Prognostic Impact of Serum Albumin for Developing Heart Failure Remotely after Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Definition and Diagnosis of STEMI and NSTEMI
2.3. Data Collection and Outcome
2.4. Statistics
3. Results
3.1. Patient Characteristics on Admission
3.2. Procedures and Management after AMI
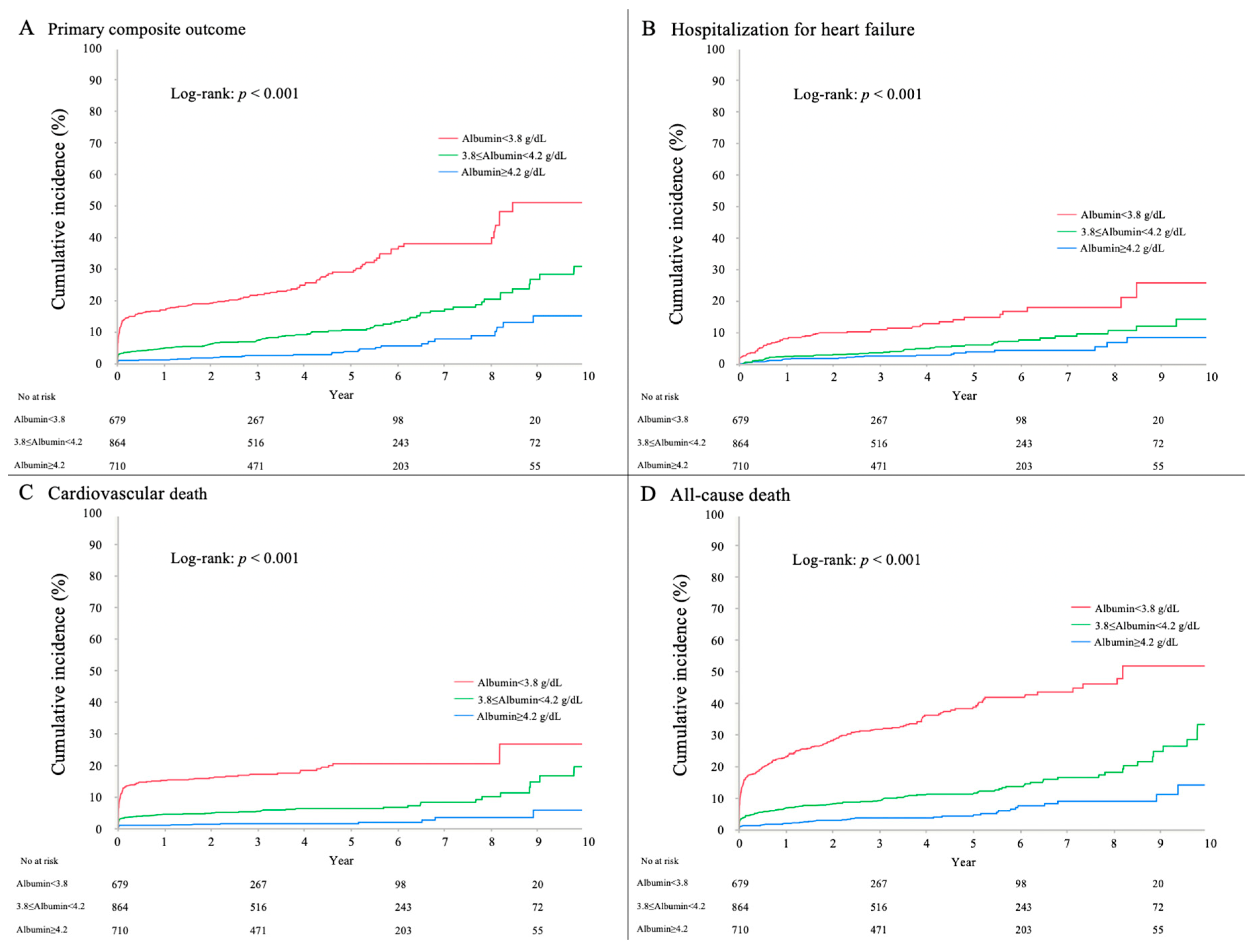
3.3. Clinical Outcome
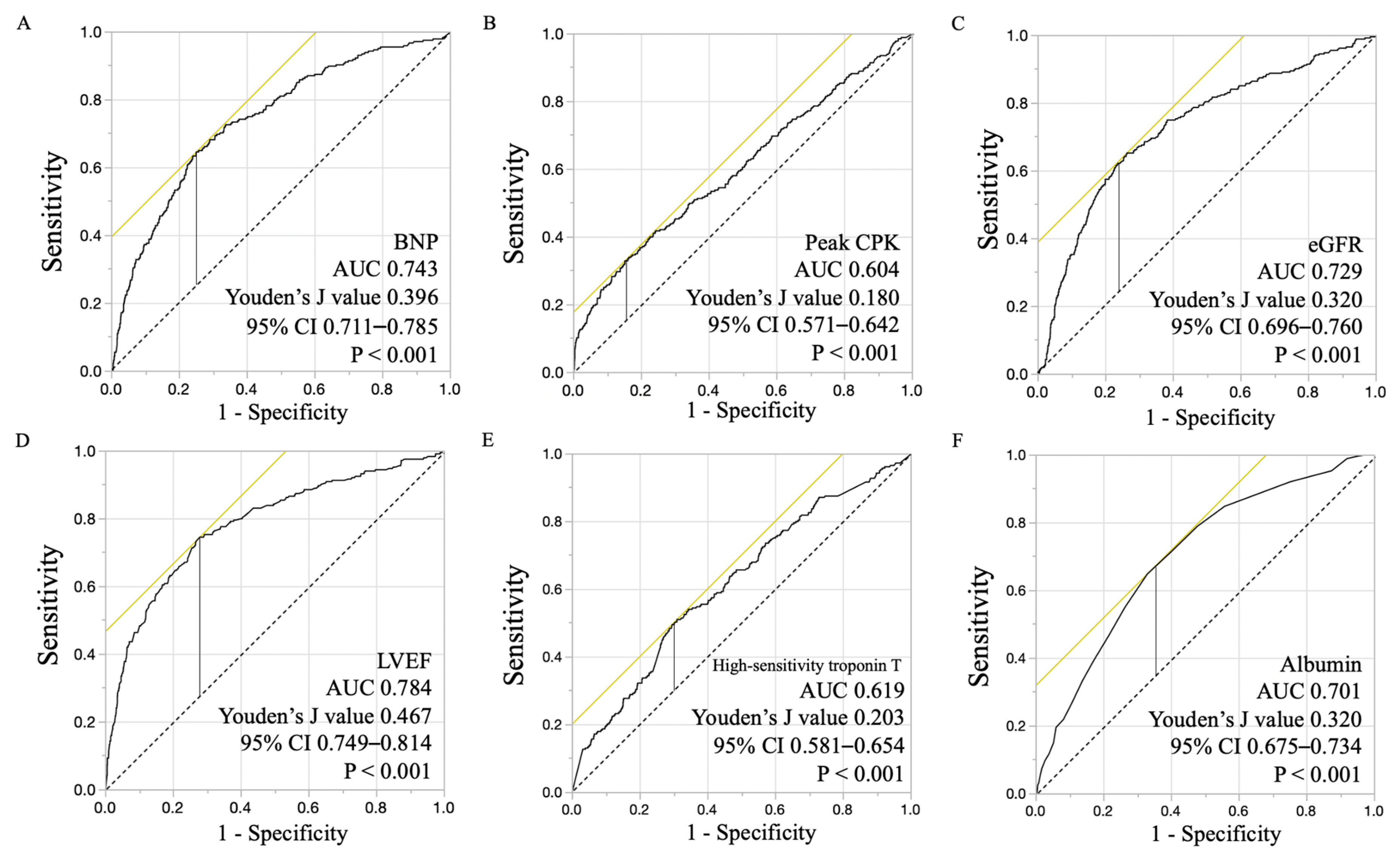
3.4. Subgroup Analysis for Primary Composite Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decramer, M.; Devos, P.; De Roo, M.; Piessens, J.; De Geest, H. Evaluation of Bedside Myocardial Scintigraphy with 201Tl in Acute Myocardial Infarction. Eur. J. Nucl. Med. Mol. Imaging 1982, 7, 200–203. [Google Scholar] [CrossRef]
- Cannon, C.P.; Gibson, C.M.; Lambrew, C.T.; Shoultz, D.A.; Levy, D.; French, W.J.; Gore, J.M.; Weaver, W.D.; Rogers, W.J.; Tiefenbrunn, A.J. Relationship of Symptom-Onset-to-Balloon Time and Door-to-Balloon Time with Mortality in Patients Undergoing Angioplasty for Acute Myocardial Infarction. JAMA 2000, 283, 2941–2947. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; E Casey, D.; Chung, M.K.; A De Lemos, J.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; A Franklin, B.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Catheter. Cardiovasc. Interv. 2013, 82, E1–E27. [Google Scholar] [CrossRef]
- Zijlstra, F.; Hoorntje, J.C.; De Boer, M.-J.; Reiffers, S.; Miedema, K.; Ottervanger, J.P.; Hof, A.W.V.T.; Suryapranata, H. Long-Term Benefit of Primary Angioplasty As Compared with Thrombolytic Therapy for Acute Myocardial Infarction. New Engl. J. Med. 1999, 341, 1413–1419. [Google Scholar] [CrossRef]
- Effect of Metoprolol CR/XL in Chronic Heart Failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [CrossRef]
- Shimizu, A. What Are the Most Useful Predictors of Cardiac Mortality in Patients Post Myocardial Infarction? Circ. J. 2013, 77, 319–320. [Google Scholar] [CrossRef]
- Nishihira, K.; Yoshioka, G.; Kuriyama, N.; Ogata, K.; Kimura, T.; Matsuura, H.; Furugen, M.; Koiwaya, H.; Watanabe, N.; Shibata, Y. Impact of Frailty on Outcomes in Elderly Patients with Acute Myocardial Infarction Who Undergo Percutaneous Coronary Intervention. Eur. Hear. J. Qual. Care Clin. Outcomes 2020. [Google Scholar] [CrossRef] [PubMed]
