Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis
Abstract
1. Introduction
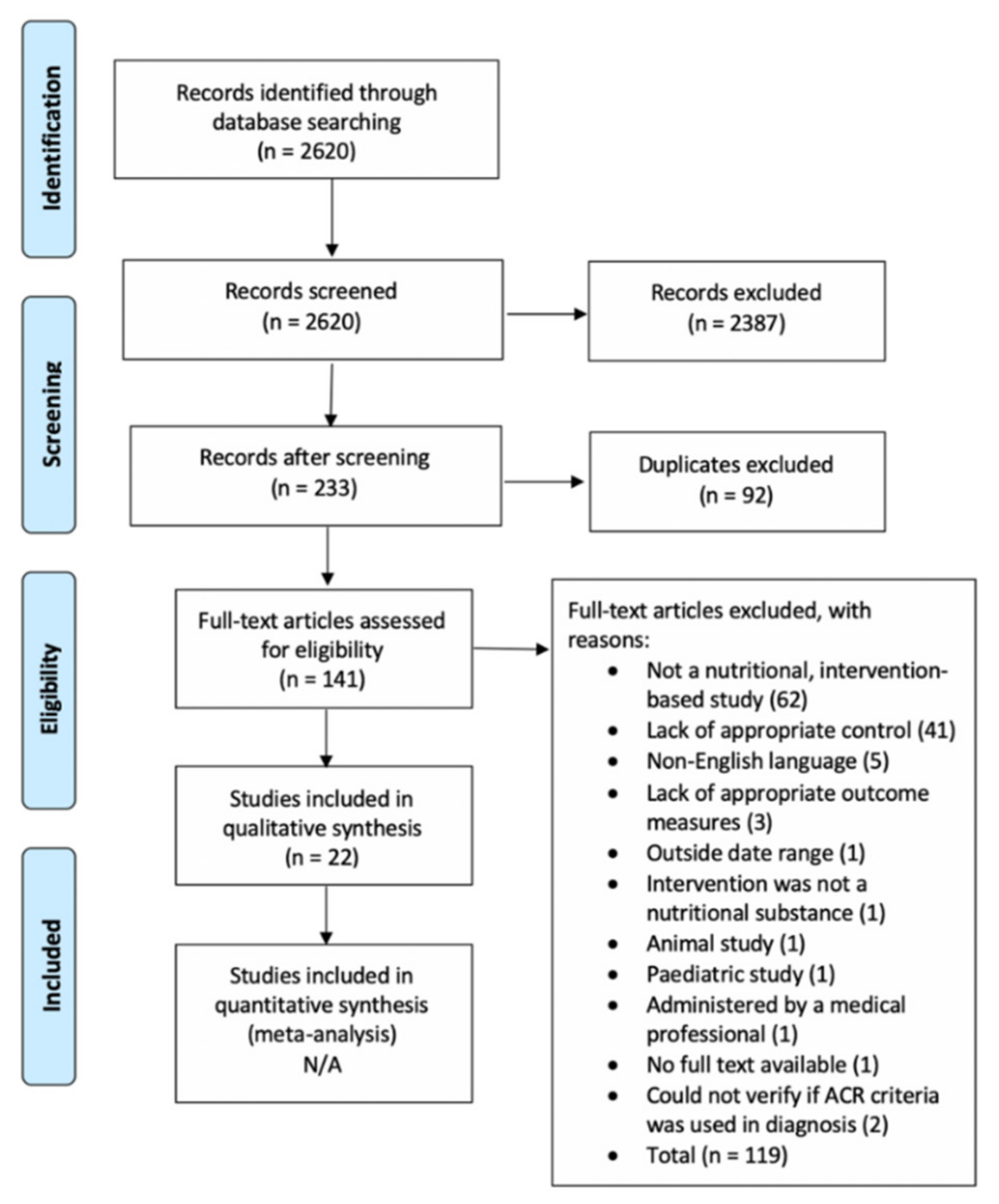
2. Methods
3. Results
3.1. Intervention Characteristics
3.2. Study Quality
3.3. Outcome Measures
3.4. Pain
3.5. Fibromyalgia Severity
3.6. General Health
3.7. Mental Health
3.8. Sleep, Fatigue & Tiredness
3.9. Strength, Stiffness & Exercise Tolerance
3.10. Gastrointestinal Symptoms or Comorbidities
3.11. Cognitive Function
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012. [Google Scholar] [CrossRef] [PubMed]
- Weir, P.T.; Harlan, G.A.; Nkoy, F.L.; Jones, S.S.; Hegmann, K.T.; Gren, L.H.; Harlan, G.A.; Nkoy, F.L.; Jones, S.S. The incidence of fibromyalgia and its associated comorbidities: A population-based retrospective cohort study based on International Classification of Diseases, 9th revision codes. J. Clin. Rheumatol. 2006, 12, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Nimigion-Young, J.; Brennenstuhl, S. Individuals with fibromyalgia and depression: Findings from a nationally representative Canadian survey. Rheumatol. Int. 2006, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.S.; Pimentel, M.J.; Rizzatti-Barbosa, C.M. Temporomandibular disorders in fibromyalgia: A short communication. Rev. Bras. Reumatol. 2015, 55, 189–194. [Google Scholar] [CrossRef]
- Rusu, C.; Gee, M.E.; Lagace, C.; Parlor, M. Chronic fatigue syndrome and fibromyalgia in Canada: Prevalence and associations with six health status indicators. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Marcus, D.A.; Bernstein, C.; Rudy, T.E. Fibromyalgia and headache: And epidemiological study supporting migraine as part of fibromyalgia syndrome. Clin. Rheumatol. 2005, 24, 595–601. [Google Scholar] [CrossRef]
- Bettendorf, E.; Belaskova, S.; Krashin, D.; Murinova, N. Pain compounding pain: Fibromyalgia and migraine comorbidity. Neurology 2019, 92, 26–59. [Google Scholar]
- Yang, T.Y.; Chen, C.S.; Lin, C.L.; Lin, W.M.; Kuo, C.N.; Kao, C.H. Risk for irritable bowel syndrome in fibromyalgia patients: A national database study. Medicine (Baltimore) 2015, 94, 1–6. [Google Scholar] [CrossRef]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheumatol. 2014, 44, 68–75. [Google Scholar] [CrossRef]
