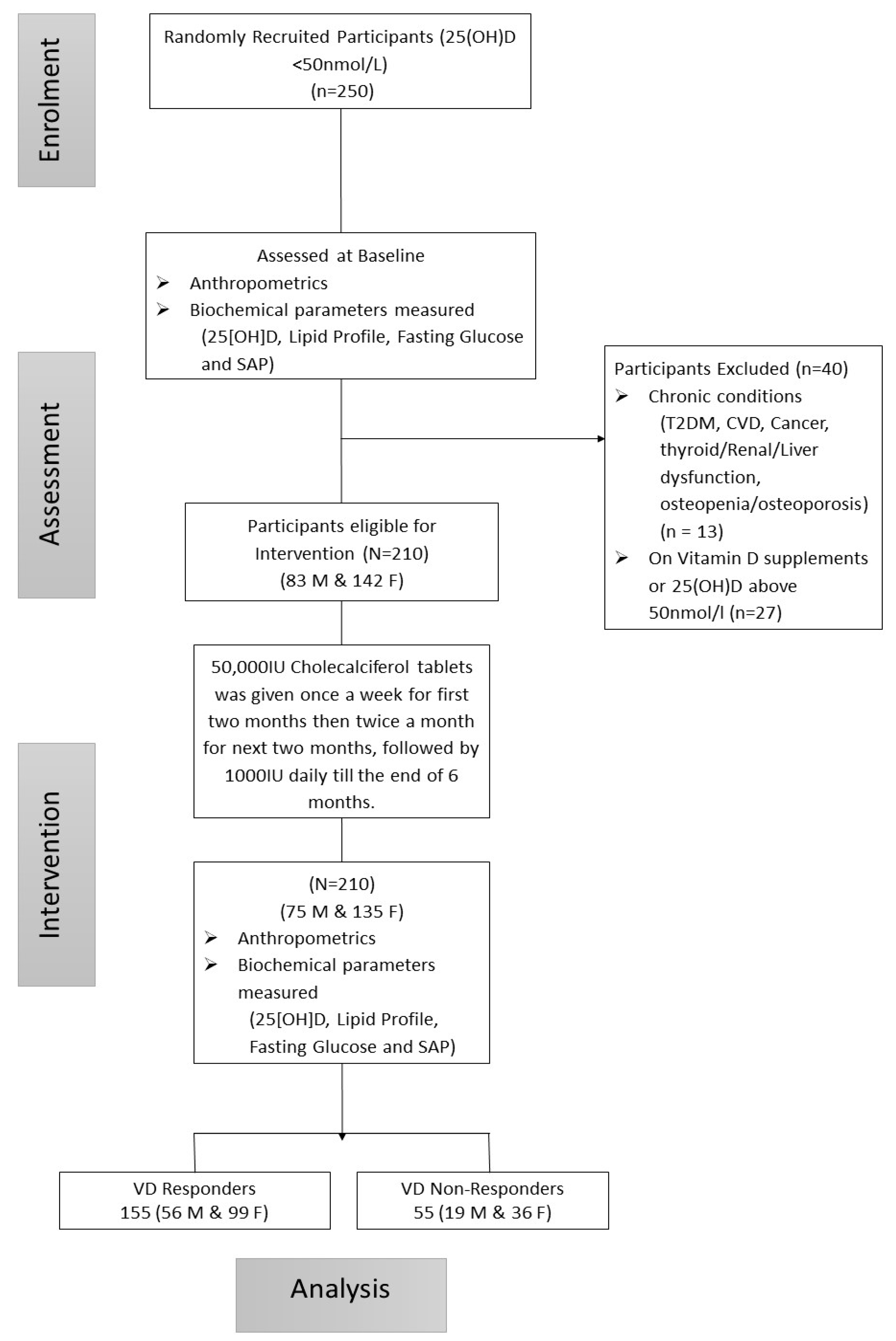
2.1. Study Design and Participants
2.2. Anthropometry and Biochemical Assessments
2.3. Intervention
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanchinathan, V.; Lim, H.W. A dermatologist’s perspective on vitamin D. Mayo Clin. Proc. 2012, 87, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos. Int. 2002, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Lahav, M.; Douvdevani, A.; Chaimovitz, C.; Shany, S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J. Steroid Biochem. Mol. Biol. 2007, 103, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Gysemans, C.A.; Cardozo, A.K.; Callewaert, H.; Giulietti, A.; Hulshagen, L.; Bouillon, R.; Eizirik, D.L.; Mathieu, C. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 2005, 146, 1956–1964. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Mirzaei, K.; Keshavarz, S.A.; Ansar, H.; Saboori, S.; Tootee, A. Evidences of dual role of vitamin D through cellular energy homeostasis and inflammation pathway in risk of cancer in obese subjects. Minerva Med. 2013, 104, 295–307. [Google Scholar]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Z.; Han, S.-F.; Li, B.-Y.; Zhang, Z.-L.; Qin, L.-Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Soares, M.J. The potential regulatory role of vitamin D in the bioenergetics of inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.J.; Mousa, A.; Ebeling, P.R.; Scott, D.; de Courten, B. Effects of vitamin D supplementation on inflammatory markers in heart failure: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2018, 8, 1169. [Google Scholar] [CrossRef] [PubMed]
- Christner, R.B.; Mortensen, R.F. Specificity of the binding interaction between human serum amyloid P-component and immobilized human C-reactive protein. J. Biol. Chem. 1994, 269, 9760–9766. [Google Scholar] [PubMed]
- Lu, J.; Marjon, K.D.; Mold, C.; Du Clos, T.W.; Sun, P.D. Pentraxins and Fc receptors. Immunol. Rev. 2012, 250, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Deban, L.; Bottazzi, B.; Garlanda, C.; de la Torre, Y.M.; Mantovani, A. Pentraxins: Multifunctional proteins at the interface of innate immunity and inflammation. Biofactors 2009, 35, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Steel, D.M.; Whitehead, A.S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 1994, 15, 81–88. [Google Scholar] [CrossRef]
- Ravnsborg, T.; Svaneklink, S.; Andersen, L.L.T.; Larsen, M.R.; Jensen, D.M.; Overgaard, M. First-trimester proteomic profiling identifies novel predictors of gestational diabetes mellitus. PLoS ONE 2019, 14, e0214457. [Google Scholar] [CrossRef]
