The Association between 25-Hydroxyvitamin D Concentration and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design (The Newcastle 85+ Study)
2.2. Ethical Approval
2.3. Measurement of Serum 25(OH)D Concentration
2.4. Disability Measures and Scores
2.5. Other Measures/Confounders
2.6. Statistical Analysis
2.7. Sensitivity Analysis
3. Results
3.1. 25(OH)D Concentration and Disability at Baseline
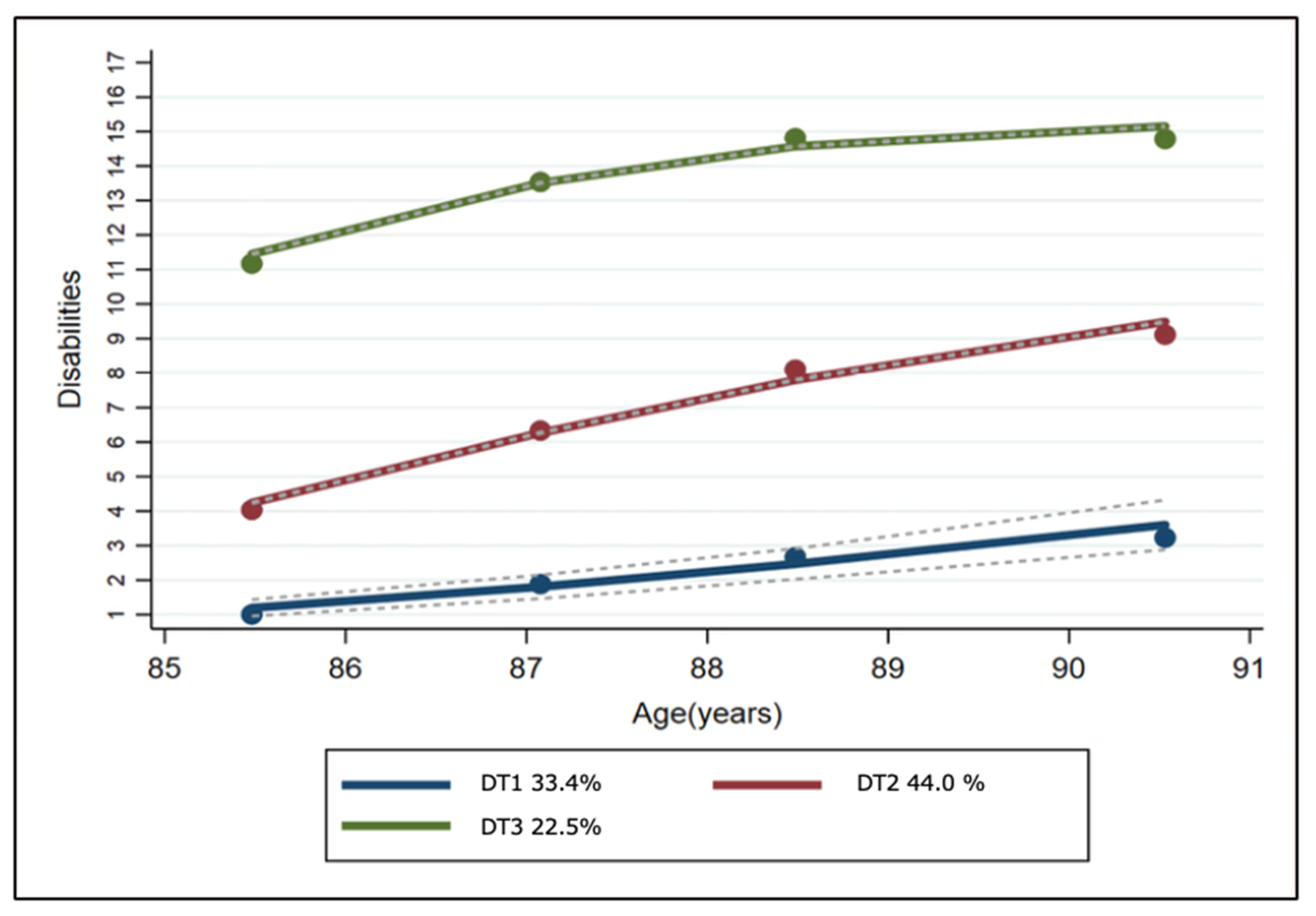
3.2. Disability Trajectories
3.3. The Differences in Socioeconomic, Lifestyle and Health Factors between Disability Trajectories
3.4. 25(OH)D Concentration and Disability Trajectory
3.5. 25(OH)D Concentration and Disability Trajectory by Sex
3.6. Sensitivity Analysis
4. Discussion
4.1. Main Findings
4.2. Evidence from Other Studies
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gobbens, R.J.; van Assen, M.A. The prediction of ADL and IADL disability using six physical indicators of frailty: A longitudinal study in the Netherlands. Curr. Gerontol. Geriatr. Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wong, M.; Chang, B.; Lai, X.; Lum, C.M.; Auyeung, T.W.; Lee, J.; Tsoi, K.; Lee, R.; Woo, J. Trends in activities of daily living disability in a large sample of community-dwelling Chinese older adults in Hong Kong: An age-period-cohort analysis. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Millán-Calenti, J.C.; Tubío, J.; Pita-Fernández, S.; González-Abraldes, I.; Lorenzo, T.; Fernández-Arruty, T.; Maseda, A. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch. Gerontol. Geriatr. 2010, 50, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Majer, I.M.; Nusselder, W.J.; Mackenbach, J.P.; Klijs, B.; van Baal, P.H.M. Mortality risk associated with disability: A population-based record linkage study. Am. J. Public Health 2011, 101, e9–e15. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z. Economics of Disability in Bangladesh; The National Archives: Richmond, UK, 2014.