- González-Pacheco, H.; Amezcua-Guerra, L.M.; Sandoval, J.; Martínez-Sánchez, C.; Ortiz-León, X.A.; Peña-Cabral, M.A.; Bojalil, R. Prognostic Implications of Serum Albumin Levels in Patients with Acute Coronary Syndromes. Am. J. Cardiol. 2017, 119, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Rothman, K.J.; Cupples, L.A.; Levy, D.; Ellison, R.C. Serum Albumin and Risk of Myocardial Infarction and All-Cause Mortality in the Framingham Offspring Study. Circulation 2002, 106, 2919–2924. [Google Scholar]
- Nelson, J.J.; Liao, D.; Sharrett, A.R.; Folsom, A.R.; Chambless, L.E.; Shahar, E.; Szklo, M.; Eckfeldt, J.; Heiss, G. Serum Albumin Level As a Predictor of Incident Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2000, 151, 468–477. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart. J. 2016, 37, 2129–2200. [Google Scholar]
- DeGeare, V.S.; A Boura, J.; Grines, L.L.; O’Neill, W.W.; Grines, C.L. Predictive Value of the Killip Classification in Patients Undergoing Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction. Am. J. Cardiol. 2001, 87, 1035–1038. [Google Scholar] [CrossRef]
- Kernis, S.J.; Harjai, K.J.; Stone, G.W.; Grines, L.L.; A Boura, J.; Yerkey, M.W.; O’Neill, W.; Grines, C.L. The Incidence, Predictors, and Outcomes of Early Reinfarction After Primary Angioplasty for Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2003, 42, 1173–1177. [Google Scholar] [CrossRef]
- Nienhuis, M.B.; Ottervanger, J.P.; Dambrink, J.-H.E.; De Boer, M.-J.; Hoorntje, J.C.; Gosselink, A.M.; Suryapranata, H.; HofA, W.V. Comparative Predictive Value of Infarct Location, Peak CK, and Ejection Fraction After Primary PCI for ST Elevation Myocardial Infarction. Coron. Artery Dis. 2009, 20, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sung, S.-H.; Cheng, H.; Hsu, P.; Guo, C.; Yu, W.; Chen, C. Prognostic Nutritional Index and the Risk of Mortality in Patients with Acute Heart Failure. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kook, H.