Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants Eligibility
2.2. Design of Human Study
2.3. Study Diets
2.4. Measurements of Body Composition
2.5. Measurements of Vascular Function
2.6. Measurements of Biomarkers in Blood and Urine
2.7. Extraction and Quantification of Polyphenols
2.8. Assessments of HRQOL
2.9. Statistical Analysis
3. Results
3.1. Nutritional Composition, Polyphenol Content, and Antioxidant Capacity of Test Cookies
3.2. General Characteristics of Participants
3.3. Vascular Function
3.4. Oxidative Stress Markers in Plasma and Urine
3.5. NO Concentration in the Plasma and Urine
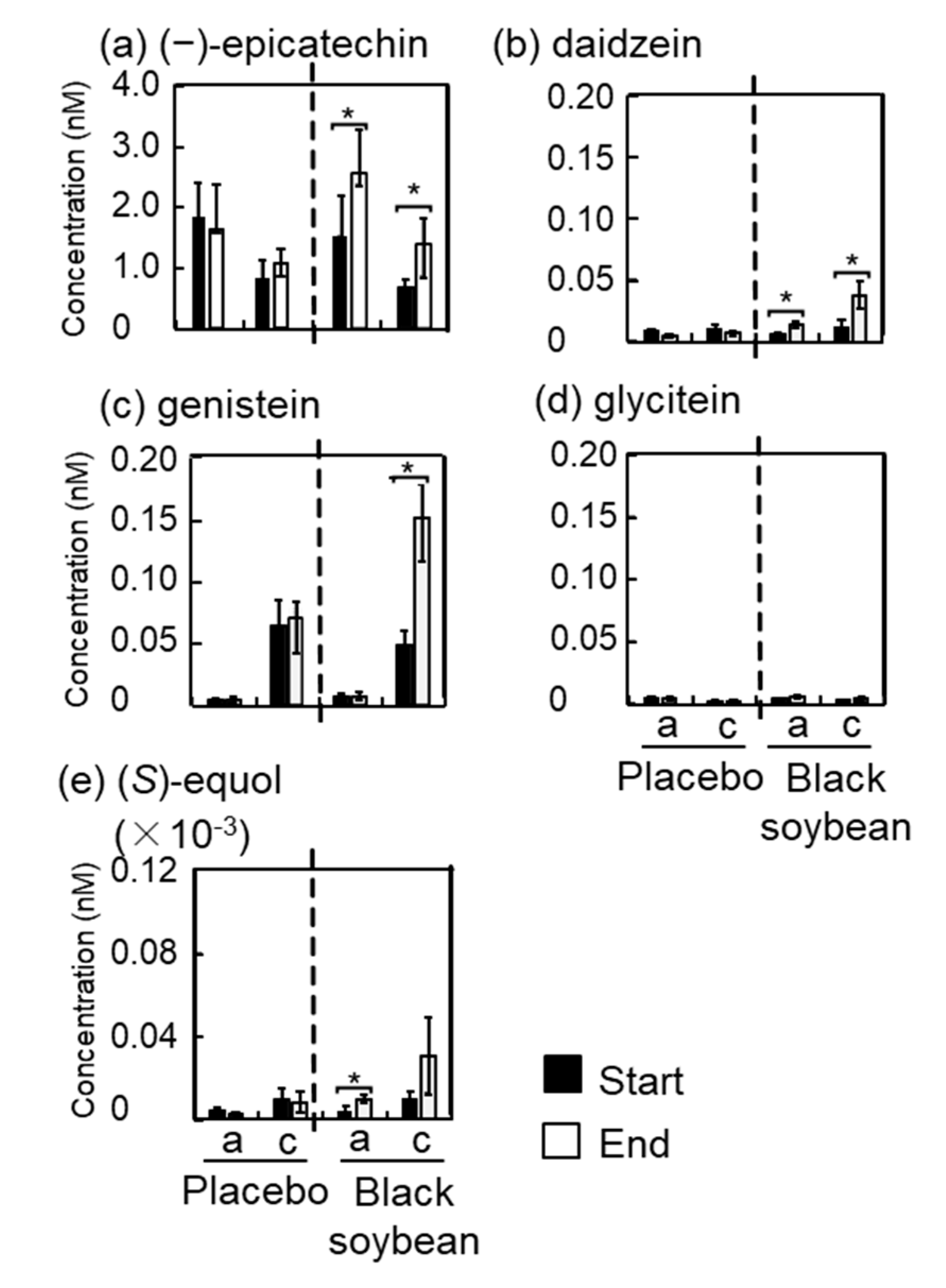
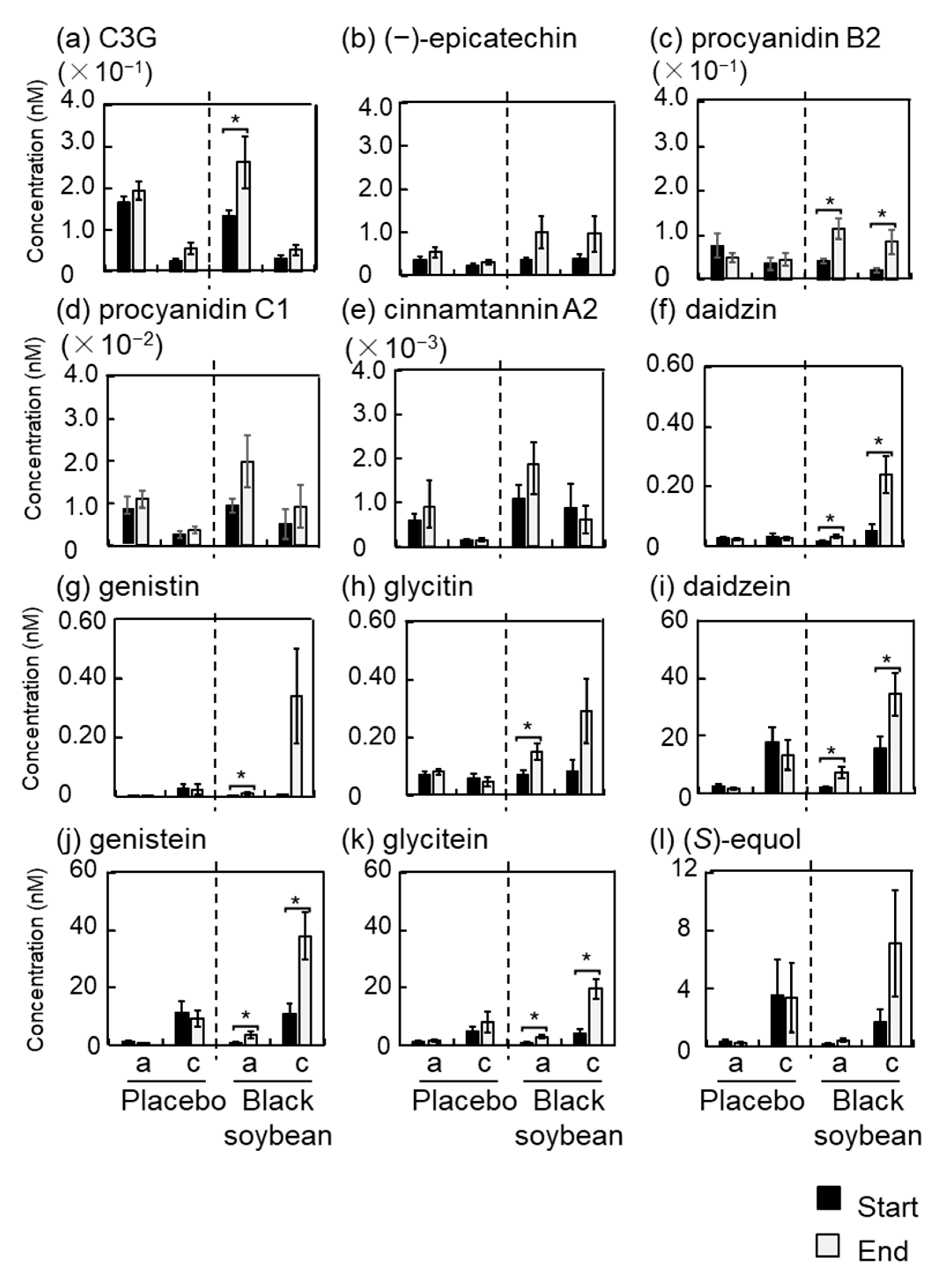
3.6. Polyphenol Concentrations in Plasma and Urine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAPH | 2, 2′−Azobis (2−amidinopropane) dihydrochloride |
| APG | acceleration plethysmogram, BMI: body mass index |
| CVD | cardiovascular diseases |
| C3G | cyanidin-3-O-glucoside 2′-deoxyguanosine |
| GLP-1 | Glucagon-like peputide-1 |
| HPLC | high-performance liquid chromatography |
| HRQOL | health-related quality of life |
| HEL | hexanoyl-lysine |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| MPO | myeloperoxidase |
| NO | nitric oxide. |
References
- Jensen-Urstad, K.; Johansson, J.; Jensen-Urstad, M. Vascular function correlates with risk factors for cardiovascular disease in a healthy population of 35-year-old subjects. J. Intern. Med. 1997, 241, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol. Res. 2020, 152, 104625. [Google Scholar] [CrossRef]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. 2019, 25, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Todd, J.J.; Vodkin, L.O. Pigmented Soybean (Glycine max) Seed Coats Accumulate Proanthocyanidins during Development. Plant Physiol. 1993, 102, 663–670. [Google Scholar] [CrossRef]
- Choung, M.G.; Baek, I.Y.; Kang, S.T.; Han, W.Y.; Shin, D.C.; Moon, H.P.; Kang, K.H. Isolation and Determination of Anthocyanins in Seed Coats of Black Soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Li, X.; Zhang, T.; Mitani, T.; Yasuda, M.; Nanba, F.; Toda, T.; Yamashita, Y.; Ashida, H. Black Soybean Seed Coat Polyphenols Prevent AAPH-induced Oxidative DNA-damage in HepG2 Cells. Clin. Biochem. Nutr. 2017, 60, 108–114. [Google Scholar] [CrossRef]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food. Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef]