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metabol. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef]
- Zamboni, M.; Zoico, E.; Tosoni, P.; Zivelonghi, A.; Bortolani, A.; Maggi, S.; Di Francesco, V.; Bosello, O. Relation between vitamin D, physical performance, and disability in elderly persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M7–M11. [Google Scholar] [CrossRef]
- Orces, C.H. Prevalence of clinically relevant muscle weakness and its association with vitamin D status among older adults in Ecuador. Aging Clin. Exp. Res. 2017, 29, 943–949. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Durmuller, U.; Stahelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Simão, A.N.C.; Alfieri, D.F.; Flauzino, T.; Kallaur, A.P.; Mezzaroba, L.; Lozovoy, M.A.B.; Sabino, B.S.; Ferreira, K.P.Z.; Pereira, W.L.C.J.; et al. Vitamin D deficiency is associated with disability and disease progression in multiple sclerosis patients independently of oxidative and nitrosative stress. J. Neurol. Sci. 2017, 381, 213–219. [Google Scholar] [CrossRef]
- Valderrama-Hinds, L.M.; Al Snih, S.; Rodriguez, M.A.; Wong, R. Association of arthritis and vitamin D insufficiency with physical disability in Mexican older adults: Findings from the Mexican Health and Aging Study. Rheumatol. Int. 2017, 37, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Garrett, E.; Johnson, B.A.; Guralnik, J.M.; Fried, L.P. Vitamin D deficiency among older women with and without disability. Am. J. Clin. Nutr. 2000, 72, 1529–1534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; Siervo, M.; Mathers, J.C.; Adamson, A.J.; Francis, R.M.; Pearce, S.H.; et al. Serum 25-hydroxyvitamin D concentration and its determinants in the very old: The Newcastle 85+ Study. Osteoporos. Int. 2016, 27, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Kirkwood, T.B.L.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Saxby, B.K.; Wesnes, K.A.; Collerton, D.; et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: The Newcastle 85+ study. Eur. J. Neurol. 2015, 22, e6–e7. [Google Scholar] [CrossRef]
- Granic, A.; Aspray, T.; Hill, T.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Kirkwood, T.B.; Mathers, J.C.; Jagger, C. 25-hydroxyvitamin D and increased all-cause mortality in very old women: The Newcastle 85+ study. J. Int. Med. 2015, 277, 456–467. [Google Scholar] [CrossRef]
- Collerton, J.; Davies, K.; Jagger, C.; Kingston, A.; Bond, J.; Eccles, M.P.; Robinson, L.A.; Martin-Ruiz, C.; von Zglinicki, T.; James, O.F.W.; et al. Health and disease in 85 year olds: Baseline findings from the Newcastle 85+ cohort study. BMJ 2009, 339. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Jagger, C.; Kingston, A.; Collerton, J.; Catt, M.; Davies, K.; Dunn, M.; Hilkens, C.; Keavney, B.; Pearce, S.H.S.; et al. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech. Ageing Dev. 2011, 132, 496–502. [Google Scholar] [CrossRef]
- Kempen, G.I.J.M.; Miedema, I.; Ormel, J.; Molenaar, W. The assessment of disability with the Groningen Activity Restriction Scale. Conceptual framework and psychometric properties. Soc. Sci. Med. 1996, 43, 1601–1610. [Google Scholar] [CrossRef]
- Collerton, J.; Barrass, K.; Bond, J.; Eccles, M.; Jagger, C.; James, O.; Martin-Ruiz, C.; Robinson, L.; von Zglinicki, T.; Kirkwood, T. The Newcastle 85+ study: Biological, clinical and psychosocial factors associated with healthy ageing: Study protocol. BMC Geriatr. 2007, 7, 14. [Google Scholar] [CrossRef]
- Mendonça, N.; Hill, T.; Granic, A.; Mathers, J.; Wrieden, W.; Siervo, M.; Seal, C.; Jagger, C.; Adamson, A. Micronutrient intake and food sources in the very old. Br. J. Nutr. 2016, 16, 751–761. [Google Scholar]
- Vieth, R.; Holick, M.F. Chapter 57B—The IOM—Endocrine Society Controversy on Recommended Vitamin D Targets: In Support of the Endocrine Society Position. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1091–1107. [Google Scholar] [CrossRef]
- Mendonça, N.; Granic, A.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Kingston, A.; Jagger, C. Protein Intake and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study. J. Am. Geriatr. Soc. 2018, 67, 50–56. [Google Scholar] [CrossRef]
- Kingston, A.; Davies, K.; Collerton, J.; Robinson, L.; Duncan, R.; Kirkwood, T.B.; Jagger, C. The enduring effect of education-socioeconomic differences in disability trajectories from age 85 years in the Newcastle 85+ Study. Arch. Gerontol. Geriatr. 2015, 60, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition. Vitamin D and Health. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf (accessed on 7 September 2020).