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Influence of Undernutrition at Admission on Clinical Outcomes in Patients with Acute Myocardial Infarction. J. Cardiol. 2017, 69, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The Prognostic Impact of Objective Nutritional Indices in Elderly Patients with ST-Elevation Myocardial Infarction Undergoing Primary Coronary Intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kim, W.-J.; Kim, J.-Y.; Chin, J.-H.; Choi, D.-K.; Choo, S.J.; Chung, C.-H.; Lee, J.-W.; Choi, I.-C.; Sim, J.-Y. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-Pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less than 4.0 g/Dl. Anesthesiol. 2016, 124, 1001–1011. [Google Scholar] [CrossRef]
- Alfonso, A.I.Q.; Fernández-Castillo, R.; Gallegos, R.F.; Jimenez, F.J.G. [Study of Serum Albumin and BMI as Nutritional Markers in Hemodialysis patients]. Nutrición Hospitalaria 2014, 31, 1317–1322. [Google Scholar]
- Ancion, A.; Allepaerts, S.; Oury, C.; Gori, A.-S.; Piérard, L.A.; Lancellotti, P. Serum Albumin Level and Hospital Mortality in Acute non-ischemic Heart Failure. ESC Hear. Fail. 2017, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Mehran, R.; Dangas, G.D.; Nikolsky, E.; Witzenbichler, B.; Guagliumi, G.; Dudek, D.; Généreux, P.; Caixeta, A.; Rabbani, L.; et al. Impact of Leukocyte Count on Mortality and Bleeding in Patients with Myocardial Infarction Undergoing Primary Percutaneous Coronary Interventions. Circulation 2011, 123, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Arquès, S.; Ambrosi, P.; Gélisse, R.; Luccioni, R.; Habib, G. Hypoalbuminemia in Elderly Patients with Acute Diastolic Heart Failure. J. Am. Coll. Cardiol. 2003, 42, 712–716. [Google Scholar] [CrossRef]
- Suzuki, S.; Hashizume, N.; Kanzaki, Y.; Maruyama, T.; Kozuka, A.; Yahikozawa, K. Prognostic Significance of Serum Albumin in Patients with Stable Coronary Artery Disease Treated by Percutaneous Coronary Intervention. PLoS ONE 2019, 14, e0219044. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Dohi, T.; Miyauchi, K.; Shitara, J.; Endo, H.; Doi, S.; Konishi, H.; Naito, R.; Tsuboi, S.; Ogita, M.; et al. Long-Term Clinical Impact of Serum Albumin in Coronary Artery Disease Patients with Preserved Renal Function. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, C.; Gu, J.; Chen, J.; Wang, L.-C.; Lu, Y.; Huang, C.-Y.; He, Y.-M.; Yang, X.-J. Impact of Serum Albumin Levels on Long-Term All-Cause, Cardiovascular, and Cardiac Mortality in Patients with First-Onset Acute Myocardial Infarction. Clin. Chim. Acta 2018, 477, 89–93. [Google Scholar] [CrossRef]
- He, Y.-M.; Yang, Q.; Yang, X.-J.; Zhao, X.; Xu, H.-F.; Qian, Y.-X. Serum Albumin Concentrations, Effect Modifiers and First Incident Acute Myocardial Infarction: A Cross-Sectional Study of 1552 Cases and 6680 Controls. Clin. Chim. Acta 2016, 454, 49–56. [Google Scholar] [CrossRef]
- Grodin, J.L.; Lala, A.; Stevens, S.R.; Devore, A.D.; Cooper, L.B.; AbouEzzeddine, O.F.; Mentz, R.J.; Groarke, J.D.; Joyce, E.; Rosenthal, J.L.; et al. Clinical Implications of Serum Albumin Levels in Acute Heart Failure: Insights From DOSE-AHF and ROSE-AHF. J. Card. Fail. 2016, 22, 884–890. [Google Scholar] [CrossRef]
- Mann, D.L. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, O.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation As a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
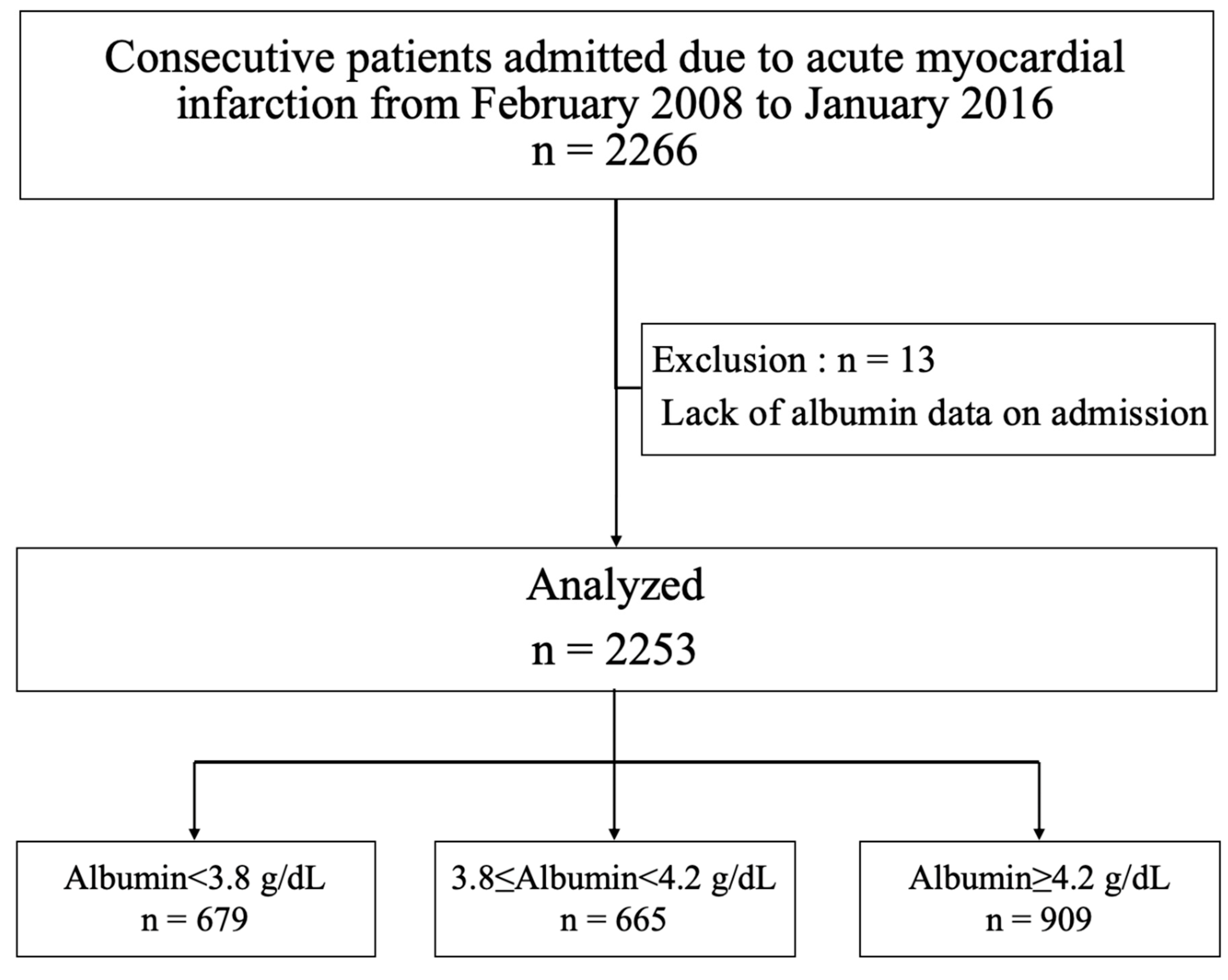
| Variables | Total | Alb < 3.8 g/dL | 3.8 ≤ Alb < 4.2 g/dL | Alb ≥ 4.2 g/dL | p |
|---|---|---|---|---|---|
| (n = 2253) | (n = 679) | (n = 665) | (n = 909) | ||
| Male | 1571 (69.7) | 397 (58.5) | 470 (70.7) | 704 (77.5) | <0.001 |
| Age, years | 70.1 ± 12.7 | 77.0 ± 10.9 | 71.4 ± 11.5 | 64.1 ± 12.0 | <0.001 |
| Body mass index, kg/m2 | 23.7 ± 3.8 | 22.4 ± 3.6 | 23.7 ± 3.7 | 24.7 ± 3.7 | <0.001 |
| Heart rate, /min | 79.0 ± 21.4 | 80.9 ± 26.0 | 77.4 ± 21.2 | 78.7 ± 17.3 | 0.019 |
| Systolic blood pressure, mm Hg | 137.9 ± 31.2 | 127 ± 33 | 136 ± 30 | 147 ± 28 | <0.001 |
| Medical history | |||||
| Hypertension | 1568 (69.6) | 494 (72.8) | 446 (67.1) | 628 (69.1) | 0.069 |
| Dyslipidemia | 1068 (47.4) | 243 (35.8) | 314 (47.2) | 511 (56.2) | <0.001 |