- Maekawa, Y.; Nagai, T.; Anzai, A. Pentraxins: CRP and PTX3 and cardiovascular disease. Inflamm. Allergy Drug Targets 2011, 10, 229–235. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Wani, K.; Alnaami, A.M.; Sabico, S.; Al-Ajlan, A.; Chrousos, G.P.; Alokail, M.S. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc. Diabetol. 2015, 14, 101. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Othman, A.; Albanyan, A.; Al-Attas, O.S.; Alokail, M.S.; Sabico, S.; Chrousos, G.P. Perceived stress scores among Saudi students entering universities: A prospective study during the first year of university life. Int. J. Environ Res. Public Health 2014, 11, 3972–3981. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Sulimani, R.; Al-Othman, A.M.; Alfawaz, H.; Fouda, M.; Al-Amri, F.; Shahrani, A.; Alharbi, M.; et al. Vitamin D status correction in Saudi Arabia: An experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch. Osteoporos. 2017, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Al Saleh, Y.; Beshyah, S.A.; Hussein, W.; Almadani, A.; Hassoun, A.; Al Mamari, A.; Ba-Essa, E.; Al-Dhafiri, E.; Hassanein, M.; Fouda, M.A.; et al. Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: An expert consensus summary statement from the GCC vitamin D advisory board. Arch. Osteoporos. 2020, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.R.; Tennent, G.A.; Sethi, D.; Gower, P.E.; Ballardie, F.W.; Amatayakul-Chantler, S.; Pepys, M.B. Serum amyloid P component in chronic renal failure and dialysis. Clin. Chim. Acta 1991, 200, 191–199. [Google Scholar] [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; White, H.E.; O’hara, B.P.; Oliva, G.; Srinivasan, N.; Tickle, I.J.; Blundell, T.L.; Pepys, M.B.; Wood, S.P. Structure of pentameric human serum amyloid P component. Nature 1994, 367, 338–345. [Google Scholar] [CrossRef]
- Ashton, A.W.; Boehm, M.K.; Gallimore, J.R.; Pepys, M.B.; Perkins, S.J. Pentameric and decameric structures in solution of serum amyloid P component by X-ray and neutron scattering and molecular modelling analyses. J. Mol. Biol. 1997, 272, 408–422. [Google Scholar] [CrossRef]
- Li, X.A.; Yutani, C.; Shimokado, K. Serum amyloid P component associates with high density lipoprotein as well as very low density lipoprotein but not with low density lipoprotein. Biochem. Biophys. Res. Commun. 1998, 244, 249–252. [Google Scholar] [CrossRef]
