Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids
Abstract
1. Introduction
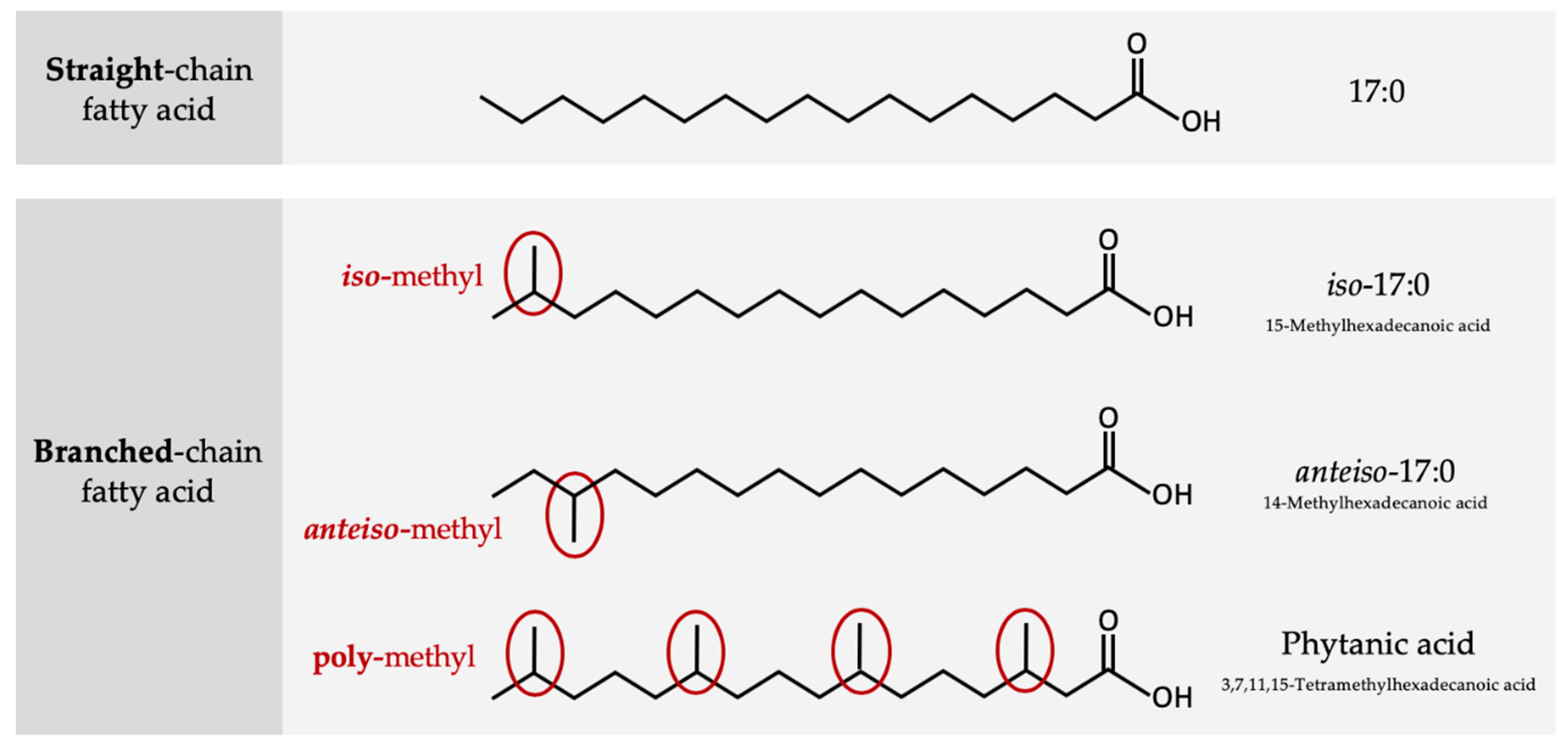
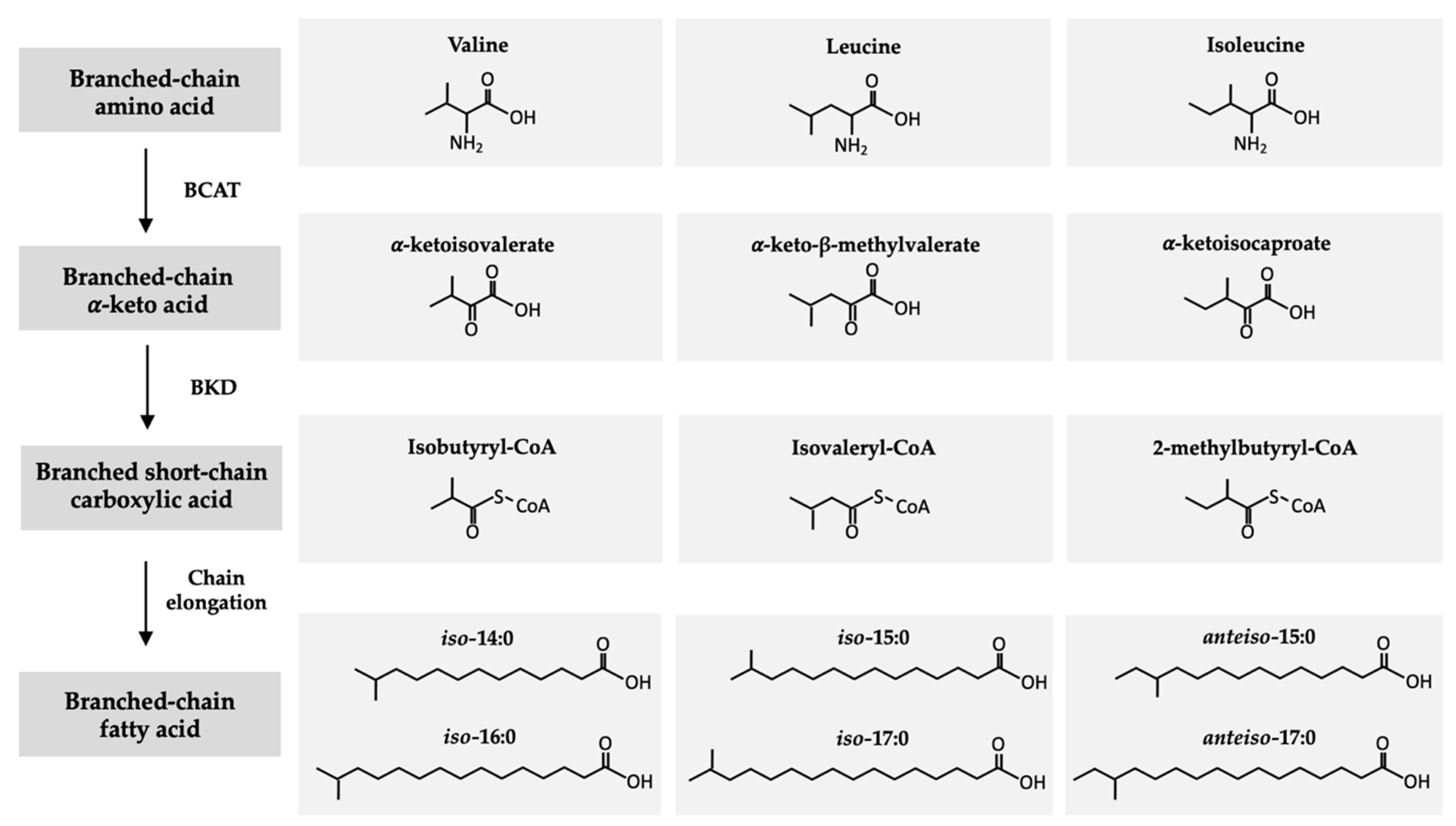
2. Structure and Origin of BCFAs in Ruminants
3. Occurrence and Metabolism of BCFAs in Humans
4. Occurrence of BCFAs in Dairy Products
4.1. Content and Composition of BCFAs in Milk
4.2. BCFAs in Milk across Ruminant Species
4.3. Comparison of BCFAs among Dairy Products
5. Human Trials Assessing Health Effects of BCFAs
5.1. Obesity
5.2. Insulin Sensitivity
5.3. Limitations of Current Evidence Available in Humans
6. Role of Dairy-Derived BCFAs in Health: Potential Mechanisms
6.1. Inflammation
6.2. Anticarcinogenic Properties
6.3. Energy and Glucose Homeostasis
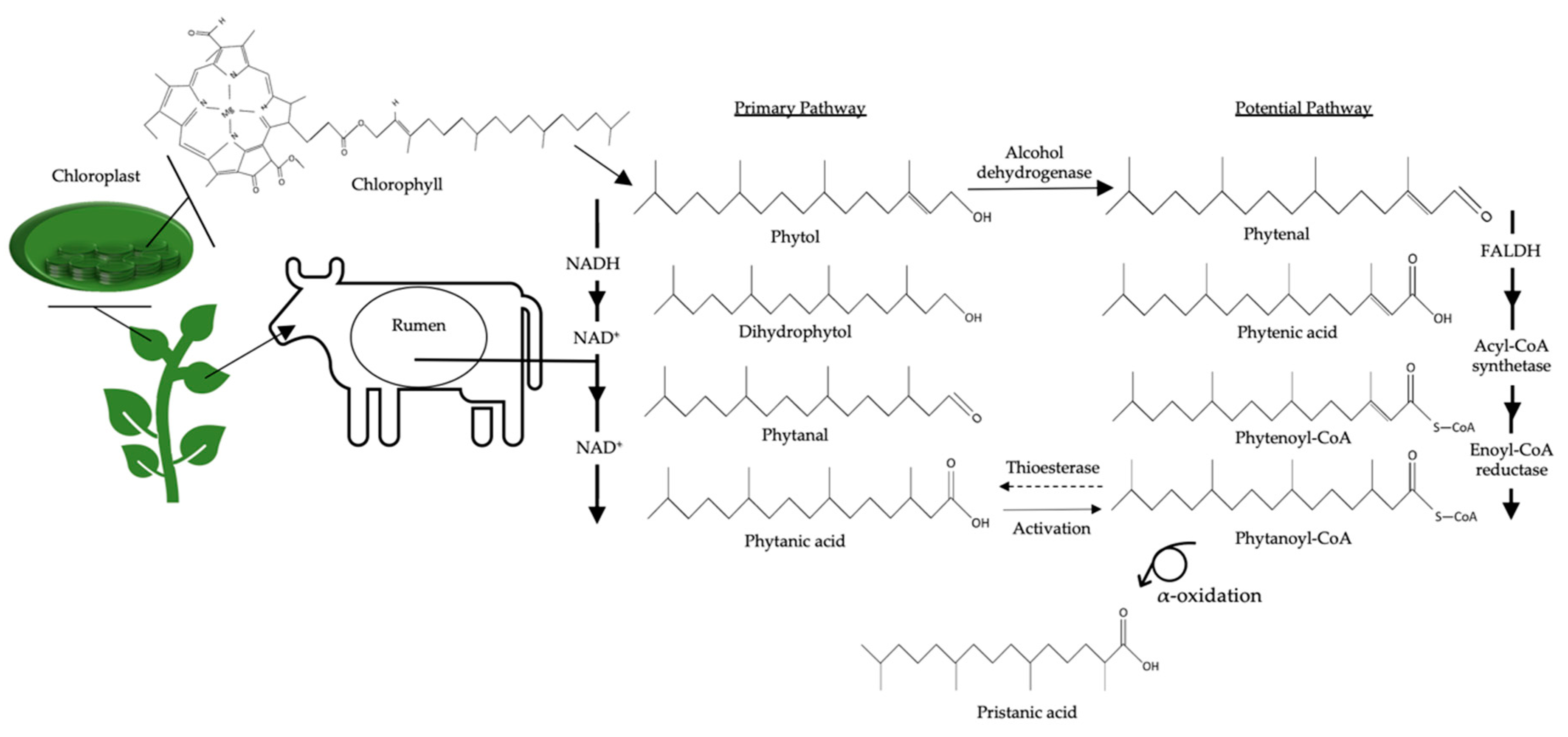
6.4. Biological Functions of Polymethyl BCFAs: Phytanic Acid
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J.; Ward, R.E. Bioactive components in milk. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 653–658. [Google Scholar] [CrossRef]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Unger, A.L.; Bourne, D.E.; Walsh, H.; Kraft, J. Fatty acid content of retail cow’s milk in the northeastern United States—What’s in it for the consumer? J. Agric. Food Chem. 2020, 68, 4268–4276. [Google Scholar] [CrossRef]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Rajion, M.A.; Meng, G.Y.; Boo, L.J.; Ebrahimi, M.; Royan, M.; Sahebi, M.; Azizi, P.; Abiri, R.; Jahromi, M.F. Conjugated linoleic acid: A potent fatty acid linked to animal and human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Pariza, M.W.; Ha, Y.L. Conjugated dienoic derivatives of linoleic acid: A new class of anticarcinogens. Med. Oncol. Tumor Pharmacother. 1990, 7, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Kim Ha, J.; Lindsay, R.C. Method for the quantitative analysis of volatile free and total branched-chain fatty acids in cheese and milk fat. J. Dairy Sci. 1990, 73, 1988–1999. [Google Scholar] [CrossRef]
- Alonso, L.; Fontecha, J.; Lozada, L.; Fraga, M.J.; Juárez, M. Fatty acid composition of caprine milk: Major, branched-chain, and trans fatty acids. J. Dairy Sci. 1999, 82, 878–884. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Thomas Brenna, J. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Corazzin, M.; Romanzin, A.; Sepulcri, A.; Pinosa, M.; Piasentier, E.; Bovolenta, S. Fatty acid profiles of cow’s milk and cheese as affected by mountain pasture type and concentrate supplementation. Animals 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.-D.G.; Kraft, J. Content and composition of branched-chain fatty acids in bovine milk are affected by lactation stage and breed of dairy cow. PLoS ONE 2016, 11, e0150386. [Google Scholar] [CrossRef] [PubMed]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Fong, B.