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Karasik, D. Bone geometry and skeletal fragility. Curr. Osteoporos. Rep. 2006, 4, 49–56. [Google Scholar] [CrossRef]
- Van de Peppel, J.; Franceschi, R.T.; Li, Y.; van der Eerden, B.C.J. Chapter 17—Vitamin D Regulation of Osteoblast Function A2—Feldman, David. In Vitamin D, 4th ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 295–308. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Dawson-Hughes, B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am. J. Med. 2004, 116, 634–639. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and skeletal muscle tissue and function. Mol. Aspects Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Kotlarczyk, M.P.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos. Int. 2017, 28, 1347–1353. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥ 60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Al-Eisa, E.S.; Alghadir, A.H.; Gabr, S.A. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging 2016, 11, 513. [Google Scholar] [PubMed]
- Van den Heuvel, E.; Van Schoor, N.; De Jongh, R.T.; Visser, M.; Lips, P. Cross-sectional study on different characteristics of physical activity as determinants of vitamin D status; inadequate in half of the population. Eur. J. Clin. Nutr. 2013, 67, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; Barreto, W.D. Association between physical activity and vitamin D: A narrative literature review. Rev. Assoc. Med. Bras. 2017, 63, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.W.; Alekel, D.L.; Ritland, L.M.; Van Loan, M.; Gertz, E.; Genschel, U. Serum 25-hydroxyvitamin D is related to indicators of overall physical fitness in healthy postmenopausal women. Menopause 2009, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Toffanello, E.D.; Perissinotto, E.; Sergi, G.; Zambon, S.; Musacchio, E.; Maggi, S.; Coin, A.; Sartori, L.; Corti, M.-C.; Baggio, G. Vitamin D and physical performance in elderly subjects: The Pro. VA study. PLoS ONE 2012, 7, e34950. [Google Scholar] [CrossRef]
- Dunlop, D.D.; Hughes, S.L.; Manheim, L.M. Disability in activities of daily living: Patterns of change and a hierarchy of disability. Am. J. Public Health 1997, 87, 378–383. [Google Scholar] [CrossRef]
- Kyle, U.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663. [Google Scholar] [CrossRef]
- Sternfeld, B.; Ngo, L.; Satariano, W.A.; Tager, I.B. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am. J. Epidemiol. 2002, 156, 110–121. [Google Scholar] [CrossRef]
- Visser, M.; Harris, T.; Langlois, J.; Hannan, M.; Roubenoff, R.; Felson, D.; Wilson, P.; Kiel, D. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M214–M221. [Google Scholar] [CrossRef]
- Visser, M.; Langlois, J.; Guralnik, J.M.; Cauley, J.A.; Kronmal, R.A.; Robbins, J.; Williamson, J.D.; Harris, T.B. High body fatness, but not low fat-free mass, predicts disability in older men and women: The Cardiovascular Health Study. Am. J. Clin. Nutr. 1998, 68, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Davies, K.; Jagger, C.; Kirkwood, T.B.; Syddall, H.E.; Sayer, A.A. Grip strength decline and its determinants in the very old: Longitudinal findings from the Newcastle 85+ Study. PLoS ONE 2016, 11, e0163183. [Google Scholar]
| Low-to-Mild (n = 249) | Mild-to-Moderate (n = 351) | Moderate-to-Severe (n = 175) | p | |
|---|---|---|---|---|
| Women % (n) | 48.4 (121) | 56.6 (231) | 69.3 (122) | <0.001 |