- Byun, J.S.; Han, Y.S.; Lee, S.S. The effects of yellow soybean, black soybean, and sword bean on lipid levels and oxidative stress in ovariectomized rats. Int. J. Vitam. Nutr. Res. 2010, 80, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Lim, K.M.; Kim, C.W.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Noh, J.Y.; Bae, O.N.; Shin, S.; Chung, J.H. Black soybean extract can attenuate thrombosis through inhibition of collagen-induced platelet activation. J. Nutr. Biochem. 2011, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black soybean improves the vascular function through an increase in nitric oxide and a decrease in oxidative stress in healthy women. Arch. Biochem. Biophys. 2020, 688, 108408. [Google Scholar] [CrossRef]
- Domae, C.; Nanba, F.; Maruo, T.; Suzuki, T.; Ashida, H.; Yamashita, Y. Black soybean seed coat polyphenols promote nitric oxide production in the aorta through glucagon-like peptide-1 secretion from the intestinal cells. Food. Funct. 2019, 10, 7875–7882. [Google Scholar] [CrossRef]
- G*Power: Heinrich-Heine-Universität Düsseldorf. Available online: https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html (accessed on 8 September 2020).
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food. Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef]
- Takazawa, K.; Tanaka, N.; Fujita, M.; Matsuoka, O.; Saiki, T.; Aikawa, M.; Tamura, S.; Ibukiyama, C. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 1998, 32, 365–370. [Google Scholar] [CrossRef]
- Ahn, J.M. Wave detection in acceleration plethysmogram. Healthc. Inform. Res. 2015, 21, 111–117. [Google Scholar] [CrossRef]
- Ahn, J.M. New Aging Index Using Signal Features of Both Photoplethysmograms and Acceleration Plethysmograms. Healthc. Inform. Res. 2017, 23, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Yamashita, Y.; Saito, A.; Ashida, H. An analysis method for flavan-3-ol using high performance liquid chromatography coupled with a fluorescence detector. J. Food. Drug. Anal. 2017, 25, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Bito, S.; Grenn, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Umans, J.G.; Levi, R. Nitric oxide in the regulation of blood flow and arterial pressure. Annu. Rev. Physiol. 