- Keskindag, B.; Karaaziz, M. The association between pain and sleep in fibromyalgia. Saudi Med. J. 2017, 38, 465–475. [Google Scholar] [CrossRef]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheumatol. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russel, A.S.; Russel, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (Hoboken) 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russel, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russel, I.J.; Wallit, B. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheumatol. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010 and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Rampakakis, E.; Ste-Marie, P.A.; Sampalis, J.S.; Shir, Y. The association of socioeconomic status and symptom severity in persons with fibromyalgia. J. Rheumatol. 2014, 41, 1398–1404. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheumatol. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Okifuji, A.; Gao, J.; Bokat, C.; Hare, B.D. Management of fibromyalgia syndrome in 2016. Pain Manag. 2016, 6, 383–400. [Google Scholar] [CrossRef]
- Walitt, B.; Fitzcharles, M.A.; Hassett, A.L.; Katz, R.S.; Häuser, W.; Wolfe, F. The longitudinal outcome of fibromyalgia: A study of 1555 patients. J. Rheumatol. 2011, 38, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.K.; Lafferty, W.E.; Tyree, P.T.; Diehr, P.K.; Grembowski, D.E. Use of complementary and alternative medicine providers by fibromyalgia patients under insurance coverage. Arthritis Rheum. 2007, 57, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Smedslund, G.; Byfuglien, M.G.; Olsen, S.U.; Hagen, K.B. Effectiveness and safety of dietary interventions from rheumatoid arthritis: A systematic review of randomized controlled trials. J. Am. Diet. Assoc. 2010, 110, 727–735. [Google Scholar] [CrossRef]
- Arranz, L.I.; Canela, M.Á.; Rafecas, M. Dietary aspects in fibromyalgia patients: Results of a survey on food awareness, allergies, and nutritional supplementation. Rheumatol. Int. 2012, 32, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. McMaster Critical Review Form–Quantitative Studies. McMaster University Occupational Therapy Evidence-Based Practice Research Group. 1998. Available online: https://srs-mcmaster.ca/wp-content/uploads/2015/04/Critical-Review-Form-Quantitative-Studies-English.pdf (accessed on 23 March 2020).