| Diabetes mellitus | 738 (32.8) | 250 (36.8) | 205 (30.8) | 283 (31.1) | 0.027 |
| Smoking | 981 (43.5) | 230 (33.9) | 293 (44.0) | 458 (50.4) | <0.001 |
| Family history of cardiovascular disease | 194 (8.6) | 38 (5.6) | 68 (10.2) | 88 (9.7) | <0.001 |
| Myocardial infarction | 115 (5.1) | 53 (7.8) | 36 (5.4) | 26 (2.9) | <0.001 |
| Malignancy | 87 (3.9) | 37 (5.5) | 25 (3.8) | 25 (2.8) | 0.023 |
| WBC, ×103/mL | 92 (56.4–120) | 96 (72–126) | 91 (70–117) | 90 (69–118) | 0.315 |
| Hemoglobin, g/dL | 13.3 ± 2.3 | 11.8 ± 2.2 | 13.3 ± 1.9 | 14.5 ± 1.8 | <0.001 |
| eGFR, mL/min/1.73 m2 | 63.1 ± 23.9 | 51.8 ± 23.9 | 62.0 ± 22.9 | 72.3 ± 20.5 | <0.001 |
| Alanine aminotransferase, U/L | 22.0 (15–35) | 24.0 (17–37) | 21.0 (15–31) | 22.5 (17–37) | <0.001 |
| Triglyceride, mg/dL | 107 (74–158) | 87 (65–117) | 103 (74–148) | 132 (89–200) | <0.001 |
| Total-cholesterol, mg/dL | 194.5 ± 48.9 | 172.6 ± 43.8 | 194.2 ± 42.6 | 210.3 ± 50.7 | <0.001 |
| LDL-cholesterol, mg/dL | 121.3 ± 37.9 | 105.0 ± 33.4 | 121.8 ± 35.7 | 132.5 ± 38.4 | <0.001 |
| HDL-cholesterol, mg/dL | 46.6 ± 13.4 | 43.9 ± 13.7 | 67.4 ± 13.3 | 48.6 ± 13.3 | 0.248 |
| High-sensitivity troponin T, ng/mL (upper limit of normal: 0.032) | 0.33 (0.05–2.07) | 1.25 (0.14–8.47) | 0.27 (0.06–2.00) | 0.15 (0.03–1.15) | <0.001 |
| Brain natriuretic peptide, pg/mL (upper limit of normal: 18.4) | 77.8 (24.0–287.2) | 335.9 (97.7–790.5) | 73.1 (26.9–221.8) | 34.9 (14.5–93.9) | <0.001 |
| STEMI | 1558 (69.2) | 624 (68.7) | 471 (70.8) | 463 (68.2) | 0.526 |
| NSTEMI | 695 (30.8) | 285 (31.3) | 194 (29.2) | 216 (31.8) | |
| Onset-to-admission time, min | 275 (160–632) | 335 (184–1071) | 260 (160–600) | 246 (159–503) | <0.001 |
| Delayed arrival (≥48 h from onset) | 64 (2.8) | 29 (4.3) | 20 (3.0) | 15 (1.7) | <0.001 |
| Killip class ≥3 | 250 (11.5) | 166 (25.5) | 64 (9.9) | 20 (2.3) | <0.001 |
| LVEF, % | 55.3 ± 13.1 | 50.3 ± 14.6 | 56.1 ± 12.5 | 58.3 ± 11.1 | <0.001 |
| Pre-TIMI grade 0.1 | 1181 (52.4) | 335 (49.3) | 343 (51.6) | 499 (54.9) | 0.346 |
| Peak creatine kinase, IU/L | 1423 (499–3250) | 1077 (372–2588) | 1522 (593–3460) | 1620 (517–3382) | 0.118 |