| Weight (kg) mean (SD) | 63.9 (11.8) | 63.5 (13.4) | 63.9 (14.3) | 0.732 |
| BMI mean (SD) | 23.8 (3.8) | 24.7 (4.4) | 24.9 (5.2) | 0.029 |
| Fat-free mass (kg) mean (SD) | 46.5 (9.2) | 44.4 (9.1) | 45 (8.9) | 0.151 |
| Total number of years in education % (n) 0–9 years 10–11 years 12–20 years | 61.9 (153) 23.9 (59) 14.2 (35) | 62.1 (213) 24.2 (83) 13.7 (47) | 70.3 (111) 22.2 (35) 7.6 (12) | 0.241 |
| Physical activity % (n) Low Moderate High | 2.4 (6) 15.8 (55) 63.2 (110) | 27.3 (68) 58.7 (205) 33.9 (59) | 70.3 (175) 25.5 (89) 2.9 (5) | <0.001 |
| Alcohol drinkers % (n) | 80 (156) | 72.4 (168) | 55.3 (52) | <0.001 |
| Smoking % (n) | 3.6 (9) | 8 (28) | 4.5 (8) | 0.124 |
| Vitamin D containing medication % (n) | 10 (25) | 13.4 (47) | 31.8 (56) | <0.001 |
| Supplement users % (n) | 23.3 (58) | 20.8 (73) | 12 (21) | 0.012 |
| Serum 25(OH)D nmol/L median (IQR) | 42 (29–59) | 36 (23–58) | 39 (21–70) | 0.178 |
| 25(OH)D <25 nmol/L (low) % (n) 25–50 nmol/L (moderate) % (n) >50 nmol/L (high) % (n) | 26.4 (66) 34.7 (122) 38.1 (67) | 36 (90) 31.8 (112) 19.9 (35) | 37.6 (94) 33.5 (118) 42 (74) | 0.02 |
| Chronic disease count mean (SD) | 4.1 (1.5) | 4.93 (1.75) | 5.6 (1.9) | <0.001 |
| Impaired cognitive status % (n) | 12 (30) | 23.3 (82) | 57.5 (100) | <0.001 |
| Living in institution % (n) | 0.4 (1) | 3.4 (12) | 30.7 (54) | <0.001 |
| Trajectories | 25(OH)D | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| DT1: Low-to-mild | (ref) | (ref) | (ref) | (ref) | (ref) | ||||||||
| DT2: Mild-to-moderate | <25 nmol/L | 2.01 | 1.29–3.14 | 0.002 | 2.01 | 1.27–3.19 | 0.003 | 1.97 | 1.22–3.17 | 0.005 | 1.61 | 0.95–2.74 | 0.074 |
| 25–50 nmol/L | (ref) | (ref) | (ref) | (ref) | |||||||||
| >50 nmol/L | 1.05 | 0.73–1.52 | 0.774 | 0.94 | 0.64–1.38 | 0.771 | 0.92 | 0.61–1.38 | 0.707 | 1.07 | 0.69–1.67 | 0.749 | |
| DT3: Moderate-to-severe | <25 nmol/L | 3.39 | 1.99–5.76 | 0.001 | 3.02 | 1.70–5.38 | 0.001 | 3.12 | 1.67–5.85 | 0.001 | 1.95 | 0.94–4.06 | 0.071 |
| 25–50 nmol/L | (ref) | (ref) | (ref) | (ref) | |||||||||
| >50 nmol/L | 1.94 | 1.23–3.06 | 0.004 | 1.34 | 0.80–2.22 | 0.254 | 0.83 | 0.45–1.55 | 0.577 | 1.02 | 0.49–2.12 | 0.945 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakeem, S.; Mendonca, N.; Aspray, T.; Kingston, A.; Ruiz-Martin, C.; Jagger, C.; Mathers, J.C.; Duncan, R.; Hill, T.R. The Association between 25-Hydroxyvitamin D Concentration and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study. Nutrients 2020, 12, 2742. https://doi.org/10.3390/nu12092742
Hakeem S, Mendonca N, Aspray T, Kingston A, Ruiz-Martin C, Jagger C, Mathers JC, Duncan R, Hill TR. The Association between 25-Hydroxyvitamin D Concentration and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study. Nutrients. 2020; 12(9):2742. https://doi.org/10.3390/nu12092742
Chicago/Turabian StyleHakeem, Sarah, Nuno Mendonca, Terry Aspray, Andrew Kingston, Carmen Ruiz-Martin, Carol Jagger, John C. Mathers, Rachel Duncan, and Tom R. Hill. 2020. "The Association between 25-Hydroxyvitamin D Concentration and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study" Nutrients 12, no. 9: 2742. https://doi.org/10.3390/nu12092742
APA StyleHakeem, S., Mendonca, N., Aspray, T., Kingston, A., Ruiz-Martin, C., Jagger, C., Mathers, J. C., Duncan, R., & Hill, T. R. (2020). The Association between 25-Hydroxyvitamin D Concentration and Disability Trajectories in Very Old Adults: The Newcastle 85+ Study. Nutrients, 12(9), 2742. https://doi.org/10.3390/nu12092742