- Bialocerkowski, A.E.; Vladusic, S.L.; Howell, S.M. Conservative interventions for positional plagiocephaly: A systematic review. Dev. Med. Child. Neurol. 2005, 47, 563–570. [Google Scholar] [CrossRef]
- Daly, A.E.; Bialocerkowski, A.E. Does evidence support physiotherapy management of adult complex regional pain syndrome type one? A systematic review. Eur. J. Pain 2009, 13, 339–353. [Google Scholar] [CrossRef]
- Lamb, J.J.; Konda, V.R.; Quig, D.W.; Desai, A.; Minich, D.M.; Bouillon, L.; Chang, J.-L.; Hsi, A.; Lerman, R.H.; Kornberg, J.; et al. A program consisting of a phytonutrient-rich medical food and an elimination diet ameliorated fibromyalgia symptoms and promoted toxic-element detoxification in a pilot trial. Altern Ther. Health Med. 2011, 17, 36–44. [Google Scholar] [PubMed]
- Elliot, D.L.; Kuehl, K.S.; Kerry, S.; Jones, K.D.; Dulacki, K. Using an eccentric exercise-testing protocol to assess the beneficial effects of tart cherry juice in fibromyalgia patients. Integr. Med. Clin. J. 2010, 9, 24–29. [Google Scholar] [CrossRef]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30, S10–S17. [Google Scholar] [PubMed]
- Umeda, M.; Kempka, L.; Weatherby, A.; Greenlee, B.; Mansion, K. Effects of caffeinated chewing gum on muscle pain during submaximal isometric exercise in individuals with fibromyalgia. Physiol. Behav. 2016, 157, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.E.; Arnspider, S.A. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J. Clin. Rheumatol. 2008, 14, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wahner-Roedler, D.L.; Thompson, J.M.; Luedtke, C.A.; King, S.M.; Cha, S.S.; Elkin, P.L.; Bruce, B.K.; Townsend, C.O.; Bergeson, J.R.; Eickhoff, A.L.; et al. Dietary soy supplement on fibromyalgia symptoms: A randomized, double-blind, placebo-controlled, early phase trial. Evid. Based Complement. Altern. Med. 2011, 350697. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A.; Wise, C.M. Nutritional supplementation with Chlorella pyrenoidosa for fibromyalgia syndrome: A double-blind, placebo-controlled, crossover study. J. Musculoskelet. Pain 2001, 9, 37–54. [Google Scholar] [CrossRef]
- Vellisca, M.Y.; Latorre, J.I. Monosodium glutamate and aspartame in perceived pain in fibromyalgia. Rheumatol. Int. 2014, 34, 1011–1013. [Google Scholar] [CrossRef]
- Rus, A.; Molina, F.; Ramos, M.M.; Martínez-Ramírez, M.J.; Del Moral, M.L. Extra Virgin olive oil improves oxidative stress, functional capacity, and health-related psychological status in patients with fibromyalgia. Biol. Res. Nurs. 2017, 19, 106–115. [Google Scholar] [CrossRef]
- Alcocer-Gomez, E.; Sanchez-Alcazar, J.A.; Cordero, M.D. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: Results of a small clinical trial. J. Clin. Psychopharmacol. 2014, 34, 277–278. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; De Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullon, P.; Battino, M.; Rodriguez-Fernandez, A.; Sanchez-Alcazar, J.A. Can coenzyme Q10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Sign. 2013, 19, 1356–1361. [Google Scholar] [CrossRef]
- Roman, P.; Estevez, A.F.; Miras, A.; Sanchez-Labraca, N.; Canadas, F.; Vivas, A.B.; Cardona, D. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Nutr. Hosp. 2017, 34, 1246–1251. [Google Scholar] [CrossRef]
- Di Pierro, F.; Rossi, A.; Consensi, A.; Giacomelli, C.; Bazzichi, L. Role for a water-soluble form of CoQ10 in female subjects affected by fibromyalgia. A preliminary study. Clin. Exp. Rheumatol. 2017, 35, 20–27. [Google Scholar] [PubMed]