| Revascularization | 2048 (90.9) | 574 (84.0) | 623 (93.7) | 851 (93.6) | <0.001 |
| PCI | 1965 (87.2) | 540 (79.5) | 597 (89.8) | 828 (91.1) | <0.001 |
| CABG | 83 (3.6) | 34 (5.0) | 26 (4.0) | 23 (2.7) | <0.001 |
| IABP | 286 (12.7) | 151 (22.8) | 68 (10.4) | 67 (7.3) | <0.001 |
| ECMO | 64 (2.8) | 40 (5.9) | 11 (1.7) | 13 (1.4) | <0.001 |
| Length of hospital stay, days | 15 (12–20) | 17 (11–26) | 15 (12–21) | 14 (12–17) | <0.001 |
| Medication at discharge | |||||
| Antiplatelet | 2026 (96.5) | 537 (93.4) | 616 (97.5) | 873 (97.5) | <0.001 |
| Statin | 1753 (83.5) | 435 (75.9) | 511 (80.9) | 807 (90.2) | <0.001 |
| β-blocker | 987 (47.0) | 272 (47.5) | 281 (44.5) | 434 (48.5) | 0.288 |
| ACE-I | 534 (25.4) | 107 (18.7) | 162 (25.6) | 265 (29.6) | <0.001 |
| ARB | 853 (40.6) | 213 (37.2) | 277 (43.8) | 363 (40.6) | 0.063 |
| MRA | 258 (12.3) | 125 (21.8) | 83 (13.1) | 50 (5.6) | <0.001 |
| Diuretic | 475 (22.6) | 227 (39.6) | 145 (22.9) | 103 (11.5) | <0.001 |
| Primary composite outcome | 305 (13.5) | 168 (24.7) | 91 (13.7) | 46 (5.1) | <0.001 |
| Hospitalization for heart failure | 146 (6.5) | 70 (10.3) | 46 (6.9) | 30 (3.3) | <0.001 |
| Cardiovascular death | 192 (8.5) | 116 (17.1) | 53 (8.0) | 23 (2.5) | <0.001 |
| Secondary outcome, n (%) | |||||
| All-cause death | 375 (16.6) | 227 (33.4) | 97 (14.6) | 51 (5.6) | <0.001 |
| In-hospital death | 154 (6.8) | 107 (15.8) | 33 (5.0) | 14 (1.5) | <0.001 |
| Outcomes | Albumin < 3.8 g/dL | 3.8 ≤ Albumin < 4.2 g/dL | Albumin ≥ 4.2 g/dL (Reference) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | |
| Primary composite outcome | |||||||
| Crude | 4.89 | 1.64–6.67 | <0.001 | 2.70 | 1.92–3.80 | <0.001 | 1.00 |
| Model 1 | 4.69 | 3.29–6.81 | <0.001 | 1.85 | 1.29–2.70 | <0.001 | 1.00 |
| Model 2 | 4.52 | 3.11–6.63 | <0.001 | 1.65 | 1.12–2.47 | 0.010 | 1.00 |
| Model 3 | 3.44 | 1.97–6.90 | <0.001 | 1.81 | 1.05–3.20 | 0.033 | 1.00 |
| Model 4 | 2.94 | 1.37–6.51 | <0.005 | 2.84 | 1.41–5.93 | 0.003 | 1.00 |
| Hospitalization for heart failure | |||||||
| Crude | 3.12 | 2.06–4.74 | <0.001 | 2.10 | 1.33–3.28 | <0.001 | 1.00 |
| Model 1 | 3.12 | 1.93–5.15 | <0.001 | 1.56 | 0.96–2.55 | 0.068 | 1.00 |