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Pasture feeding conventional cows removes differences between organic and conventionally produced milk. Food Chem. 2017, 229, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Schwendel, B.H.; Morel, P.C.H.; Wester, T.J.; Tavendale, M.H.; Deadman, C.; Fong, B.; Shadbolt, N.M.; Thatcher, A.; Otter, D.E. Fatty acid profile differs between organic and conventionally produced cow milk independent of season or milking time. J. Dairy Sci. 2015, 98, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Wang, X.; Wang, Y.; Xiang, J.; Kothapalli, K.S.D.; Thomas Brenna, J. Branched chain fatty acids positional distribution in human milk fat and common human food fats and uptake in human intestinal cells. J. Funct. Foods 2017, 29, 172–177. [Google Scholar] [CrossRef]
- Or-Rashid, M.M.; Odongo, N.E.; McBride, B.W. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids. J. Anim. Sci. 2007, 85, 1228–1234. [Google Scholar] [CrossRef]
- Kaneda, T. Iso-and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Ann. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Harfoot, C.G. Lipid metabolism in ruminant animals. In Lipid Metabolism in Ruminant Animals; Christie, W.W., Ed.; Pergamon Press Ltd: Oxford, UK, 1981; pp. 1–19. [Google Scholar]
- Jansen, G.A.; Wanders, R.J.A. Alpha-oxidation. Biochim. Biophys. Acta 2006, 1763, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Vetter, W.; Schröder, M. Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chem. 2010, 119, 746–752. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef]
- Gloerich, J.; Van Den Brink, D.M.; Ruiter, J.P.N.; Van Vlies, N.; Vaz, F.M.; Wanders, R.J.A.; Ferdinandusse, S. Metabolism of phytol to phytanic acid in the mouse, and the role of PPARa in its regulation. J. Lipid Res. 2007, 48, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lough, A.K. The phytanic acid content of the lipids of bovine tissues and milk. Lipids 1977, 12, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Harfoot, C.; Hazelwood, G. Lipid metabolism in the rumen. In The Rumen Microbial Ecosystem; Hobson, P., Stewart, C., Eds.; Blackie Academic & Professional: London, UK, 1997; pp. 382–426. [Google Scholar]
- Kniazeva, M.; Crawford, Q.T.; Seiber, M.; Wang, C.Y.; Han, M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004, 2, e257. [Google Scholar] [CrossRef]
- Garcia-Caraballo, S.C.; Comhair, T.M.; Verheyen, F.; Gaemers, I.; Schaap, F.G.; Houten, S.M.; Hakvoort, T.B.M.; Dejong, H.C.; Lamers, W.H.; Koehler, S.E. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim. Biophys. Acta 2013, 1832, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caraballo, S.C.; Comhair, T.M.; Houten, S.M.; Dejong, H.C.; Lamers, W.H.; Koehler, S.E. High-protein diets prevent steatosis and induce hepatic accumulation of monomethyl branched-chain fatty acids. J. Nutr. Biochem. 2014, 25, 1263–1274. [Google Scholar] [CrossRef]
- Hirosuke, O.; Noriyasu, Y.; Junichi, N.; Isao, C. Precursor role of branched-chain amino acids in the biosynthesis of iso and anteiso fatty acids in rat skin. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1994, 1214, 279–287. [Google Scholar] [CrossRef]