- Rossini, M.; Di Munno, O.; Valentini, G.; Bianchi, G.; Biasi, G.; Cacace, E.; Malesci, D.; La Montagna, G.; Viapiana, O.; Adami, S. Double-blind, multicenter trial comparing acetyl l-carnitine with placebo in the treatment of fibromyalgia patients. Clin. Exp. Rheumatol. 2007, 25, 182–188. [Google Scholar] [PubMed]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Mughal, M.S.; Asghar, M.N.; Shaheen, N.; Ahmad, N.M.; Farman, S.; Saeed, M.A.; Khan, I.U.; Arshad, M. Effect of vitamins C, E and nigella sativa seeds on antioxidant activity in fibromyalgia patients. Pak. J. Zool. 2015, 47, 7–13. [Google Scholar]
- Kaartinen, K.; Lammi, K.; Hypen, M.; Nenonen, M.; Hänninen, O. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheumatol. 2000, 29, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wepner, F.; Scheuer, R.; Schuetz-Wieser, B.; Machacek, P.; Pieler-Bruha, E.; Cross, H.S.; Cross, H.S.; Hahne, J.; Friedrich, M. Effects of vitamin D on patients with fibromyalgia syndrome: A randomized placebo-controlled trial. Pain 2014, 155, 261–268. [Google Scholar] [CrossRef]
- Barmaki, M.; Maindet-Dominci, C.; Nizard, J.; Baron, D.; Russ, I.; Fardellone, P.; Ginies, P.; Marc, J.-F.; Conrozier, T.; Bertin, P. Multicenter, prospective, controlled double-blind study comparing Fib-19-01, a phytotherapy treatment, to a dietary supplement and to conventional care in patients suffering from fibromyalgia. Altern. Ther. Health Med. 2019, 25, 46–53. [Google Scholar] [PubMed]
- Alves, C.R.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.G.; Calich, A.L.; Tritto, A.C.C.; de Sa Pinto, A.L.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine supplementation in fibromyalgia: A randomized, double-blind, placebo-controlled trial. Arthritis Care Res. 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Naziroǧlu, M.; Akkuş, S.; Soyupek, F.; Yalman, K.; Çelik, O.; Eriş, S.; Uslusoy, G.A. Vitamins C and e treatment combined with exercise modulates oxidative stress markers in blood of patients with fibromyalgia: A controlled clinical pilot study. Stress 2010, 13, 498–505. [Google Scholar] [CrossRef]
- Edwards, A.M.; Blackburn, L.; Christie, S.; Townsend, S.; David, J. Food supplements in the treatment of primary fibromyalgia: A double-blind, crossover trial of anthocyanidins and placebo. J. Nutr. Environ. Med. 2000, 10, 189–199. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K. Assessment of pain in rheumatoid arthritis: Minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J. Rheumatol. 2007, 34, 1674–1683. [Google Scholar] [PubMed]
- Bennett, R.M.; Bushmakin, A.G.; Cappelleri, J.C.; Zlateva, G.; Sadosky, A.B. Minimal clinically important difference in the fibromyalgia impact questionnaire. J. Rheumatol. 2009, 36, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Arnold, L.M. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arhritis Care Res. (Hoboken) 2011, 63, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Gostine, M.; Davis, F.; Roberts, B.A.; Risko, R.; Asmus, M.; Cappelleri, J.C.; Sadosky, A. Clinical characteristics of fibromyalgia in a chronic pain population. Pain Pract. 2018, 18, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Leppink, J.; Winston, K.; O’Sullivan, P. Statistical significance does not imply a real effect. Perspect. Med. Educ. 2016, 5, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutritional effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Robinson, M.E.; Staud, R. Pain and fatigue variability patterns distinguish subgroups of fibromyalgia patients. J. Pain 2018, 19, 372–381. [Google Scholar] [CrossRef]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Perrot, S.; Häuser, W. Comorbid fibromyalgia: A qualitative review of prevalence and importance. Eur. J. Pain 2018, 22, 1565–1576. [Google Scholar] [CrossRef]