| Model 2 | 3.12 | 1.89–5.25 | <0.001 | 1.56 | 0.94–2.60 | 0.079 | 1.00 |
| Model 3 | 4.03 | 2.04–8.19 | <0.001 | 1.97 | 1.03–3.87 | 0.040 | 1.00 |
| Model 4 | 3.69 | 1.56–9.15 | 0.003 | 2.55 | 1.15–5.92 | 0.021 | 1.00 |
| Cardiovascular death | |||||||
| Crude | 6.75 | 4.37–10.4 | <0.001 | 3.15 | 1.95–5.09 | <0.001 | 1.00 |
| Model 1 | 6.15 | 3.84–10.2 | <0.001 | 2.12 | 1.29–3.59 | 0.003 | 1.00 |
| Model 2 | 5.80 | 3.54–9.85 | <0.001 | 1.71 | 1.01–2.99 | 0.048 | 1.00 |
| Model 3 | 5.07 | 2.01–13.8 | <0.001 | 2.00 | 0.79–5.06 | 0.142 | 1.00 |
| Model 4 | 4.77 | 1.39–18.4 | 0.012 | 2.14 | 0.64–7.11 | 0.211 | 1.00 |
| All-cause death | |||||||
| Crude | 5.96 | 4.47–7.94 | <0.001 | 2.60 | 1.88–3.59 | <0.001 | 1.00 |
| Model 1 | 5.61 | 4.06–7.88 | <0.001 | 1.80 | 1.27–2.58 | <0.001 | 1.00 |
| Model 2 | 5.55 | 3.93–7.99 | <0.001 | 1.69 | 1.16–2.48 | 0.006 | 1.00 |
| Model 3 | 5.30 | 3.26–8.86 | <0.001 | 1.79 | 1.08–3.03 | 0.023 | 1.00 |
| Model 4 | 3.99 | 2.19–7.45 | <0.001 | 1.70 | 1.01–2.90 | 0.045 | 1.00 |
| In-hospital death | |||||||
| Crude | 10.2 | 5.91–17.7 | <0.001 | 3.22 | 1.74–5.97 | <0.001 | 1.00 |
| Model 1 | 8.16 | 4.64–15.4 | <0.001 | 2.21 | 1.18–4.37 | 0.013 | 1.00 |
| Model 2 | 1.41 | 1.25–1.59 | <0.001 | 0.97 | 0.87–1.82 | 0.603 | 1.00 |
| Model 3 | 1.26 | 1.09–1.46 | 0.002 | 0.96 | 0.85–1.08 | 0.485 | 1.00 |
| Model 4 | 3.00 | 1.11–8.66 | <0.001 | 0.80 | 0.24–0.91 | 0.541 | 1.00 |
| Subgroups | Albumin < 3.8 g/dL | 3.8 ≤ Albumin < 4.2 g/dL | Albumin ≥ 4.2 g/dL (Reference) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | |
| High-risk group (n = 611) | |||||||
| Crude | 1.87 | 1.17–3.02 | 0.001 | 1.59 | 0.95–2.66 | 0.066 | 1.00 |
| Model 1 | 2.38 | 1.38–4.36 | 0.001 | 1.17 | 0.65–2.18 | 0.592 | 1.00 |
| Model 2 | 2.38 | 1.35–4.46 | 0.002 | 1.11 | 0.59–2.16 | 0.740 | 1.00 |
| Model 3 | 3.96 | 1.52–11.4 | 0.004 | 1.15 | 0.45–3.14 | 0.763 | 1.00 |
| Model 4 | 6.41 | 2.01–21.1 | <0.001 | 2.30 | 0.78–7.36 | 0.128 | 1.00 |
| Non-high-risk group (n = 1642) | |||||||
| Crude | 6.10 | 4.07–9.15 | <0.001 | 2.79 | 1.79–4.36 | <0.001 | 1.00 |