- Nicolaides, N. The structures of the branched fatty acids in the wax esters of vernix caseosa. Lipids 1971, 6, 901–905. [Google Scholar] [CrossRef]
- Ran-Ressler, R.; Devapatla, S.; Lawrence, P.; Brenna, J.T. Comparison of BCFA types in vernix and meconium of healthy neonates. FASEB J. 2008, 22. [Google Scholar] [CrossRef]
- Egge, H.; Murawski, U.; Ryhage, R.; György, P.; Chatranon, W.; Zilliken, F. Minor constitutents of human milk IV: Analysis of the branched chain fatty acids. Chem. Phys. Lipids 1972, 8, 42–55. [Google Scholar] [CrossRef]
- Gibson, R.A.; Kneebone, G.M. Fatty acid composition of human colostrum and mature breast milk. Am. J. Clin. Nutr. 1981, 43, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dingess, K.A.; Valentine, C.J.; Ollberding, N.J.; Davidson, B.S.; Woo, J.G.; Summer, S.; Peng, Y.M.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Ran-Ressler, R.R.; et al. Branched-chain fatty acid composition of human milk and the impact of maternal diet: The global exploration of human milk (GEHM) study. Am. J. Clin. Nutr. 2017, 105, 177–184. [Google Scholar] [CrossRef]
- Su, X.; Magkos, F.; Zhou, D.; Eagon, J.C.; Fabbrini, E.; Okunade, A.L.; Klein, S. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity 2015, 23, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Stepnowski, P.; Kaska, L.; Proczko, M.; Wisniewski, P.; Sledzinski, M.; Sledzinski, T. A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity 2016, 24, 1669–1676. [Google Scholar] [CrossRef]
- Nicolaides, N.; Fu, H.C.; Ansari, M.N.A.; Rice, G.R. The fatty acids of wax esters and sterol esters from vernix caseosa and from human skin surface lipid. Lipids 1972, 7, 506–517. [Google Scholar] [CrossRef]
- Nicolaides, N.; Apon, J.M.B. The saturated methyl branched fatty acids of adult human skin surface lipid. Biol. Mass Spectrom. 1977, 4, 337–347. [Google Scholar] [CrossRef]
- Nicolaides, N.; Kaitaranta, J.K.; Rawdah, T.N.; Macy, J.I.; Boswell, F.M.; Smith, R.E. Meibomian gland studies: Comparison of steer and human lipids. Investig. Ophthalmol. Vis. Sci. 1981, 20, 522–536. [Google Scholar]
- Horning, M.G.; Martin, D.B.; Karmen, A.; Vagelos, P.R. Fatty Acid Synthesis in Adipose Tissue. II. Enzymatic Synthesis of Branched Chain and Odd-Numbered Fatty Acids. J. Biol. Chem. 1961, 236, 669–672. [Google Scholar]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Sim, D.; O’Donnell-Megaro, A.M.; Bauman, D.E.; Barbano, D.M.; Brenna, J.T. Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 2011, 46, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.S.D.; Park, H.G.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J.T. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Wang, D.; Lawrence, P.; Wang, X.; Kothapalli, K.S.D.; Greenwald, J.; Liu, R.; Park, H.G.; Brenna, J.T. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatric Res. 2018, 83, 874–879. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Park, H.G.; Xu, C.; Lawrence, P.; Su, X.; Wijendran, V.; Walker, W.A.; Kothapalli, K.S.D.; Brenna, J.T. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 32–39. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Inafuku, M.; Toda, T.; Yanagita, T. Incorporation of branched-chain fatty acid into cellular lipids and caspase-independent apoptosis in human breast cancer cell line, SKBR-3. Lipids Health Dis. 2005, 4, 29. [Google Scholar] [CrossRef]
- Vahmani, P.; Salazar, V.; Rolland, D.C.; Gzyl, K.E.; Dugan, M.E.R. Iso- but not anteiso-branched chain fatty acids exert growth-inhibiting and apoptosis-inducing effects in MCF-7 cells. J. Agric. Food Chem. 2019, 67, 10042–10047. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans fatty acids and bioactive lipids in ruminant milk. In Bioactive Components in Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; pp. 3–66. [Google Scholar]
- Leiber, F.; Kreuzer, M.; Nigg, D.; Wettstein, H.R.; Scheeder, M.R.L. A study on the causes for the elevated n-3 fatty acids in cows’ milk of alpine origin. Lipids 2005, 40, 191–202. [Google Scholar] [CrossRef]
- Schröder, M.; Larissa Lutz, N.; Chick Tangwan, E.; Hajazimi, E.; Vetter, W. Phytanic acid concentrations and diastereomer ratios in milk fat during changes in the cow’s feed from concentrate to hay and back. Eur. Food Res. Technol. 2012, 234, 955–962. [Google Scholar] [CrossRef]
- Che, B.N.; Kristensen, T.; Nebel, C.; Dalsgaard, T.K.; Hellgren, L.I.; Young, J.F.; Larsen, M.K. Content and distribution of phytanic acid diastereomers in organic milk as affected by feed composition. J. Agric. Food Chem. 2013, 61, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Baars, T.; Schröder, M.; Kusche, D.; Vetter, W. Phytanic acid content and SRR/RRR diastereomer ratio in milk from organic and conventional farms at low and high level of fodder input. Org. Agric. 2012, 2, 13–21. [Google Scholar] [CrossRef]
- Vetter, W.; Schröder, M. Phytanic acid—A tetramethyl-branched fatty acid in food. Lipid Technol. 2011, 23, 175–178. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Sun, Q.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am. J. Clin. Nutr. 2016, 104, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef]
- Santaren, I.D.; Watkins, S.M.; Liese, A.D.; Wagenknecht, L.E.; Rewers, M.J.; Haffner, S.M.; Lorenzo, C.; Hanley, A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [Google Scholar] [CrossRef]
- Mozaffarian, D.; De Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Hotamisligil, G.; Tsai, M.Y.; Siscovick, D.S.; Nettleton, J.A. Trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The multi-ethnic study of atherosclerosis (MESA). Am. J. Clin. Nutr. 2013, 97, 854–861. [Google Scholar] [CrossRef]