1995, 57, 771–790. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Guseva, Canu, I.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Kato, Y.; Mori, Y.; Makino, Y.; Morimitsu, Y.; Hiroi, S.; Ishikawa, T.; Osawa, T. Formation of Nepsilon-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J. Biol. Chem. 1999, 274, 20406–20414. [Google Scholar] [CrossRef]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef]
- Lai, C.Q.; Tucker, K.L.; Parnell, L.D.; Adiconis, X.; García-Bailo, B.; Griffith, J.; Meydani, M.; Ordovás, J.M. PPARGC1A variation associated with DNA damage, diabetes, and cardiovascular diseases: The boston puerto rican health study. Diabetes 2008, 57, 809–816. [Google Scholar] [CrossRef][Green Version]
- Urquiaga, I.; Strobel, P.; Perez, D.; Adiconis, X.; García-Bailo, B.; Griffith, J.; Meydani, M.; Ordovás, J.M. Mediterranean diet and red wine protect against oxidative damage in young volunteers. Atherosclerosis 2010, 211, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.; Silva, T.R.; Maturana, M.A.; Spritzer, P.M. Dietary intake of isoflavones is associated with a lower prevalence of subclinical cardiovascular disease in postmenopausal women: Cross-sectional study. J. Hum. Nutr. Diet. 2019, 32, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.H.; Noh, H.; Kim, H.W.; Cho, S.Y.; Kim, H.J.; Lee, S.H.; Lee, S.H.; Gunter, M.J.; Ferrari, P.; Scalbert, A.; et al. Metabolic tracking of isoflavones in soybean products and biosamples from healthy adults after fermented soybean consumption. Food. Chem. 2020, 330, 12731. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A. Effect of soy isoflavone supplementation on endothelial dysfunction and oxidative stress in equol-producing postmenopausal women. Endocr. Metab. Immune. Disord. Drug. Targets. 2015, 15, 71–79. [Google Scholar]
- Ohkurab, Y.; Obayashi, S.; Yamada, K.; Yamada, M.; Kubota, T. S-equol Partially Restored Endothelial Nitric Oxide Production in Isoflavone-deficient Ovariectomized Rats. J. Cardiovasc. Pharmacol. 2015, 65, 500–507. [Google Scholar] [CrossRef]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef]