- Li, X.A.; Hatanaka, K.; Ishibashi-Ueda, H.; Yutani, C.; Yamamoto, A. Characterization of serum amyloid P component from human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 252–257. [Google Scholar] [CrossRef]
- Cubedo, J.; Padró, T.; Badimon, L. Coordinated proteomic signature changes in immune response and complement proteins in acute myocardial infarction: The implication of serum amyloid P-component. Int. J. Cardiol. 2013, 168, 5196–5204. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, T.; Zeng, C.; Li, X.; Li, X.; Wen, D.; Ji, T.; Lan, T.; Xing, L.; Li, J.; et al. SAP deficiency mitigated atherosclerotic lesions in ApoE(-/-) mice. Atherosclerosis 2016, 244, 179–187. [Google Scholar] [CrossRef]
- Xi, D.; Luo, T.; Xiong, H.; Liu, J.; Lu, H.; Li, M.; Hou, Y.; Guo, Z. SAP: Structure, function, and its roles in immune-related diseases. Int. J. Cardiol. 2015, 187, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Kawano, Y.; Imamura, T.; Kawakita, K.; Sagara, M.; Matsuo, T.; Kakitsubata, Y.; Ishikawa, T.; Kitamura, K.; Hatakeyama, K.; et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity 2010, 18, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Johnston, H.E.; Singhania, A.; Alokail, M.S.; Alkharfy, K.M.; Abd-Alrahman, S.H.; Sabico, S.L.; Roumeliotis, T.I.; Manousopoulou-Garbis, A.; et al. Whole serum 3D LC-nESI-FTMS quantitative proteomics reveals sexual dimorphism in the milieu intérieur of overweight and obese adults. J. Proteome Res. 2014, 13, 5094–5105. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019, 63–64, 148–154. [Google Scholar] [CrossRef]
| Parameters | Responders | Non-Responders | * p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | p-Value | Before | After | Change | p-Value | ||
| N (M/F) | 155 (56/99) | 55 (19/36) | |||||||
| Age (year) | 39.9 ± 10.6 | 34.6 ± 11.2 | 0.007 | ||||||
| BMI (kg/m2) | 29.2 ± 4.9 | 27.1 ± 5.1 | 0.98 | ||||||
| WHR | 0.94 ± 0.13 | 0.87 ± 0.11 | <0.001 | ||||||
| SBP (mmHg) | 124.5 ± 13.6 | 125.3 ± 15.4 | 0.73 | ||||||
| DBP (mmHg) | 77.4 ± 8.9 | 78.4 ± 9.5 | 0.50 | ||||||
| TC (mmol/L) | 5.15 ± 1.2 | 5.16 ± 1.2 | 0.02 (−0.16–0.19) | 0.86 | 5.10 ± 0.9 | 5.51 ±1.2 | 0.44 (0.13–0.8) | 0.006 | 0.67 |
| HDL-C (mmol/L) | 1.04 ± 0.4 | 1.16 ± 0.4 | 0.11 (0.03–0.19) | 0.007 | 1.1 ± 0.5 | 1.3 ± 0.4 | 0.22 (0.08–0.37) | 0.003 | 0.88 |
| LDL-C (mmol/L) | 3.26 ± 0.9 | 3.15 ± 1.0 | −0.11 (−0.26–0.05) | 0.17 | 3.3 ± 0.8 | 3.4 ± 0.9 | 0.14 (−0.14–0.41) | 0.33 | 0.79 |
| TG (mmol/L) | 1.72 ± 1.1 | 1.75 ± 1.0 | 0.03 (−0.13–0.19) | 0.70 | 1.4 ± 0.8 | 1.7 ± 0.9 | 0.29 (0.04–0.54) | 0.02 | 0.08 |