- Rautiainen, S.; Wang, L.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older women: A prospective cohort study. Am. J. Clin. Nutr. 2016, 103, 979–988. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Pakiet, A.; Wilczynski, M.; Rostkowska, O.; Korczynska, J.; Jabłonska, P.; Kaska, L.; Proczko-Stepaniak, M.; Sobczak, E.; Stepnowski, P.; Magkos, F.; et al. The effect of one anastomosis gastric bypass on branched-chain fatty acid and branched-chain amino acid metabolism in subjects with morbid obesity. Obes. Surg. 2020, 30, 304–312. [Google Scholar] [CrossRef]
- Mika, A.; Wilczynski, M.; Pakiet, A.; Kaska, L.; Proczko-stepaniak, M.; Stankiewicz, M.; Stepnowski, P.; Sledzinski, T. Short-term effect of one-anastomosis gastric bypass on essential fatty acids in the serum of obese patients. Nutrients 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Unger, A.L.; Eckstrom, K.; Jetton, T.L.; Kraft, J. Colonic bacterial composition is sex-specific in aged CD-1 mice fed diets varying in fat quality. PLoS ONE 2019, 14, e0226635. [Google Scholar] [CrossRef] [PubMed]
- Pellattiero, E.; Cecchinato, A.; Tagliapietra, F.; Schiavon, S.; Bittante, G. The use of 2-dimensional gas chromatography to investigate the effect of rumen-protected conjugated linoleic acid, breed, and lactation stage on the fatty acid profile of sheep milk. J. Dairy Sci. 2015, 98, 2088–2102. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Cívico, A.; De La Fuente, M.A.; Núñez Sánchez, N.; Peña Blanco, F.; Martinez Marin, A.L. Effects of dietary concentrate composition and linseed oil supplementation on the milk fatty acid profile of goats. Animal 2018, 12, 2310–2317. [Google Scholar] [CrossRef]
- Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. Fatty acid composition and CLA content in goat milk and cheese samples from Umbrian market. Eur. Food Res. Technol. 2014, 239, 905–911. [Google Scholar] [CrossRef]
- Hauff, S.; Vetter, W. Quantification of branched chain fatty acids in polar and neutral lipids of cheese and fish samples. J. Agric. Food Chem. 2010, 58, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Wang, Z.; Brenna, J.T. Gas chromatography chemical ionization mass spectrometry and tandem mass spectrometry for identification and straightforward quantification of branched chain fatty acids in foods. J. Agric. Food Chem. 2020, 68, 4973–4980. [Google Scholar] [CrossRef]
- Goudjil, H.; Fontecha, J.; Luna, P.; De La Fuente, M.A.; Alonso, L.; Juárez, M. Quantitative characterization of unsaturated and trans fatty acids in ewe’s milk fat. Lait 2004, 84, 473–482. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Orford, M.; Miltiadou, D.; Papachristoforou, C. Partial suckling of lambs reduced the linoleic and conjugated linoleic acid contents of marketable milk in Chios ewes. J. Dairy Sci. 2015, 98, 1739–1749. [Google Scholar] [CrossRef]
- Lopez, A.; Vasconi, M.; Moretti, V.M.; Bellagamba, F. Fatty acid profile in goat milk from high- and low-input conventional and organic systems. Animal 2019, 9, 452. [Google Scholar] [CrossRef]