- Streppel, M.T.; Arends, L.R.; van’t Veer, P.; Grobbee, D.E.; Geleijnse, J.M. Dietary fiber and blood pressure: A meta-analysis of randomized placebo-controlled trials. Arch. Intern. Med. 2005, 165, 150–156. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||
| Content (g) | ||
| Placebo | Black Soybean | |
| Wheat flour | 20 | 0 |
| Roasted black soybean powder | 0 | 20 |
| Vegetable oil | 8.3 | 7.3 |
| Water | 6.1 | 6.1 |
| Liquid sucrose | 5.3 | 5.3 |
| Sugar | 2.3 | 2.3 |
| Indigestible dextrin | 0.1 | 0.1 |
| Salt | 0.1 | 0.1 |
| Baking powder | 4 | 7.3 |
| Total | 46.3 | 48.5 |
| Weight after baking | 39 | 42 |
| (B) | ||
| Placebo | Black Soybean | |
| Calories | 201.7 kcal | 192.5 kcal |
| Protein | 2.0 g | 7.5 g |
| Fat | 8.9 g | 9.7 g |
| Carbohydrate | 28.4 g | 18.8 g |
| Ash | 0.2 g | 1.1 g |
| Sodium | 49.6 mg | 54.2 mg |
| Dietary fiber | 0.6 g | 3.5 g |
| Salt equivalents | 0.1 g | 0.1 g |
| Content (mg) | ||
|---|---|---|
| Placebo | Black Soybean | |
| Anthocyanidin | ||
| Cyanidin-3-O-glucoside | ND | 1.12 |
| Flavan-3-ols | ||
| (−)-Epicatechin | ND | 0.038 |
| Procyanidin B2 | ND | 0.049 |
| Procyanidin C1 | ND | 0.064 |
| Cinnamtannin A2 | ND | 0.044 |
| Isoflavones | ||
| Daizein | ND | 0.046 |
| Daidzin | ND | 5.99 |
| Glycitein | ND | 0.04 |
| Glycitin | ND | 0.324 |
| Genistein | ND | 0.085 |
| Genistin | ND | 2.27 |
| Total polyphenol (mg gallic acid equivalent) | 3.20 ± 0.02 | 20.0 ± 3.99 |
| Antioxidant activity (mg Trolox equivalent) | 0.98± 0.03 | 24.6 ± 0.23 |
| Placebo | Black Soybean | |||
|---|---|---|---|---|
| Anthropometric parameters | Start | End | Start | End |
| Body weight (kg) | 63.8 ± 2.7 | 64.3 ± 2.7 * | 64.0 ± 2.8 | 63.9 ± 2.7 |
| BMI | 23.1 ± 0.8 | 23.2 ± 0.8 * | 23.1 ± 0.8 | 23.1 ± 0.8 |
| Body fat (%) | 23.9 ± 1.7 | 25.4 ± 1.5 * | 24.3 ± 1.7 | 24.3 ± 1.7 |
| Visceral fat (%) | 8.4 ± 1.0 | 8.4 ± 1.0 | 8.5 ± 1.0 | 8.5 ± 1.0 |
| Biological age | 36.3 ± 2.8 | 37.3 ± 2.8 | 37.1 ± 2.7 | 37.0 ± 2.8 |
| Basal metabolic rate (kcal/day) | 1368 ± 53 | 1360 ± 52 | 1366 ± 52 | 1362 ± 51 |
| Estimated bone mass (kg) | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 |
| Muscle mass (%) | 45.7 ± 2.0 | 45.1 ± 2.0 | 45.7 ± 2.0 | 45.5 ± 2.0 |
| Vascular Function | Placebo | Black Soybean | ||
|---|---|---|---|---|
| Start | End | Start | End | |
| APG | ||||
| Vascular age | 52.5 ± 11.0 | 52.2 ± 11.2 | 52.2 ± 10.2 | 49.3 ± 9.3 †,* |