| Model 1 | 4.74 | 2.98–7.70 | <0.001 | 1.84 | 1.15–2.99 | 0.011 | 1.00 |
| Model 2 | 4.40 | 2.67–7.40 | <0.001 | 1.45 | 0.86–2.45 | 0.156 | 1.00 |
| Model 3 | 2.22 | 0.75–6.32 | 0.146 | 1.72 | 0.76–3.99 | 0.191 | 1.00 |
| Model 4 | 2.54 | 1.02–6.61 | 0.044 | 2.11 | 0.68–6.54 | 0.193 | 1.00 |
| STEMI (n = 1558) | 1.00 | ||||||
| Crude | 5.45 | 3.82–7.93 | <0.001 | 2.33 | 1.58–3.47 | <0.001 | 1.00 |
| Model 1 | 3.67 | 2.46–5.57 | <0.001 | 1.60 | 1.07–2.43 | 0.002 | 1.00 |
| Model 2 | 3.40 | 2.22–5.28 | <0.001 | 1.49 | 0.97–2.30 | 0.069 | 1.00 |
| Model 3 | 3.47 | 1.71–7.14 | <0.001 | 1.89 | 1.03–3.53 | 0.038 | 1.00 |
| Model 4 | 3.75 | 1.70–8.68 | 0.001 | 3.51 | 1.31–9.64 | 0.012 | 1.00 |
| NSTEMI (n = 695) | 1.00 | ||||||
| Crude | 14.8 | 6.80–39.1 | <0.001 | 6.61 | 2.82–18.1 | <0.001 | 1.00 |
| Model 1 | 11.0 | 4.79–29.8 | <0.001 | 4.09 | 1.66–11.6 | <0.001 | 1.00 |
| Model 2 | 11.0 | 4.72–30.3 | <0.001 | 2.74 | 1.05–8.12 | 0.038 | 1.00 |
| Model 3 | 12.0 | 3.38–57.8 | <0.001 | 3.02 | 0.68–17.5 | 0.149 | 1.00 |
| Model 4 | 7.04 | 1.21–63.8 | 0.0284 | 6.45 | 0.01–2201 | 0.531 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, G.; Tanaka, A.; Nishihira, K.; Shibata, Y.; Node, K. Prognostic Impact of Serum Albumin for Developing Heart Failure Remotely after Acute Myocardial Infarction. Nutrients 2020, 12, 2637. https://doi.org/10.3390/nu12092637
Yoshioka G, Tanaka A, Nishihira K, Shibata Y, Node K. Prognostic Impact of Serum Albumin for Developing Heart Failure Remotely after Acute Myocardial Infarction. Nutrients. 2020; 12(9):2637. https://doi.org/10.3390/nu12092637
Chicago/Turabian StyleYoshioka, Goro, Atsushi Tanaka, Kensaku Nishihira, Yoshisato Shibata, and Koichi Node. 2020. "Prognostic Impact of Serum Albumin for Developing Heart Failure Remotely after Acute Myocardial Infarction" Nutrients 12, no. 9: 2637. https://doi.org/10.3390/nu12092637
APA StyleYoshioka, G., Tanaka, A., Nishihira, K., Shibata, Y., & Node, K. (2020). Prognostic Impact of Serum Albumin for Developing Heart Failure Remotely after Acute Myocardial Infarction. Nutrients, 12(9), 2637. https://doi.org/10.3390/nu12092637