- Rajaej, E.; Mowla, K.; Ghorbani, A.; Bahadoram, S.; Bahadoram, M.; Dargahi-Malamir, M. The effect of omega-3 fatty acids in patients with active rheumatoid arthritis receiving DMARDs Therapy: Double-blind randomized controlled trial. Glob. J. Health Sci 2016, 8, 18–25. [Google Scholar] [CrossRef]
- Felson, D.T.; Bischoff-Ferrari, H.A. Dietary fatty acids for the treatment of OA, including fish oil. Ann. Rheumatol. Dis. 2016, 75, 1–2. [Google Scholar] [CrossRef]
- Senftleber, N.; Nielsen, S.; Andersen, J.; Bliddal, H.; Tarp, S.; Lauritzen, L.; Furst, D.E.; Suarez-Almazor, M.E.; Lyddiatt, A.; Christensen, R. Marine oil supplements for arthritis pain: A systematic review and meta-analysis of randomized trials. Nutrients 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sanchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Oktayoglu, P.; Em, S.; Caglayan, M.; Yuksel, H.; Uçar, D.; Batmatz, I.; Akif Sariyildiz, M.; Karatoprak, S.; Nas, K. Serum Coenzyme Q10 Levels and Oxidative Status in Patients with Fibromyalgia Syndrome. J. Musculoskelet. Pain 2013, 22, 27–32. [Google Scholar] [CrossRef]
- Kasznicki, J.; Kosmalski, M.; Sliwinska, A.; Mrowicka, M.; Stanczyk, M.; Majsterek, I.; Drzewoski, J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol. Biol. Rep. 2012, 39, 8669–8678. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.H.; Hankerd, K.M.; Kim, H.H.; Chung, J.M.; La, J.H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, F.; Anelli, M.G.; Rizzo, D.; Morelli, E.; Scioscia, C.; Mazzotta, D.; Iannone, F.; Lapadula, G. Influence of TNF-α inhibition on oxidative stress of rheumatoid arthritis patients. Reumatismo 2015, 67, 97–102. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immunol. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Groven, N.; Fors, E.A.; Reitan, S.K. Patients with fibromyalgia and chronic fatigue syndrome show increased hsCRP compared to healthy controls. Brain Behav. Immunol. 2019, 81, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Haynes, W.L.; Michalek, J.E.; Russell, I.J. Elevated serum high-sensitivity C-reactive protein levels in fibromyalgia syndrome patients correlate with body mass index, interleukin-6, interleukin-8, erythrocyte sedimentation rate. Rheumatol. Int. 2013, 33, 1259–1264. [Google Scholar] [CrossRef]
- Bazzichi, L.; Rossi, A.; Massimetti, G.; Giannaccini, G.; Giuliano, T.; Feo, F.D.; Ciapparelli, A.; Dell’Osso, L.; Bombardieri, S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 2007, 25, 225–230. [Google Scholar] [PubMed]
- Biswas, S.K. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4 T cells in neurodegenerative diseases. Front. Cell Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Backryd, E.; Tanum, L.; Lind, A.L.; Larsson, A.; Gordh, T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J. Pain Res. 2017, 10, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Hoglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia–A multi-site positron emission tomography investigation. Brain Behav. Immunol. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and oxidative stress: A systematic review of human studies. Int. J. Food Sci. Nutr. 2013, 64, 312–326. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. Modulation of C-Reactive protein, tumor necrosis factor-α, and Adiponectin by diet, exercise, and weight loss. J. Nutr. 2008, 138, 2293–2296. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Palo, C.D.; Giugliano, F.; Darmiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [CrossRef]