- Serment, A.; Schmidely, P.; Giger-Reverdin, S.; Chapoutot, P.; Sauvant, D. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats. J. Dairy Sci. 2011, 94, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Valenti, B.; Luciano, G.; Morbidini, L.; Rossetti, U.; Codini, M.; Avondo, M.; Priolo, A.; Bella, M.; Natalello, A.; Pauselli, M. Dietary pomegranate pulp: Effect on ewe milk quality during late lactation. Animals 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Currò, S.; Manuelian, C.; De Marchi, M.; Claps, S.; Rufrano, D.; Neglia, G. Effects of breed and stage of lactation on milk fatty acid composition of Italian goat breeds. Animals 2019, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.; Doreau, M.; Ferlay, A.; Chilliard, Y. Technical note: Estimation of milk fatty acid yield from milk fat data. J. Dairy Sci. 2007, 90, 2302–2304. [Google Scholar] [CrossRef] [PubMed]
- FoodData Central—Butterkase. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/522677/nutrients (accessed on 11 May 2020).
- FoodData Central—Cheese, Brie. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172177/nutrients (accessed on 11 May 2020).
- FoodData Central—Cheese, Swiss. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171251/nutrients (accessed on 7 May 2020).
- FoodData Central—Ricotta Cheese, Ricotta. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/678748/nutrients (accessed on 14 May 2020).
- FoodData Central—Cheese, Camembert. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172178/nutrients (accessed on 11 May 2020).
- FoodData Central—Cheese, Cottage, Creamed, Large or Small Curd. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172179/nutrients (accessed on 7 May 2020).
- FoodData Central—Cheese, Gouda. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171241/nutrients (accessed on 11 May 2020).
- FoodData Central—Cheese, Limburger. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172175/nutrients (accessed on 11 May 2020).
- FoodData Central—Cheese, Mozzarella, Whole Milk. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170845/nutrients (accessed on 7 May 2020).
- FoodData Central—Cheese, Pasteurized Process, American, Fortified with Vitamin D. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170853/nutrients (accessed on 7 May 2020).
- FoodData Central—Cheese, Provolone. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170850/nutrients (accessed on 7 May 2020).
- FoodData Central—Cheese, Ricotta, Whole Milk. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170851/nutrients (accessed on 7 May 2020).
- FoodData Central—Yogurt, Plain, Whole Milk. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171284/nutrients (accessed on 4 May 2020).
- FoodData Central—Butter, Salted. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173410/nutrients (accessed on 4 May 2020).
- FoodData Central—Milk, Sheep, Fluid. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170882/nutrients (accessed on 5 May 2020).
- FoodData Central—Milk, Goat, Fluid, with Added Vitamin D. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171278/nutrients (accessed on 5 May 2020).