| 25(OH)D (nmol/L) | 31.7 ± 11.7 | 63.8 ± 19.8 | 32.1 (29.0–35.2) | <0.001 | 31.9 ± 15.3 | 29.1 ± 12.4 | −2.8 (−6.2–0.7) | 0.11 | 0.60 |
| Glucose (mmol/L) | 5.48 ± 0.9 | 5.55 ± 0.9 | 0.07 (−0.10–0.23) | 0.43 | 5.4 ± 0.9 | 5.5 ± 0.8 | 0.13 (−0.17–0.44) | 0.39 | 0.93 |
| SAP (mg/L) | 44.9 (3.1–84.8) | 41.2 (2.5–69.1) | −2.8 (−18.2–1.74) | 0.002 | 40.0 (16.9–57.6) | 42.4 (21.7–67.3) | 1.42(−5.6–8.4) | 0.29 | 0.44 |
| CRP (µg/mL) | 22.8 (4.5–53.5) | 13.5 (4.9–34.5) | −8.2 (−16.6–2.24) | 0.014 | 39.9(14.9–75.9) | 34.7 (8.0–80.4) | −3.5 (−20.1–6.9) | 0.74 | 0.038 |
| Parameters | 25(OH) D (nmol/L) | Group Effect | Group Effect (Adjusted) | |
|---|---|---|---|---|
| Responders | Non-Responders | |||
| Baseline | 31.7 ± 11.7 | 31.9 ± 15.3 | <0.001 | <0.001 |
| 6 month | 63.8 ± 19.8 | 29.1 ± 12.4 | ||
| Mean Difference | 32.1 (29.0–35.2) | −2.8 (−6.2–0.7) | ||
| Change (%) | 103% | 8.8% | ||
| Time effect | <0.001 | |||
| Time effect (adjusted) | <0.001 | |||
| Serum Amyloid P Component (mg/L) | ||||
| Responders | Non-Responders | 0.004 | 0.001 | |
| Baseline | 44.9 (3.1–84.8) | 40.0 (16.9–57.6) | ||
| 6 month | 41.2 (2.5–69.1) | 42.4 (21.7–67.3) | ||
| Mean Difference | −2.8 (−18.2–1.74) | 1.42(−5.6–8.4) | ||
| Change (%) | 6.2% | 3.6% | ||
| Time effect | 0.017 | |||
| Time effect (adjusted) | <0.001 | |||
| Parameters | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| N (M/F) | 56 | 99 | ||||||
| Age (year) | 41.9 ± 9.6 | 37.9 ± 11.0 | ||||||
| BMI (kg/m2) | 28.4 ± 3.8 | 29.6 ± 5.4 | ||||||
| WHR | 0.98 ± 0.10 | 0.92 ± 0.15 | ||||||
| SBP (mmHg) | 130.4 ± 11.5 | 120.3 ± 13.6 | ||||||
| DBP (mmHg) | 81.2 ± 7.1 | 74.8 ± 9.1 | ||||||
| Baseline | 6-Month | Δ | p-Value | Baseline | 6-Month | Δ | p-Value | |
| TC (mmol/L) | 5.02 ± 1.2 | 5.11 ± 1.4 | 0.09 (−0.19–0.37) | 0.52 | 5.22 ± 1.2 | 5.20 ± 1.1 | −0.03 (−0.24–0.19) | 0.79 |
| HDL-C (mmol/L) | 0.94 ± 0.3 | 1.02 ± 0.4 | 0.08 (0.001–0.16) | 0.05 | 1.11 ± 0.5 | 1.24 ± 0.4 | 0.13 (0.01–0.25) | 0.03 |
| LDL-C (mmol/L) | 3.16 ± 0.8 | 3.06 ± 1.04 | −0.10 (−0.4–0.19) | 0.50 | 3.32 ± 0.9 | 3.20 ± 0.9 | −0.12 (−0.31–0.07) | 0.23 |
| TG (mmol/L) | 1.89 ± 1.4 | 1.97 ± 1.2 | 0.08 (0.001–0.16) | 0.54 | 1.61 ± 0.9 | 1.62 ± 0.8 | 0.003 (−0.19–0.20) | 0.98 |
| 25(OH)D (nmol/L) | 34.5 ± 10.3 | 64.6 ± 17.8 | 30.1 (24.9–35.3) | <0.001 | 30.1 ± 12.2 | 63.3 ± 20.9 | 33.2 (29.3–37.1) | <0.001 |
| Glucose (mmol/L) | 5.61 ± 0.9 | 5.87 ± 0.9 | 0.25 (0.03–0.47) | 0.029 | 5.41 ± 1.0 | 5.38 ± 0.8 | −0.03 (−0.26–0.20) | 0.80 |
| SAP (mg/L) | 57.3 (27.7–100.9) | 55.7 (31.2–78.4) | −1.75 (−21.7–7.4) | 0.011 | 38.4 (1.3–74.1) | 28.9 (1.4–62.4) | −0.57 (−16.5–1.2) | 0.046 |