- Cordero, M.D.; de Miguel, M.; Fernández, A.M.M.; López, I.M.C.; Maraver, J.G.; Cotán, D.; Izquierdo, L.G.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Moreno-Fernández, A.M.; Demiguel, M.; Bonal, P.; Campa, F.; Jimenez-Jimenez, L.M.; Ruiz-Losada, A.; Sanchez-Dominguez, B.; Sanchez-Alcazar, J.A.; Salviati, L.; et al. Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin. Biochem. 2009, 42, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Cano-Garcia, F.J.; Alcocer-Gomez, E.; De Miguel, M.; Sanchez-Alcazar, J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q (1)(0) effect on clinical improvement. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Sepand, M.R.; Razavi-Azarkhiavi, K.; Omidi, A.; Zirak, M.R.; Sabzevari, S.; Kazemi, A.R.; Sabzevari, O. Effect of acetyl-L-carnitine on antioxidant status, lipid peroxidation, and oxidative damage of arsenic in rat. Biol. Trace Elem. Res. 2016, 171, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lin, J.S.; Lin, T.C.; Lin, P.T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef]
- Silva, A.R.; Bernardo, A.; Costa, J.; Cardoso, A.; Santos, P.; de Mesquita, M.F.; Patto, J.V.; Moreira, P.; Silva, M.L.; Padrão, P. Dietary interventions in fibromyalgia: A systematic review. Ann. Med. 2019, 51, 2–14. [Google Scholar] [CrossRef]
| Search Number | Search Terms |
|---|---|
| Search #1 | “Fibromyalgia” OR “FMS” OR “Fibrositis” |
| Search #2 | “Diet therapy” OR “Diet” OR “Nutrition” OR “Vitamin(s)” OR “Minerals” OR “Micronutrients” OR “Macronutrients” OR “Dietary Supplement” OR “Dietary Supplementation” OR “Food and Beverages” OR “Vegetarian” OR “Vegan” OR “Dietary Fats” OR “Dietary Carbohydrates” OR “Dietary Proteins” OR “Coenzyme(s)” |
| Search #3 | Search #1 AND Search #2 |
| Paper | Intervention | ACR Criteria | Sample (n) | Age (Years), Mean (SD) | % Female | |||
|---|---|---|---|---|---|---|---|---|
| Tr | C | Tr | C | Tr | C | |||
| [45] | Vegan diet * | 1990 | 18 | 15 | 51 | 52 | 100 | 100 |
| [30] | Tart cherry juice * | 1990 | 8 | 7 | 51* | 100 | 100 | |
| [43] | Low-FODMAP diet | 2011 | 38 | 51* | 100 | |||
| [29] | Phytonutrient supplement | 1990 | 8 | 55.6 (9.4) | 100 | |||
| [34] | Soy | 1990 | 25 | 25 | Median +,*, 47.7 | 98 | ||
| [37] | Extra-Virgin olive oil | 1990 | 11 | 12 | 53.63 (5.50) | 48.16 (7.96) | 100 | 100 |
| [36] | Elimination of MSG and aspartame | 1990 | 36 | 36 | 42.33 (8.43) | 39.64 (8.16) | 100 | 100 |
| [38] | Coenzyme Q10 | 1990 | 10 | 10 | -- | -- | -- | -- |
| [33] | Vitamin D | 1990 | 20 | 22 | 58.0 (7.3) | 56.7 (11.3) | 98 | 87 |
| [44] | Vitamin C, E & Nigella seeds | 1990 | 50 | 42.93 (1.59) | 100 | |||
| [32] | Caffeine | 1990 | 23 | 43.57 (18.49) | 86.96 | |||
| [46] | Vitamin D | 1990 + 2010 | 15 | 15 | 48.37 (5.301) | 90 | ||
| [41] | Coenzyme Q10 | 2010 | 12 | 10 | 52.5 (10.4) | 53.6 (7.8) | 100 | 100 |
| [48] | Creatine | 1990 | 15 | 13 | 48.7 (8.4) | 49.0 (10.1) | 100 | 100 |
| [31] | Elimination of MSG | 1990 | 31 | 53.4 (13) | 90 | |||