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, P.; Després, J.P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Couillard, C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity 2006, 14, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.C.; Steiner, M.J.; Henderson, F.W.; Perrin, E.M. Multiple markers of inflammation and weight status: Cross-sectional analyses throughout childhood. Pediatrics 2010, 125, e801–e809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Chao, J.; Wolfaardt, G.M.; Arts, M.T. Characterization of pseudomonas aeruginosa fatty acid profiles in biofilms and batch planktonic cultures. Can. J. Microbiol. 2010, 56, 1028–1039. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, H.; Qian, D.; Chen, K.; Luo, J.; Tian, Y.; Lin, T.; Lin, T. 13-methyltetradecanoic acid exhibits anti-tumor activity on T-cell lymphomas in vitro and in vivo by down-regulating p-AKT and activating caspase-3. PLoS ONE 2013, 8, e65308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, S.; Chen, X.; Chen, H.; Huang, M.; Zheng, J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000, 60, 505–509. [Google Scholar] [PubMed]
- Lin, T.; Yin, X.B.; Cai, Q.; Fan, X.; Xu, K.; Huang, L.; Luo, J.; Zheng, J.; Huang, J. 13-Methyltetradecanoic acid induces mitochondrial-mediated apoptosis in human bladder cancer cells. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 339–345. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.C.; Yang, P.; Van Pelt, C.S.; Hicks, M.E.; Collin, P.; Newman, R.A. Evaluation of targeted arterial delivery of the branched chain fatty acid 12-methyltetradecanoic acid as a novel therapy for solid tumors. J. Exp. Ther. Oncol. 2005, 5, 55–68. [Google Scholar] [PubMed]
- Yu, J.; Yang, L.N.; Wu, Y.Y.; Li, B.H.; Weng, S.M.; Hu, C.L.; Han, Y.L. 13-Methyltetradecanoic acid mitigates cerebral ischemia/reperfusion injury. Neural Regen. Res. 2016, 11, 1431–1437. [Google Scholar] [CrossRef]
- Cole, N.; Hume, E.B.; Jalbert, I.; Vijay, A.K.; Krishnan, R.; Willcox, M.D. Effects of topical administration of 12-methyl tetradecanoic acid (12-MTA) on the development of corneal angiogenesis. Angiogenesis 2007, 10, 47–54. [Google Scholar] [CrossRef]
- Brooks, K.K.; Liang, B.; Watts, J.L. The influence of bacterial diet on fat storage in C. elegans. PLoS ONE 2009, 4, e7545. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, D.; Lei, H.; Peng, T.; Li, J.; Cheng, T.; He, J. Branched-chain fatty acids lower triglyceride levels in a fatty liver model in vitro. FASEB J. 2017, 31. [Google Scholar] [CrossRef]
- Kraft, J.; Jetton, T.; Satish, B.; Gupta, D. Dairy-derived bioactive fatty acids improve pancreatic ß-cell function. FASEB J. 2015, 29. [Google Scholar] [CrossRef]
- Roca-Saavedra, P.; Mariño-Lorenzo, P.; Miranda, J.M.; Porto-Arias, J.J.; Lamas, A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A. Phytanic acid consumption and human health, risks, benefits and future trends: A review. Food Chem. 2017, 221, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sahu, U.; Kar, S.; Parvez, S. Phytanic acid-induced neurotoxicological manifestations and apoptosis ameliorated by mitochondria-mediated actions of melatonin. Mol. Neurobiol. 2017, 54, 6960–6969. [Google Scholar] [CrossRef]
- Chaudhary, S.; Parvez, S. Phytanic acid induced neurological alterations in rat brain synaptosomes and its attenuation by melatonin. Biomed. Pharmacother. 2017, 95, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Refsum Disease—Genetics Home Reference—NIH. Available online: https://ghr.nlm.nih.gov/condition/refsum-disease#statistics (accessed on 30 June 2020).
- Nakanishi, T.; Motoba, I.; Anraku, M.; Suzuki, R.; Yamaguchi, Y.; Erickson, L.; Eto, N.; Sugamoto, K.; Matsushita, Y.; Kawahara, S. Naturally occurring 3RS, 7R, 11R-phytanic acid suppresses in vitro T-cell production of interferon-gamma. Lipids Health Dis. 2018, 17, 147. [Google Scholar] [CrossRef]
- An, J.-Y.; Jheng, H.-F.; Nagai, H.; Sanada, K.; Takahashi, H.; Iwase, M.; Watanabe, N.; Kim, Y.-I.; Teraminami, A.; Takahashi, N.; et al. A Phytol-Enriched Diet Activates PPAR-α in the Liver and Brown Adipose Tissue to Ameliorate Obesity-Induced Metabolic Abnormalities. Mol. Nutr. Food Res. 2018, 62, 1700688. [Google Scholar] [CrossRef]