| CRP (µg/mL) | 26.8 (4.7–48.5) | 22.1 (5.2–34.8) | −2.42 (−10.9–3.6) | 0.20 | 22.2 (3.9–61.6) | 7.8 (4.4–32.4) | −0.10 (−28.9–2.20) | 0.036 |
| Parameters | Baseline | 6-Month | ||||
|---|---|---|---|---|---|---|
| All | Males | Females | All | Males | Females | |
| Age (year) | 0.08 | −0.04 | 0.04 | |||
| BMI (kg/m2) | −0.03 | −0.05 | 0.04 | |||
| WHR | −0.07 | −0.17 | −0.18 | |||
| Systolic BP | 0.20 * | 0.19 | 0.03 | |||
| Diastolic BP | 0.33 ** | 0.30 * | 0.20 | |||
| Total Cholesterol | −0.06 | −0.08 | −0.03 | 0.04 | 0.03 | 0.10 |
| HDL-C | −0.30 ** | 0.11 | −0.37 ** | −0.06 | 0.22 | −0.10 |
| LDL-C | −0.05 | −0.18 | 0.02 | 0.02 | 0.14 | 0.01 |
| Triglycerides | 0.10 | −0.03 | 0.14 | 0.12 | −0.19 | 0.23 * |
| 25(OH)D | −0.04 | 0.11 | −0.16 | −0.17 * | −0.27 * | −0.16 |
| Glucose | 0.13 | 0.32 * | 0.04 | 0.12 | 0.04 | 0.14 |
| CRP | 0.55 ** | 0.54 ** | 0.55 ** | 0.39 ** | 0.61 ** | 0.47 ** |
| All Participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Δ SAP | Δ CRP | Δ Cholesterol | Δ HDL | Δ LDL | Δ Triglycerides | Δ VD | Δ Glucose | |
| Δ SAP | 1.00 | |||||||
| Δ CRP | 0.28 ** | 1.00 | ||||||
| Δ TC | −0.05 | 0.26 * | 1.00 | |||||
| Δ HDL-C | −0.30 ** | −0.16 | 0.33 ** | 1.00 | ||||
| Δ LDL-C | 0.11 | 0.31 ** | 0.75 ** | −0.01 | 1.00 | |||
| Δ Triglycerides | −0.05 | 0.05 | 0.29 ** | −0.06 | −0.15 | 1.00 | ||
| Δ 25(OH)D | 0.01 | −0.12 | −0.04 | 0.15 | 0.00 | −0.10 | 1.00 | |
| Δ Glucose | 0.06 | 0.34 ** | 0.19 * | −0.09 | 0.15 | 0.16 | −0.19 * | 1.00 |
| Males | ||||||||
| Δ SAP | 1.00 | |||||||
| Δ CRP | 0.11 | 1.00 | ||||||
| Δ TC | −0.07 | 0.17 | 1.00 | |||||
| Δ HDL-C | −0.31 * | 0.10 | 0.00 | 1.00 | ||||
| Δ LDL-C | 0.01 | 0.31 | 0.83 ** | 0.17 | 1.00 | |||
| Δ Triglycerides | −0.27 * | −0.11 | 0.25 | −0.18 | −0.24 | 1.00 | ||
| Δ 25(OH)D | −0.02 | −0.13 | −0.21 | 0.15 | −0.25 | 0.00 | 1.00 | |
| Δ Glucose | 0.22 | 0.06 | −0.11 | −0.04 | −0.08 | −0.09 | −0.05 | 1.00 |
| Females | ||||||||
| Δ SAP | 1.00 | |||||||
| Δ CRP | 0.09 | 1.00 | ||||||
| Δ TC | −0.06 | 0.08 | 1.00 | |||||
| Δ HDL-C | −0.16 | −0.13 | 0.29 ** | 1.00 | ||||
| Δ LDL-C | 0.02 | 0.06 | 0.71 ** | −0.04 | 1.00 | |||
| Δ Triglycerides | −0.07 | 0.26 | 0.42 ** | −0.05 | 0.10 | 1.00 | ||
| Δ 25(OH)D | 0.10 | −0.03 | 0.07 | 0.00 | 0.02 | −0.02 | 1.00 | |
| Δ Glucose | −0.12 | 0.07 | 0.25 * | −0.17 | 0.25 * | 0.13 | −0.14 | 1.00 |
| Parameters | Male (SAP > 30 mg/L) | Female (SAP > 24 mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month | Δ | Effect Size | p-Value | Baseline | 6-Month | Δ | Effect Size | p-Value | |