| [39] | Coenzyme Q10 | 1990 | 10 | 10 | 44.3 (9.7) | 55 (5) | 100 | 100 |
| [42] | Acetyl-l-carnitine | 1990 | 50 | 52 | 47.3 (11.7) | 46.3 (10.4) | 97 | |
| [50] | Phytonutrient supplement | 1990 | 12 | 45.6 (5.9) | 100 | |||
| [49] | Vitamin C and E | 1990 | 31 | 30 | 40.1 (5.2) | 39.6 (5.8) | 100 | 100 |
| [35] | Chlorella green algae | 1990 | 30 | 47.1 (9.0) | 97 | |||
| [40] | * Phytonutrient supplement | 1990 + 2010 | 31 | 55 | 50.27 | 94 | 87 | |
| [47] | Probiotics | 1990 | 75 (3-Arm) | A: 49.6 (9.4) B: 47.4 (8.6) | 47.8 (9.0) | 100 | 100 | |
| Paper | Intervention (Dosage) | Duration | Statistically significant results (p < 0.05) |
|---|---|---|---|
| [45] | Vegan diet compared to participants normal diet | 3 months | Improved VAS-Pain; morning stiffness; GHQ; HAQ; & Sleep quality |
| [30] | Tart cherry juice (2 x 10.5 Oz bottles daily) or placebo | 2 weeks | No statistically significant changes |
| [43] | A diet low in Fermentable oligo-di-mono-saccharides and polyols (Low-FODMAP). Reducing consumption of lactose, excess fructose, fructans, galactans, polyols. | 4 weeks | Improved: VAS-pain; VAS-muscle tension; VAS-asthenia; VAS-depression; VAS-sleep quality; VAS-memory; VAS-headache; VAS-abdominal pain; VAS-constipation; VAS-diarrhoea; VAS-Bloating; FSQ; and, FIQR; IBS-SSS |
| [29] | Phytonutrient supplement containing: 3 g fat, 20 g carbs, 6 g sugars, 12 g protein; 4000 IU β-carotene; 1000 IU vitamin A; 300 mg vitamin C; 35 IU vitamin D; 42 IU vitamin E; 2 mg thiamine; 2 mg riboflavin; 7 mg niacin; 3.4 mg vitamin B6; 80 μg folate; 2.6 μg vitamin b12; 135 mg biotin; 36 mg pantothenic; 220 mg sodium; 520 mg potassium; 1 mg iron; 230 mg phosphorus; 53 μg iodine; 160 mg magnesium; 10 mg zinc; 1 mg copper; 1 mg manganese; 50 μg chromium; 20 mg sulfate; 1 g spent hops; 50 mg pomegranate rind extract 125 mg prune skin extract; 67 mg watercress whole plant extract; 15 mg decaffeinated green tea extract (2 x daily servings) Elimination of: simple sugars, artificial colours, flavours and sweeteners; caffeinated beverages; gluten; eggs, dairy; allergenic foods; or foods high in arachidonic acid | 4 weeks | Improved: FIQ subsections for pain and stiffness |
| [34] | Soy protein (20 g), soy isoflavone (160 mg) (1 serving daily) or placebo | 6 weeks | Both soy and placebo resulted in significant improvements in FIQ and CES-D. No significant differences between groups |
| [37] | Extra-virgin olive oil (50 mLs) vs. refined olive oil | 2 weeks | Improved: MCS-12 and FIQ |
| [36] | Elimination of MSG and aspartame from diet | 3 months | No statistically significant changes |
| [38] | Coenzyme Q10 (300 mg daily) or placebo | 40 days | Improved: BDI |
| [33] | Vitamin D (50,000 IU once per week) or placebo | 3 months | No significant changes compared to placebo |
| [44] | Vitamin C (200 mg daily), E (200 mg daily) & Nigella sativa seeds (13 mg 4–5 times daily) | 8 weeks | Improved: VAS-pain |
| [32] | Caffeinated chewing gum (100 mg caffeine) or placebo | 1 x serving | No significant changes compared to placebo |
| [46] | Vitamin D (1200 IU or 2400 IU daily) or placebo | 25 weeks | Improved: VAS-Pain and FIQ subsection for morning fatigue |
| [41] | Coenzyme Q10 (400 mg daily) or placebo | 6 months | Improved: SF-36 Subscale for physical pain |
| [48] | Creatine (20 g daily for 5 days; followed by 5 g daily) or placebo | 16 weeks | Increased: muscle strength leg press and chest press; and isometric strength. |