- Lamichane, S.; Lamichane, B.D.; Kwon, S.M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci. 2018, 19, 914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, X.; Du, M. Phytanic acid activates PPARα to promote beige adipogenic differentiation of preadipocytes. J. Nutr. Biochem. 2019, 67, 201–211. [Google Scholar] [CrossRef]
| FA 1 | Proportion (% of Total FAs) 2 | Content (mg/Three Daily Servings) 3 |
|---|---|---|
| iso-13:0 | 0.02–0.03 [4,13,14,15] 4 | 5–7 [4,13,14,15] 4 |
| anteiso-13:0 | 0.07–0.09 [4,13] 4 | 15–19 [4,13] 4 |
| iso-14:0 | 0.08–0.22 [4,11,12,13,14,15] 4 | 18–48 [4,11,12,13,14,15] 4 |
| iso-15:0 | 0.13–0.44 [4,11,12,13,14,15] 4 | 29–97 [4,11,12,13,14,15] 4 |
| anteiso-15:0 | 0.37–0.93 [4,11,12,13,14,15] 4 | 81–206 [4,11,12,13,14,15] 4 |
| iso-16:0 | 0.17–0.45 [4,11,12,13,14,15] 4 | 38–100 [4,11,12,13,14,15] 4 |
| iso-17:0 | 0.26–0.56 [4,11,12,13,14,15] 4 | 58–123 [4,11,12,13,14,15] 4 |
| anteiso-17:0 | 0.11–0.76 [4,11,12,13,14,15] 4 | 24–169 [4,11,12,13,14,15] 4 |
| iso-18:0 | 0.01–0.09 [4,11,12,13,14,15] 4 | 2–20 [4,11,12,13,14,15] 4 |
| Σ iso-BCFAs | 0.87–1.75 [4,11,12,13,14,15] 4 | 193–387 [4,11,12,13,14,15] 4 |
| Σ anteiso-BCFAs | 0.67–1.69 [4,11,12,13,14,15] 4 | 149–375 [4,11,12,13,14,15] 4 |
| Σ BCFAs 5 | 1.66–3.44 [4,11,12,13,14,15] 4 | 367–763 [4,11,12,13,14,15] 4 |
| FA 2 | Milk 3 | Cheese 4 | Yogurt 5 | Butter 6 | Sheep Milk 7 | Goat Milk 8 | ||
|---|---|---|---|---|---|---|---|---|
| Soft/Semi-Soft 9 | Semi-Hard/Hard 10 | Unknown 11 | ||||||
| iso-13:0 | 2 [4,13,14,15] 12 | 2 [65] | 8 [66] | 1 [67] | ||||
| anteiso-13:0 | 5–6 [4,13] 12 | 9 [65] | 5 [66] | 1–7 [67,68] | ||||
| iso-14:0 | 6–16 [4,11,12,13,14,15] 12 | 5–14 [11,69] | 6–20 [11,12,69] | 56–13 [11,69] | 9 [11] | 8–18 [11,65,70] | 8–24 [66,71,72] | 5–10 [67,68,73,74] |
| iso-15:0 | 10–32 [4,11,12,13,14,15] 12 | 6–33 [11,69] | 8–41 [11,12,69] | 12–33 [11,69] | 11 [11] | 1–22 [11,65,70] | 21–71 [66,71,72,75] | 13–36 [67,68,73,74,76] |
| anteiso-15:0 | 27–69 [4,11,12,13,14,15] 12 | 26–68 [11,69] | 33–87 [11,12,69] | 35–62 [11,69] | 47 [11] | 36–67 [11,65,70] | 45–122 [66,71,72,75] | 25–47 [67,68,73,74,76] |
| iso-16:0 | 13–33 [4,11,12,13,14,15] 12 | 12–30 [11,69] | 9–42 [11,12,69] | 11–26 [11,69] | 22 [11] | 20–36 [11,65,70] | 25–69 [66,71,72,75] | 13–22 [67,68,73,74] |
| iso-17:0 | 19–41 [4,11,12,13,14,15] 12 | 8–41 [11,69] | 8–52 [11,12,69] | 14–38 [11,69] | 19 [11] | 22–34 [11,65,70] | 34–115 [66,71,72,75] | 20–58 [67,68,73,74,76] |
| anteiso-17:0 | 8–56 [4,11,12,13,14,15] 12 | 23–71 [11,69] | 17–71 [11,12,69] | 25–61 [11,69] | 45 [11] | 34–42 [11,65,70] | 48–124 [66,71,72,75] | 25–57 [67,68,73,74,76] |
| iso-18:0 | 1–7 [4,11,12,13,14,15] 12 | <1–8 [11,69] | <1–8 [11,12,69] | <1–7 [11,69] | 3 [11] | <1–7 [11,65,70] | 17–20 [66,72] | 5 [67] |
| Σ iso-BCFAs | 64–129 [4,11,12,13,14,15] 12 | 32–127 [11,69] | 32–164 [11,12,69] | 53–116 [11,69] | 64 [11] | 68–106 [11,65,70] | 108–255 [66,71,72,75] | 53–96 [67,68,73,74,76] |
| Σ anteiso-BCFAs | 50–125 [4,11,12,13,14,15] 12 | 51–139 [11,69] | 50–158 [11,12,69] | 68–123 [11,69] | 91 [11] | 69–107 [11,65,70] | 96–247 [66,71,72,75] | 50–104 [67,68,73,74,76] |
| Σ BCFAs 13 | 123–254 [4,11,12,13,14,15] 12 | 83–264 [11,69] | 82–322 [11,12,69] | 123–239 [11,69] | 152 [11] | 137–204 [11,65,70] | 204–502 [66,71,72,75] | 109–180 [67,68,73,74,76] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients 2020, 12, 2875. https://doi.org/10.3390/nu12092875
Taormina VM, Unger AL, Schiksnis MR, Torres-Gonzalez M, Kraft J. Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients. 2020; 12(9):2875. https://doi.org/10.3390/nu12092875
Chicago/Turabian StyleTaormina, Victoria M., Allison L. Unger, Morgan R. Schiksnis, Moises Torres-Gonzalez, and Jana Kraft. 2020. "Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids" Nutrients 12, no. 9: 2875. https://doi.org/10.3390/nu12092875
APA StyleTaormina, V. M., Unger, A. L., Schiksnis, M. R., Torres-Gonzalez, M., & Kraft, J. (2020). Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients, 12(9), 2875. https://doi.org/10.3390/nu12092875