| N (M/F) | 42 | 56 | ||||||||
| Age (year) | 41.4 ± 8.8 | 38.8 ± 11.6 | ||||||||
| BMI (kg/m2) | 27.7 ± 3.0 | 29.8 ± 4.4 | ||||||||
| WHR | 0.98 ± 0.06 | 0.89 ± 0.10 | ||||||||
| SBP (mmHg) | 132.0 ± 11.2 | 121.5 ± 13.4 | ||||||||
| DBP (mmHg) | 82.6 ± 6.7 | 76.8 ± 8.4 | ||||||||
| T. Chol (mmol/L) | 4.9 ± 1.1 | 5.0 ± 1.4 | 0.1 (−0.1–0.3) | 0.12 | 0.41 | 5.2 ± 1.3 | 5.2 ± 1.2 | 0.03 (−0.3–3) | 0.026 | 0.85 |
| HDL-C (mmol/L) | 0.97 ± 0.3 | 1.1 ± 0.3 | 0.1 (0.05–0.2) | 0.52 | 0.002 | 1.0 ± 0.4 | 1.2 ± 0.5 | 0.21 (0.05–0.4) | 0.36 | 0.01 |
| LDL-C (mmol/L) | 3.1 ± 0.8 | 3.0 ± 0.9 | −0.08 (−0.3–0.2) | 0.10 | 0.51 | 3.3 ± 0.9 | 3.1 ± 1.0 | −0.12 (−0.4–0.1) | 0.13 | 0.32 |
| TG (mmol/L) | 0.03 ± 0.8 | 0.15 ± 0.5 | 0.1 (−0.05–0.3) | 0.21 | 0.16 | 0.04 ± 1.1 | 0.21 ± 0.8 | 0.17 (0.03–0.3) | 0.34 | 0.016 |
| VD (nmol/L) | 34.9 ± 9.7 | 63 ± 14.1 | 28 (23–33) | 1.61 | <0.001 | 28.6 ± 11.4 | 59.6 ± 20.7 | 30.9 (26–36) | 1.53 | <0.001 |
| Glucose (mmol/L) | 5.8 ± 0.9 | 5.9 ± 0.9 | 0.14 (−0.1–0.4) | 0.21 | 0.25 | 5.5 ± 1.1 | 5.4 ± 0.9 | −0.06 (−0.4–0.3) | 0.06 | 0.71 |
| SAP (mg/L) | 82 (53–109) | 65 (44–88) | −9.5 (−34–7) | 0.39 | 0.007 | 64 (46–104) | 58.4 (38–76) | −13.9 (−33–2.2) | 0.56 | <0.001 |
| CRP (µg/mL) | 36.2 (9–49) | 27.5 (10–41) | −3.0 (−16–4) | 0.32 | 0.07 | 27.7 (5–62) | 9.1 (5.1–33) | −0.4 (−30.1–3.6) | 0.33 | 0.038 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, O.E.; Khattak, M.N.K.; Alnaami, A.M.; Aljohani, N.J.; Al-Daghri, N.M. Vitamin D Correction Down-Regulates Serum Amyloid P Component Levels in Vitamin D Deficient Arab Adults: A Single-Arm Trial. Nutrients 2020, 12, 2880. https://doi.org/10.3390/nu12092880
Amer OE, Khattak MNK, Alnaami AM, Aljohani NJ, Al-Daghri NM. Vitamin D Correction Down-Regulates Serum Amyloid P Component Levels in Vitamin D Deficient Arab Adults: A Single-Arm Trial. Nutrients. 2020; 12(9):2880. https://doi.org/10.3390/nu12092880
Chicago/Turabian StyleAmer, Osama E., Malak N. K. Khattak, Abdullah M. Alnaami, Naji J. Aljohani, and Nasser M. Al-Daghri. 2020. "Vitamin D Correction Down-Regulates Serum Amyloid P Component Levels in Vitamin D Deficient Arab Adults: A Single-Arm Trial" Nutrients 12, no. 9: 2880. https://doi.org/10.3390/nu12092880
APA StyleAmer, O. E., Khattak, M. N. K., Alnaami, A. M., Aljohani, N. J., & Al-Daghri, N. M. (2020). Vitamin D Correction Down-Regulates Serum Amyloid P Component Levels in Vitamin D Deficient Arab Adults: A Single-Arm Trial. Nutrients, 12(9), 2880. https://doi.org/10.3390/nu12092880