| [31] | Elimination of MSG from diet | 3 days | Worsened: symptom frequency; IBS-QOL; FIQR after the consumption of MSG |
| [39] | Coenzyme Q10 (300 mg daily) or placebo | 40 days | Improved: FIQ; VAS-pain; TPE |
| [42] | Acetyl-L-carnitine (2 x 500 mg capsules daily and 1 x 500 mg IM injection weekly for 2 weeks; 3 x 500 mg capsules daily for 8 weeks) or placebo | 10 weeks | Improved: TPE; Total myalgic score; VAS-pain, VAS-depression; SF-36 |
| [50] | Colladeen™ Anthocyanidin Phytonutrient supplement: grape seeds, bilberries and cranberries. (120 mg a day/80 mg a day/40 mg a day/placebo) 12 weeks per dosage + 4 weeks baseline period | 52 weeks | Improved: Likert scale-sleep; GHQ-28 |
| [49] | Vitamin C (500 mg) & E (150 mg daily) | 12 weeks | No significant changes |
| [35] | Sun Chlorella™ green algae tablets (10 g x 50 daily) and Wakasa Gold Chlorella™ (100 mL daily) or placebo | 3 months | Improved: PAQ; VAS-Pain; TPE; Hassles scale |
| [40] | Ergyphilus Plus™ Probiotics: Lactobacillus rhamnosus GG, Casei, Acidophilus and Bifidobacterium Bifidus (2 pills with breakfast and dinner) or placebo | 8 weeks | Reduced: number of impulsive choices (within the “two-choice task”) |
| [47] | Phytonutrient supplement (Fib-19-01) morning pill: ginger extract 50 mg, acerola 240 mg, vitamin C 120 mg, meadowsweet 40 mg, royal jelly 40 mg (one capsule). Phytonutrient supplement (Fib-19-01) evening pill: passiflora 80 mg, camomile 80 mg, meadowsweet 40 mg, quackgrass 100 mg and L-tyrosine 45 mg (1 capsule). Food supplement comparator: magnesium 71 mg, valerian 65 mg, escholtzia 50 mg, white ginseng roots 83 mg, willow 50 mg, acerola 120 mg, sage 50 mg and L-tryptophan 220 mg (1 capsule in morning and 1 capsule in evening) or no supplementation at all. | 24 weeks | Improved: Pichot scale; HAD; SF-12 Subsections for Mental and social score variations when compared to food supplement comparator and no supplementation Improved: FIQ for Fib-19-01 but not significant when inter-group comparison took place |
| Intervention | Mechanism | |||
|---|---|---|---|---|
| Antioxidant | Anti-Inflammatory | Energy Production | Immuno-Neuromodultion | |
| Phytotherapy | + | |||
| Probiotic | + | + | ||
| Chlorella green algae | + | + | ||
| Vegan diet | + | + | ||
| Tart cherry juice | + | + | ||
| Low-FODMAP | + | + | ||
| Soy | + | + | ||
| Extra-virgin olive oil | + | + | ||
| Vitamin D | + | + | ||
| Caffeine | + | |||
| Vitamin C, E and Nigella sativa | + | |||
| Vitamin C and E | + | |||
| Creatine | + | |||
| Coenzyme Q10 | + | + | + | |
| Acetyl-L-carnitine | + | + | ||
| Elimination of MSG and aspartame | + | |||
| Elimination of MSG | + | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowry, E.; Marley, J.; McVeigh, J.G.; McSorley, E.; Allsopp, P.; Kerr, D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients 2020, 12, 2664. https://doi.org/10.3390/nu12092664
Lowry E, Marley J, McVeigh JG, McSorley E, Allsopp P, Kerr D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients. 2020; 12(9):2664. https://doi.org/10.3390/nu12092664
Chicago/Turabian StyleLowry, Ethan, Joanne Marley, Joseph G. McVeigh, Emeir McSorley, Philip Allsopp, and Daniel Kerr. 2020. "Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis" Nutrients 12, no. 9: 2664. https://doi.org/10.3390/nu12092664
APA StyleLowry, E., Marley, J., McVeigh, J. G., McSorley, E., Allsopp, P., & Kerr, D. (2020). Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients, 12(9), 2664. https://doi.org/10.3390/nu12092664