| a wave | 109.0 ± 10.9 | 113.0 ± 7.8 | 111.1 ± 9.5 | 109.3 ± 9.1 |
| b wave | −57.8 ± 16.2 | −59.0 ± 14.7 | −58.6 ± 13.1 | −62.3 ± 12.9 |
| c wave | −32.2 ± 17.9 | −36.3 ± 20.7 | −35.9 ± 19.3 | −27.3 ± 20.4 |
| d wave | −50.4 ± 19.3 | −55.1 ± 23.3 | −56.1 ± 22.9 | −44.1 ± 20.5 * |
| Vascular waveform | 3.8 ± 1.4 | 3.8 ± 1.3 | 3.8 ± 1.4 | 3.1 ± 1.3 † |
| Waveform score | 44.5 ± 12.8 | 43.8 ± 11.6 | 43.5 ± 12.6 | 51.0 ± 14.0 †,* |
| Peripheral vascular health | 62.0 ± 12.9 | 60.6 ± 11.9 | 62.7 ± 11.4 | 70.0 ± 15.4 †,* |
| Blood pressure | ||||
| Systolic blood pressure | 122.3 ± 3.5 | 125.7 ± 3.1 | 129.4 ± 3.9 | 121.9 ± 3.1 †,* |
| Diastolic blood pressure | 81.0 ± 2.2 | 82.5 ± 2.3 | 84.5 ± 2.7 | 80.9 ± 2.0 |
| Central blood pressure | 129.4 ± 3.5 | 132.6 ± 3.3 | 134.2 ± 4.3 | 127.6 ± 3.2 |
| Vascular Function | Placebo | Black Soybean | ||
|---|---|---|---|---|
| Start | End | Start | End | |
| Plasma | ||||
| NO2/NO3 (μM) | 29.1 ± 3.8 | 9.6 ± 1.4 | 29.1 ± 2.7 | 35.4 ± 2.9 † |
| HEL (nM) | 4.3 ± 0.2 | 4.6 ± 0.2 | 4.3 ± 0.2 | 4.5 ± 0.2 |
| MPO (ng/mL) | 88.1 ± 3.0 | 90.6 ± 3.7 | 86.6 ± 3.0 | 85.5 ± 2.8 |
| 8-OHdG (ng/mL) | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.1 *,† |
| Urine | ||||
| NO2/NO3 (μM) | 20.9 ± 5.3 | 15.5 ± 3.4 | 18.8 ± 4.2 | 31.5 ± 7.2 † |
| HEL (nM) | 110.5 ± 30.0 | 122.5 ± 41.1 | 128.5 ± 23.0 | 114.6 ± 15.6 |
| MPO (ng/mL) | 10.5 ± 0.4 | 14.0 ± 3.9 | 10.1 ± 0.1 | 0.1 ± 0.1 |
| 8-OHdG (ng/mL) | 9.4 ± 1.7 | 12.5 ± 2.6 | 8.8 ± 1.5 | 7.6 ± 0.7 † |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans. Nutrients 2020, 12, 2755. https://doi.org/10.3390/nu12092755
Yamashita Y, Nakamura A, Nanba F, Saito S, Toda T, Nakagawa J, Ashida H. Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans. Nutrients. 2020; 12(9):2755. https://doi.org/10.3390/nu12092755
Chicago/Turabian StyleYamashita, Yoko, Asuka Nakamura, Fumio Nanba, Shizuka Saito, Toshiya Toda, Junichi Nakagawa, and Hitoshi Ashida. 2020. "Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans" Nutrients 12, no. 9: 2755. https://doi.org/10.3390/nu12092755
APA StyleYamashita, Y., Nakamura, A., Nanba, F., Saito, S., Toda, T., Nakagawa, J., & Ashida, H. (2020). Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans. Nutrients, 12(9), 2755. https://doi.org/10.3390/nu12092755





